Abstract
Ethanol exposure during fetal development is a leading cause of long-term cognitive impairments. Studies suggest that ethanol exposure have deleterious effects on the hippocampus, a brain region that is important for learning and memory. Ethanol exerts its effects, in part, via alterations in glutamatergic neurotransmission, which is critical for the maturation of neuronal circuits during development. The current literature strongly supports the growing evidence that ethanol inhibits glutamate release in the neonatal CA1 hippocampal region. However, the exact molecular mechanism responsible for this effect is not well understood. In this study, we show that ethanol enhances endocannabinoid (EC) levels in cultured hippocampal neurons, possibly through calcium pathways. Acute ethanol depresses miniature postsynaptic current (mEPSC) frequencies without affecting their amplitude. This suggests that ethanol inhibits glutamate release. The CB1 receptors (CB1Rs) present on presynaptic neurons are not altered by acute ethanol. The CB1R antagonist SR 141716A reverses ethanol-induced depression of mEPSC frequency. Drugs that are known to enhance the in vivo function of ECs occlude ethanol effects on mEPSC frequency. Chelation of postsynaptic calcium by EGTA antagonizes ethanol-induced depression of mEPSC frequency. The activation of CB1R with the selective agonist WIN55,212-2 also suppresses the mEPSC frequency. This WIN55,212-2 effect is similar to the ethanol effects and is reversed by SR141716A. In addition, tetani-induced excitatory postsynaptic currents (EPSCs) are depressed by acute ethanol. SR141716A significantly reverses ethanol effects on evoked EPSC amplitude in a dual recording preparation. These observations, taken together, suggest the participation of ECs as retrograde messengers in the ethanol-induced depression of synaptic activities.
Keywords: Development, FASD, hippocampal neurons, ethanol, endocannabinoids, retrograde messenger activity
Introduction
Ethanol exposure during development can produce characteristic physiological and cognitive deficits, often termed Fetal Alcohol Spectrum Disorder (FASD) (Sokol et al. 2003). Studies suggest that the effects of ethanol in the developing CNS are, to some extent, the result of alterations in neurotransmission at glutamatergic synapses (Ikonomidou et al. 2001). Glutamate mediates fast excitatory neurotransmission in the CNS and interacts with NMDA (NMDARs), AMPA (AMPARs), and kainate receptors (KARs) (Dingledine et al. 1999). Although it is widely accepted that ethanol acutely inhibits NMDA and non-NMDARs, several studies suggest that their sensitivity varies both among different CNS regions and as a function of development. Recent studies also suggest that, compared to adult animals, hippocampal slices from juvenile/early-adolescent animals show increased sensitivity to synaptic plasticity (Pyapali et al. 1999), a cellular model for learning and memory (Bliss & Collingridge 1993). Given that hippocampal synaptic transmission and synaptic plasticity during early development contribute to synapse development (Costa et al. 2000, Hua & Smith 2004, Ikonomidou et al. 2001, Molnar et al. 2002), it is important to understand how ethanol modifies synaptic transmission during early CNS development.
Numerous studies suggest that endogenously produced cannabinoids (endocannabinoids, ECs) mediate synaptic plasticity in regions of the brain that are involved in learning and memory (Chevaleyre et al. 2006). Anandamide (N-arachidonylethanolamide, AEA) (Devane et al. 1992), 2-arachidonylglycerol (2-AG) (Mechoulam et al. 1995, Sugiura et al. 1995b), and a family of fatty acid ethanolamides have been identified as ECs (Matsuda et al. 1990, Devane et al. 1992). The EC-mediated plasticity encompasses many forms of transient and long-lasting synaptic depression, and is found at both excitatory and inhibitory synapses (Chevaleyre et al. 2006, Ohno-Shosaku et al. 2001, Wilson & Nicoll 2001). Consequently, the EC system is now seen as a major target in synaptic plasticity. Although several lines of evidence suggest that the CB1 receptors and their endogenous agonists play an important role in the pharmacological action of ethanol (Basavarajappa et al. 2000, Basavarajappa et al. 2003), the molecular mechanism underlying early CNS development has not yet been characterized.
In the present study, we have used hippocampal cultures derived from day 1 pups to investigate the possible involvement of ECs in the acute ethanol-induced inhibition of excitatory neurotransmission and synaptic plasticity using patch-clamp electrophysiological techniques. We have found that acute ethanol-induced inhibition of spontaneous neurotransmission and synaptic plasticity is mediated by a retrograde signaling via ECs in hippocampal neurons.
Methods
Cell culture
Hippocampal cultures were prepared from 1-day-old male and female C57BL/6J mouse pups, as described previously (Ninan & Arancio 2004). Animals were housed in groups under standard laboratory conditions (12 hr light/12 hr dark cycle) with food and water available ad libitum. Animal care and handling procedures followed Institutional and National Institutes of Health guidelines.
Endocannabinoid extraction and chromatography
The hippocampal neurons (15 days in vitro, DIV) (60 mm plates) were incubated with [3H]-arachidonic acid (AA) (1 μCi/ml) in neurobasal media for 22 h. Media were monitored for uptake and it was found that 95 % of the total AA was incorporated into the neurons within 22 h of incubation. The neurons were then washed three times with media containing 0.1 % BSA to remove essentially all the free AA. The neurons were then subjected to acute ethanol exposure (25 and 50 mM for 30 or 60 min) in bath solution. EGTA (10 mM) was added along with ethanol (50 mM, 30 min). The cells were scraped and then suspended in 10 mM Tris-HCl buffer pH 7.4 containing a protease inhibitor cocktail (Sigma). Lipids from cells and media were extracted using a mixture of chloroform/methanol (2:1, v/v); 2 μg of unlabeled AEA was included as a carrier. Butyrate hydroxy toluene (BHT) (0.05%) was added to prevent lipid peroxidation. Both the extraction and chromatography procedures were similar to those described previously (Basavarajappa & Hungund 1999a, Basavarajappa et al. 2000, Basavarajappa et al. 2003). Data (media and cells) are expressed as the (mean ± SEM) dpm/mg cellular protein or percentage of control.
Immunocytochemistry and immunoblot
Neurons were fixed with 4% paraformaldehyde/4% sucrose in Tris-buffered saline (TBS; 100 mM Tris, 0.9% NaCl) for 15 minutes. Permeabilization was performed in TBS with 0.1% Triton X-100 for 5 minutes. After blocking with 4% normal goat serum (NGS) for 30 minutes, incubation with primary antibodies was performed overnight at 4°C. Co-localization of CB1 receptors with pre- and postsynaptic proteins was performed using anti-synaptophysin (Zymed, San Francisco, CA) and anti-PSD-95 (Affinity Bioreagents, Golden, CO) antibodies, respectively. Cultures were washed three times in PBS for 10 minutes each time, and then incubated with secondary antibodies for 1 hour at 4°C. The secondary antibodies, either Alexa 488-conjgated-goat anti-rabbit IgG or Alexa 568-conjugated goat anti-mouse IgG (4 μg/ml; Molecular Probes), were used. The cultures were then washed five times in PBS for 10 minutes each, mounted in Fluoromont G, and examined with a Nikon D-Eclipse C1 confocal microscope. Averages of four scans were collected for each image. For all measurements, the mean results from 10 neurons in a dish were normalized to the mean from control dishes in the same culture batch. These values were used for statistical comparison (using t-tests) of dishes that received different experimental treatments. In some experiments, Alexa Fluor 568 phalloidin was used to stain F-Actin. Cells were scraped and collected by centrifugation at 3000 ×g for 5 minutes. Pellets were resuspended in PBS plus a protease inhibitor cocktail and sonicated for 1 minute as described elsewhere (Veeranna et al. 2004). Blots were incubated with primary antibody rabbit anti-mouse CB1 receptor (Affinity Bioreagents, Golden, CO) for 1 hr at room temperature and processed as described before (Veeranna et al. 2004).
Electrophysiology
Electrophysiological studies were performed on 10- to 17-day-old hippocampal neurons in vitro. Neurons were voltage clamped with the whole cell ruptured patch technique throughout the experiment, as described previously (Ninan & Arancio 2004). The bath solution consisted of (in mM) NaCl (119), KCl (5), HEPES (20), CaCl2 (2), MgCl2 (2), glucose (30), glycine (0.001), and picrotoxin (0.1); it was maintained at pH 7.3 and the osmolarity adjusted to 330 mOsm with sucrose. The solution in the whole cell patch electrode consisted of (in mM) K-gluconate (130), KCl (10), MgCl2 (5), EGTA (0.6), HEPES (5), CaCl2 (0.06), Mg-ATP (2), GTP (0.2), leupeptin (0.2), phosphocreatine (20), and creatine-phosphokinase (50 U/ml). For the miniature postsynaptic current (mEPSC) experiments, 1 μM tetrodotoxin was also added to the bath to suppress action potentials. Currents were recorded with a Warner amplifier (model PC-501A) (Hamden, CT), and were filtered at 1 kHz. In order to eliminate artifacts due to variation in the seal properties, the access resistance was monitored for constancy throughout all experiments. The recordings were digitized (Digidata 1322A, Axon Instruments) and analyzed with the Mini Analysis program (version 4.0) from Synaptosoft, Inc. (Decatur, GA).
Drugs
WIN 55,212-2 was purchased from BIOMOL, (Plymouth Meeting, PA); AM 404, AEA, and 2-AG were from Tocris Cookson Inc. (Ellisville, MO); and SR141716A was a gift from RBI (Natick, MA). URB 597 and URB 602 were from Cayman Chemical (Ann Arbor, MI). SR141716A, AM 404, URB 597, and URB 602 were dissolved in dimethylsulphoxide (DMSO) (0.001%, which was also included in the control bath). EGTA was dissolved in bath solution. The concentrations of WIN 55,212-2 (1 μM), AM 404 (1 μM), SR141716A (2 μM), URB 597 (1 μM), and URB 602 (100 μM) were selected based on their pharmacological and physiological effects in various systems published by various investigators (Diana et al. 2002, Kreitzer & Malenka 2007, Kreitzer & Regehr 2001b, Kreitzer & Regehr 2001a, Ohno-Shosaku et al. 2001, Robbe et al. 2001, Ronesi et al. 2004, Szabo et al. 2006, Yoshida et al. 2002).
Statistical analysis
The effects of all the drugs were quantified with respect to the average of control responses. The Kolmogorov-Smirnov test was used initially to test for significant differences between treatments in individual cells. Statistical comparisons of pooled data were performed by two-way ANOVA with Bonferroni’s post hoc test, one-way ANOVA, or Student’s t test. In all the cases, a p < 0.05 was considered to indicate statistical significance. Statistical analyses were performed with the Mini Analysis program (Synaptosoft, Decatur, GA) or Prism (GraphPad, San Diego, CA). All the data are presented as means ± S.E.
RESULTS
Acute ethanol enhances the formation of endocannabinoids
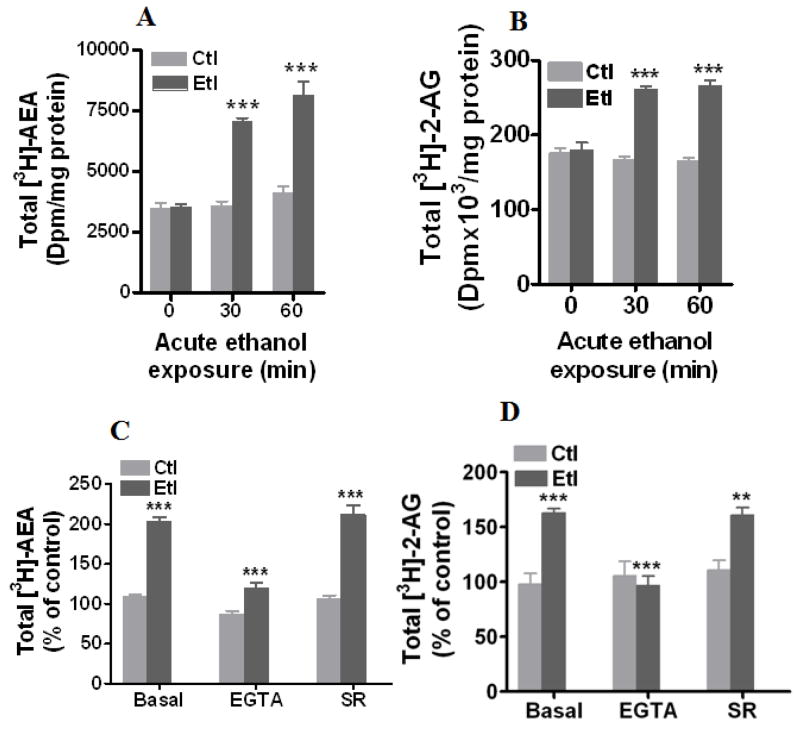
We investigated whether acute ethanol affects the formation of AEA and 2-AG in hippocampal neurons. Treatment of cultured hippocampal neurons with acute ethanol increased the formation of both [3H]-AEA (197 % ± 4 at 30 min and 197 % ± 14 at 60 min, t-test, p< 0.0001) (Fig. 1A) and [3H]-2-AG (156 % ± 4 at 30 min and 161 % ± 5 at 60 min, t-test, p< 0.0001) (Fig. 1B) at 50 mM but not at 25 mM (data not shown). We used only 50 mM ethanol in all our rest of the experiments. It was previously established that formation of ECs is regulated by intracellular calcium in various neuronal cells (Basavarajappa 2007). Hence, we next tested whether calcium chelation with EGTA (10 mM) affects an ethanol-induced increase in ECs. EGTA (10 mM) significantly blocked the ethanol (50 mM, 30 min)-induced increase in both [3H]-AEA (87 % ± 4 in the control and 113 % ± 3 in ethanol, F1, 60 = 41.72, p< 0.0001, two-way ANOVA) (Fig. 1C) and [3H]-2-AG (106 % ± 3 in the control and 97 % ± 8 in ethanol, F1, 24.6 = 30.24, p< 0.0001, two-way ANOVA) (Fig. 1D). In our previous study, co-treatment of cerebellar granular neurons with CB1 receptor antagonist SR 141716 A and ethanol (100 mM) for 72 h blocked the ethanol-induced formation of 2-AG(Basavarajappa et al. 2000). In the present study, we have examined whether SR 141716A modulate the acute ethanol-induced formation of endocannabinoids. SR 141716A (2 μM) did not modulate the acute ethanol-induced formation of [3H]-AEA (Fig. 1B) and [3H]-2-AG (Fig. 1C & D) in hippocampal neurons.
Fig. 1.
Acute ethanol-induced formation of [3H]-AEA and 2-AG. (A–D) Neurons in culture (15 DIV) were labeled with [3H]-AA (1 μCi/ml) in neurobasal media for 22 hours and then treated with or without ethanol (50 mM) for up to 60 minutes. Cells were treated with or without SR141716A(SR) (2μM) or EGTA (10 mM) in the presence or absence of ethanol (50 mM) for 30 minutes. [3H]-AEA and 2-[3H]-AG were extracted from the medium and cells (total), and separated by TLC using solvent system A (n = 8). *P< 0.05, **P< 0.001, ***P< 0.0001.
CB1 receptors are localized to presynaptic neurons and are not affected by acute ethanol
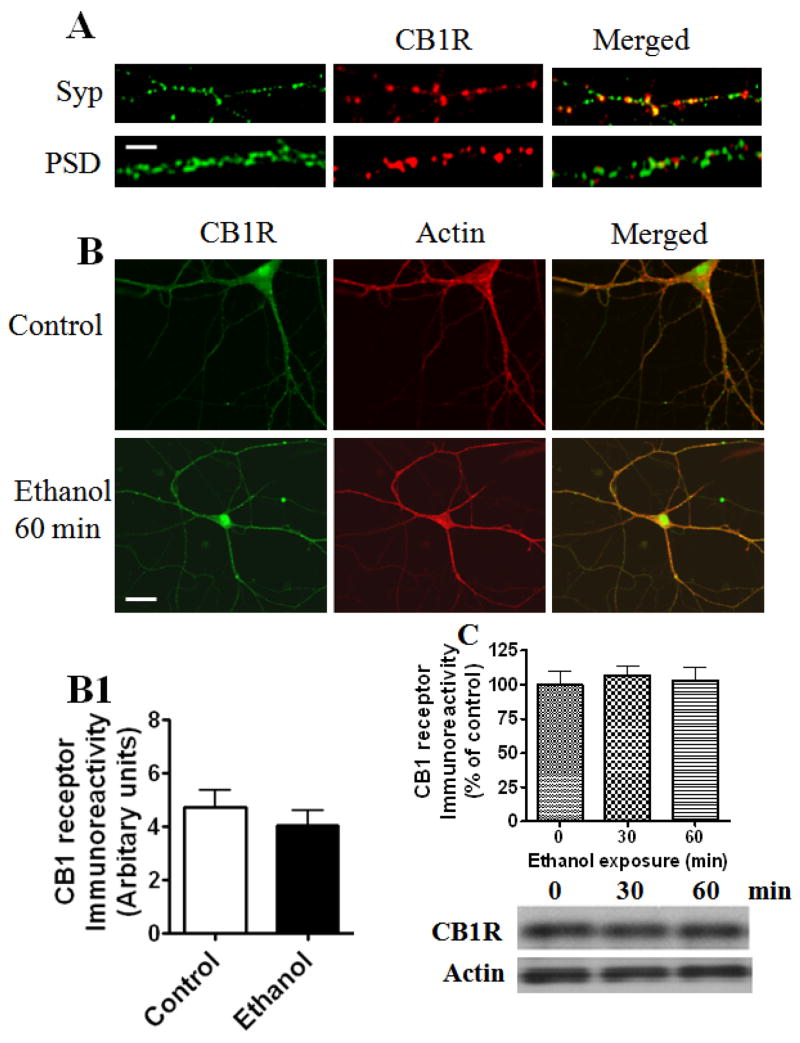
We examined the localization of CB1 receptors and their sensitivity to acute ethanol exposure. Consistent with earlier reports (Katona et al. 1999, Kawamura et al. 2006, Robbe et al. 2001), we found that CB1 receptor immunoreactivity was localized predominantly on neurites (Fig. 2A). To further verify that the observed immunolabelling for CB1 receptors is localized to pre- and/or postsynaptic neurons, we double-labeled the cultures with an antibody against synaptophysin, a vesicle-associated protein used as a presynaptic marker, or against the postsynaptic protein PSD-95. More of the CB1 receptors co-localized with synaptophysin than with the PSD-95 protein (Fig. 2A).
Fig. 2.
CB1 receptor immunoreactivity in cultured hippocampal neurons. Representative images showing immunoreactivity associated with neurites. (A) Panel shows single-plane confocal images from a dual-labeling experiment investigating the co-localization of cell surface CB1 receptors labeled with a synaptophysin (Syp) or PSD-95 antibody after permeabilization in neurons in culture (15 DIV) (n=15). Regions of overlap are in yellow (merged). Scale bars, 20 nm. (B) A representative panel shows single-plane confocal images from a dual-labeling experiment investigating the effects of acute ethanol (50 mM; 60 min) on CB1 receptors. Neurons were labeled with F-actin antibody (Alexa Fluor 568 phalloidin) and CB1 receptor antibody. Red corresponds to F-actin, green to CB1 labeling, and yellow to regions of overlap. Note the marked correspondence between the CB1 receptor label and axons and dendrites of F-actin immunoreactivity, which are not affected by acute ethanol treatment. Scale bar, 50 μm. (B1) Bar graph generated using NIH-LSM software shows the relative co-localization of CB1 receptors with F-actin in control and ethanol treated neurons. (C) There was no significant change in CB1 receptor proteins or actin in hippocampal neurons exposed to acute ethanol (50 mM) for up to 60 min (n=15).
Chronic ethanol treatment has been shown to down-regulate CB1 receptor levels in mouse brain (Basavarajappa et al. 1998, Basavarajappa & Hungund 1999b). However, it is not clear whether acute ethanol has an effect on CB1 receptor levels in the hippocampal neurons. Therefore, we tested whether acute ethanol affects CB1 receptor immunoreactivity in the hippocampal neurons. We did not find any significant difference in CB1 receptor immunoreactivity between control and ethanol-treated cultures (Fig 2B, B1). Immunoblot data further confirmed that acute ethanol has no effect on CB1 receptor levels (60 kDa) (Fig. 2C).
Acute ethanol inhibits synaptic transmission in hippocampal neurons through a presynaptic mechanism
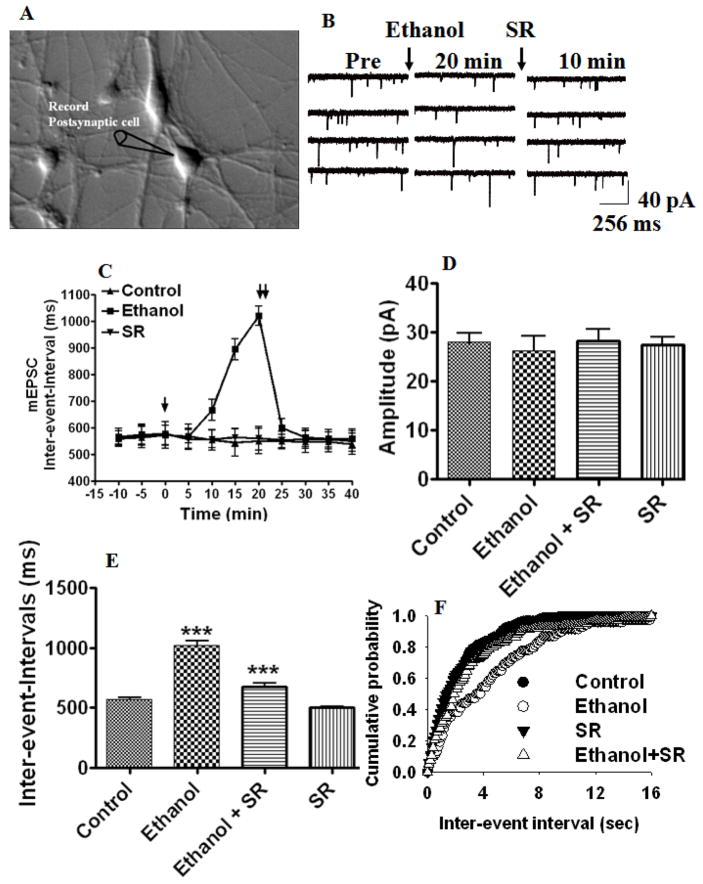
To address whether ethanol-induced ECs participate in inhibition of excitatory neurotransmission, we recorded mEPSCs in the presence of tetrodotoxin (TTX; 1 μM) and picrotoxin (100 μM) to selectively block voltage-dependent Na+ currents and GABAA receptors, respectively. As shown in Fig 3 A–F, in the presence of 50 mM ethanol, the frequency (inter-event-intervals, 178% ± 6 of control at 20 min, p< 0.0001) of mEPSCs decreased after 10-minute exposure (Fig. 3C), whereas the mEPSC amplitude remained unchanged (Fig. 3D). Accordingly, thedistribution of the mEPSC amplitude was also not modified by ethanol (data not shown), whereas the inter-event interval distribution was shifted to the right (Fig. 3F). Selective suppression of the frequency but not amplitude of mEPSCs by ethanol suggests that ethanol suppresses glutamatergic neurotransmission through a presynaptic mechanism in hippocampal neurons.
Fig. 3.
ECs mediate ethanol-induced suppression of mini excitatory postsynaptic currents. (A) Hippocampal neurons in culture (20x). Whole-cell patch-clamp recordings were made from a single pyramidal-shaped neuron. (B) Traces of continuously recorded mEPSCs before (control), during ethanol exposure, and after the addition of the CB1 receptor antagonist SR141716A (SR). (C) In the presence of 50 mM ethanol for 20 min, the frequency was reduced (inter-event intervals) without affecting the amplitude (p<0.0001) (n= 12 neurons). [↓, Vehicle, ethanol or SR was added to the bath solution; ↓↓, SR was added to the bath solution]. (D) Combined plots of the average amplitudes of mEPSCs under control, ethanol, ethanol + SR141716A, and SR141716A treatment conditions (n= 12 neurons) (E) Combined plots of the average inter-event-intervals of mEPSCs under control, ethanol, ethanol + SR141716A, and SR141716A treatment conditions (n= 12 neurons) (***p<0.0001). Average cumulative distributions of mEPSC (F) inter-event intervals (sec) (p < 0.01) in ethanol-treated cells relative to controls (Kolmogorow-Simrnov two-sample test).
Acute ethanol inhibits synaptic transmission in hippocampal neurons through the endocannabinoid system
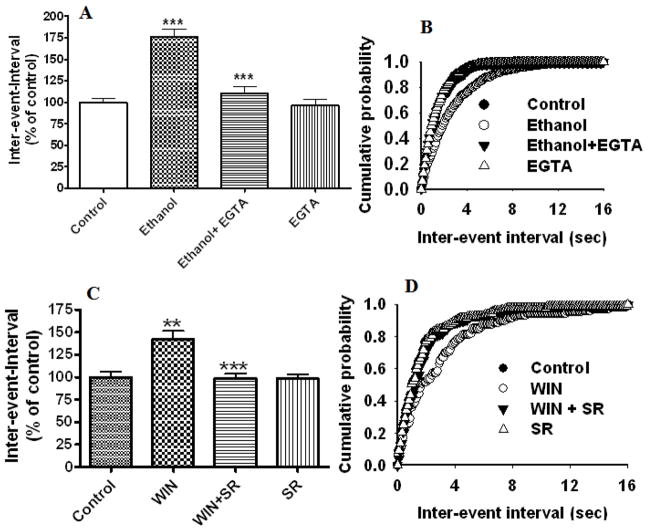
To understand whether ethanol-induced inhibition of mEPSC frequency is mediated through CB1 receptors, we used a selective CB1 receptor antagonist, SR141716A, in our next experiment. This compound antagonized the effects of ethanol on mEPSC frequency (inter-event-intervals, 118 % ± 6 of control, p< 0.0001, two-way ANOVA) (Fig. 3E). The inter-event interval distribution is shown in Fig. 3G. These results suggest that ethanol-induced depression of mEPSC frequency involves ECs in our recording conditions. Since the ethanol-induced increase in ECs is mediated through calcium signaling, we examined the role of postsynaptic calcium in ethanol-induced inhibition of mEPSC frequency. Perfusion of EGTA (10 mM) in to the postsynaptic neuron significantly reduced the ethanol-induced depression of mEPSC frequency (inter-event-intervals, 110% ± 8 of control at 20 min, F1,52 = 50, one-way ANOVA, p< 0.0001, n=6 neurons) (Fig. 4 A and B) without affecting the amplitude (data not shown). Similar to the effect of ethanol, the addition of selective exogenous CB1 receptor agonist WIN 55, 212-2 to the bath solution inhibited mEPSC frequency (Fig. 4 C & D) (inter-event-intervals, 142 % ± 9 of control at 20 min, F1,52 = 50.23, one-way ANOVA, p< 0.0001, n=10 neurons) without affecting the amplitude. This effect was reversed by SR141716A (inter-event-intervals, 101 % ± 12 of control, t-test, p< 0.0001, n=10 neurons).
Fig. 4.
Postsynaptic perfusion of EGTA into the postsynaptic neuron rescues the ethanol-induced depression of mEPSC frequency and bath application of CB1 receptor antagonist rescues WIN 55, 212-2 –induced suppression of mEPSC frequency. (A) Combined plots showing the perfusion of EGTA into the postsynaptic neuron antagonized the ethanol-induced depression of frequency (increased inter-event intervals) (p<0.0001) (n=6 neurons). EGTA alone has no significant effect on mEPSC frequency. (B) Average cumulative distributions showing significant inhibition of the ethanol-induced depression of mEPSC inter-event intervals (sec) relative to ethanol by postsynaptic perfusion of EGTA (p < 0.01; Kolmogorow-Simrnov two-sample test). (C) Combined plot showing the bath application of WIN55,212-2 suppresses mEPSC frequency without affecting the amplitude. SR141716A antagonized the WIN 55,212-2-induced depression of mEPSC frequency. SR141716A alone does not significantly affect mEPSC frequency and amplitude. (D) Average cumulative distributions of mEPSC inter-event interval (sec) showing a decrease in mEPSC frequency in WIN 55,212-2-treated cells relative to control (n = 6 neurons). (p < 0.01; Kolmogorow-Simrnov two-sample test).
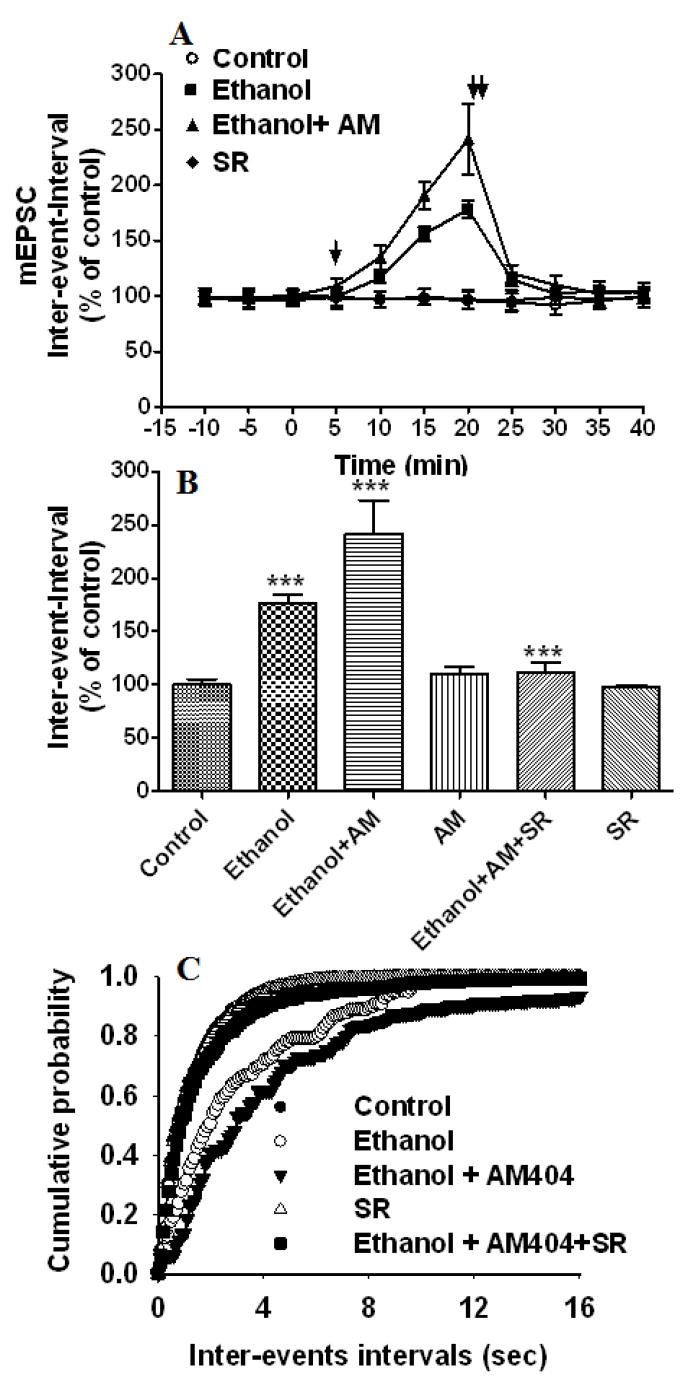
It is well established that EC function can be potentiated by blocking its re-uptake across cell membranes (Beltramo & Piomelli 2000, Beltramo et al. 1997). We tested the effect of the EC uptake inhibitor (AM 404) on ethanol-induced depression of mEPSC frequency. Application of AM 404 (Fig. 5A–C) (1 μM) (bath) further enhanced the ethanol-induced depression of mEPSC frequency after 5 minutes of exposure (Fig. 5A) (inter-event-intervals, 242 % ± 32 of control at 20 min, F3,358 = 25.83, p< 0.0001, two-way ANOVA) without affecting the mEPSC amplitude. Accordingly, the distribution of the mEPSC amplitude was not modified by ethanol and AM 404 treatments, whereas the ethanol-induced right shift in the inter-event interval distribution was further shifted to the right by AM 404 treatments (Fig. 5C). Application of SR141716A (2 μM) abolished the effects of ethanol and AM 404 (Fig. 5B) on mEPSC frequency (inter-event-intervals, 111 % ± 8 of control, t-test, p< 0.0001). As AM 404 is not a CB1 agonist (Beltramo et al. 1997), these results imply that the endogenous substrate for the AM 404-sensitive uptake is an EC that inhibits mEPSC frequency when it is allowed to accumulate in the cell culture. AM 404 (1 μM) alone had no significant effect on mEPSC frequency, suggesting that higher concentrations of AM 404 may be required to effect the mEPSC frequency in these preparations and warrant future investigation.
Fig. 5.
The EC uptake inhibitor AM 404 enhances ethanol-induced suppression of mEPSC frequency. (A) Application of 50 mM ethanol (bath) decreased the mEPSC frequency (inter-event intervals) (p<0.0001) (n= 8 neurons) of the control. Addition of AM 404 to the bath solution in the presence of 50 mM ethanol enhanced the ethanol-induced depression of frequency (inter-events intervals) (n=13 neurons). [↓, Vehicle, ethanol, AM 404 or SR141716A (SR) was added to the bath solution; ↓↓, SR141716A was added to the bath solution]. (B) Combined plots of the average inter-event-intervals of mEPSCs under control, ethanol, ethanol + AM 404 +SR141716A treatment conditions. AM 404 alone does not affect mEPSC frequency or amplitude. SR141716A antagonized the effect of AM 404 on ethanol-induced inhibition of mEPSC frequency (n=13 neurons) (**p<0.001; ***p<0.0001). SR141716A alone does not affect mEPSC frequency and amplitude. (C) Average cumulative distributions showing an increase in mEPSC inter-event intervals (sec) in ethanol + AM 404-treated cells relative to controls and ethanol (p < 0.001; Kolmogorow-Simrnov two-sample test).
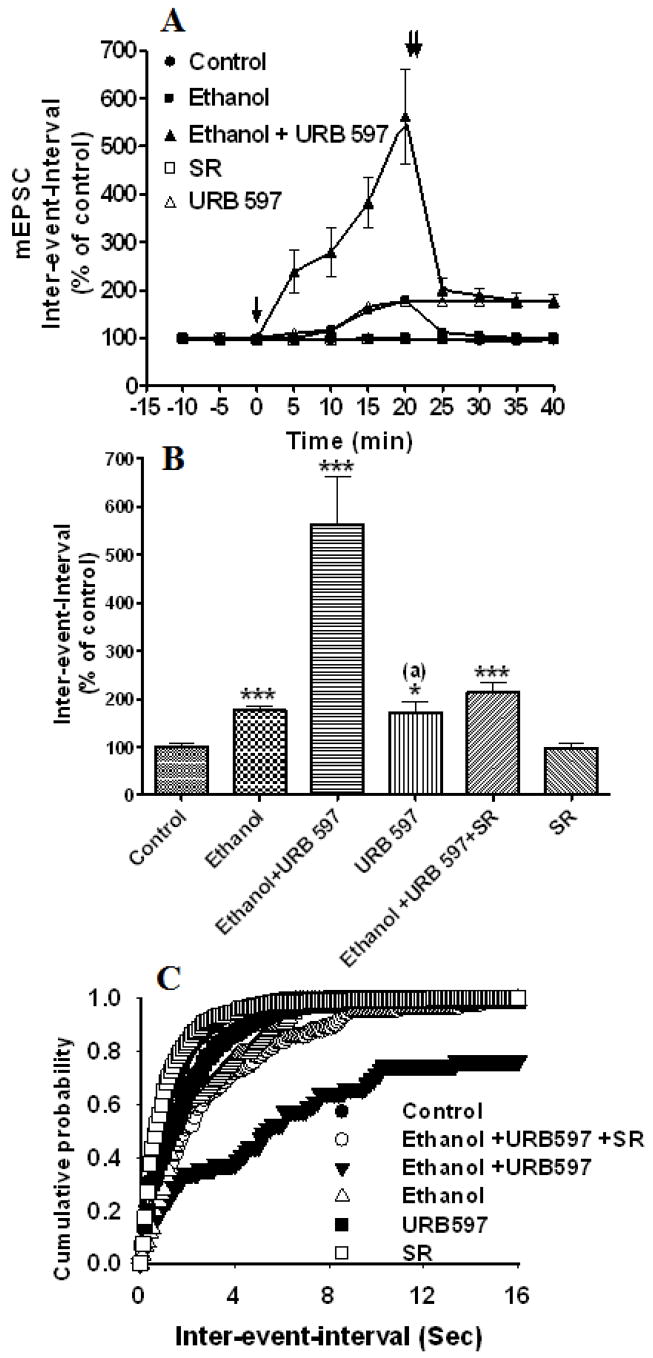
We also examined the effects of blockage of the AEA metabolizing enzyme, fatty acid amidohydrolase (FAAH), using the specific inhibitor, URB 597, on mEPSCs. We found that application of URB 597 (URB, 1 μM) (bath) immediately (< 5min, Fig. 6A) enhanced ethanol-induced depression of the mEPSC frequency (inter-event-intervals, 562 % ± 99 of control at 20 min, F1, 394 = 95.76, p< 0.0001, two-way ANOVA) (Fig. 6B). The distribution of the mEPSC amplitude was not modified by ethanol or URB 597 treatment (data not shown), whereas the ethanol-induced right shift in time interval distribution was further shifted dramatically to the right by URB 597 (Fig. 6C). Application of the selective CB1 receptor antagonist, SR 141716A (2 μM), reduced the effects of ethanol and URB 597 (Fig. 6B) on mEPSC frequency (inter-event-intervals, 213 % ± 21 of control at 20 min, p< 0.0001, one-way ANOVA). The ethanol-induced shift in the inter-event interval distribution was strongly occluded by URB 597, but not to the control levels (Fig. 6C). URB (1 μM) alone depressed mEPSC frequency (inter-event-intervals, 172% ± 23 of control at 20 min, p< 0.05, one-way ANOVA), suggesting that FAAH serves as a metabolic gatekeeper for the regulation of on-site AEA levels in order to maintain an in vivo EC tone conducive to normal neurotransmission.
Fig. 6.
FAAH inhibitor, URB597, enhances EC-mediated ethanol-induced suppression of mEPSCs. (A) Application of 50 mM ethanol (bath) decreased the mEPSC frequency (inter-event intervals) (p<0.0001) (n= 4 neurons) of the control. Addition of URB597 to the bath solution in the presence of 50 mM ethanol enhanced the ethanol-induced depression of frequency (inter-event intervals) (n=13 neurons). [↓, Vehicle, ethanol, URB597 or SR141716A (SR) was added to the bath solution; ↓↓, SR141716A was added to the bath solution]. (B) Combined plots of the average inter-event-intervals of mEPSCs under control, ethanol, ethanol + URB597 +SR141716A treatment conditions. URB597 alone significantly depressed mEPSC frequency, probably through the inhibition of endogenous AEA degradation. SR141716A antagonized the effect of URB597 on the ethanol-induced inhibition of mEPSC frequency (n=13 neurons) (***p<0.0001). (a), compared to the control. (C) Average cumulative distributions showing an increase in the mEPSC inter-event interval (sec) in ethanol + URB 597-treated cells relative to controls and ethanol (p < 0.0001; Kolmogorow-Simrnov two-sample test).
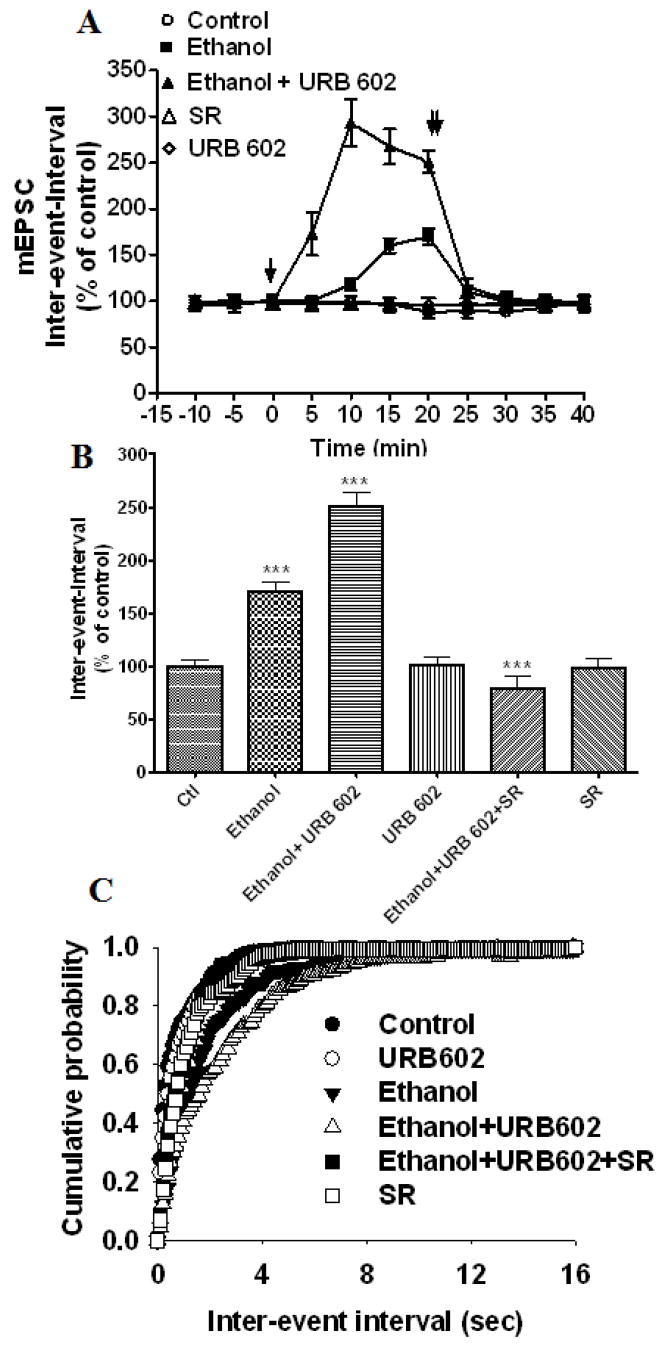
In the next experiment, we examined the effect of a 2-AG metabolizing enzyme, monoacylglycerol lipase (MAGL) inhibitor (URB 602), on ethanol-induced suppression of the mEPSC frequency in hippocampal neurons. URB 602 (100 μM) significantly enhanced ethanol-induced suppression of the mEPSC frequency (inter-event-intervals, 251 % ± 13 of control at 20 min, F1, 95 = 27.18, p< 0.0001, two-way ANOVA) (Fig. 7A–C) without affecting the amplitude (data not shown). The ethanol-induced right shift in the distribution of inter-event intervals was further shifted to the right by URB 602 (Fig. 7C). Application of selective CB1 receptor antagonist SR 141716A (2 μM) blocked the effects of ethanol and URB 602 (Fig. 7 B & C) on mEPSC frequency (inter-event-intervals, 78 % ± 12 of control at 20 min, p< 0.0001, one-way ANOVA). URB 602 (1 μM) alone had no significant effect on mEPSC frequency (inter-event-intervals, 101 % ± 7 of control at 20 min, t-test, p> 0.05) (Fig. 7B), suggesting that MAGL may not serve as a metabolic gatekeeper for the regulation of on-site 2-AG levels and 2-AG may not be involved in the in vivo EC tone conducive to normal neurotransmission in hippocampal neurons.
Fig. 7.
MAGL inhibitor, URB602, enhances EC-mediated ethanol-induced suppression of mEPSCs in cultured hippocampal neurons. (A) Application of 50 mM ethanol (bath) decreased the mEPSC frequency (inter-event intervals) (p<0.0001) (n= 6 neurons) of the control. [↓, Vehicle, ethanol, URB602, or SR141716A (SR) was added to the bath solution; ↓↓, SR141716A was added to the bath solution]. (B) Combined plots of the average inter-event-intervals of mEPSCs under control, ethanol, and ethanol + URB602 + SR141716A treatment conditions. Addition of URB602 to the bath solution in the presence of 50 mM ethanol enhanced the ethanol-induced depression of frequency (inter-event intervals) (n=13 neurons). URB602 alone had no significant effect on mEPSC frequency. SR141716A antagonized the effect of URB602 on ethanol-induced inhibition of mEPSC frequency (n=13 neurons) (***p<0.0001). (C) Average cumulative distributions showing an increase in mEPSC inter-event intervals (sec) in ethanol + URB602-treated cells relative to controls and ethanol (p < 0.001; Kolmogorow-Simrnov two-sample test).
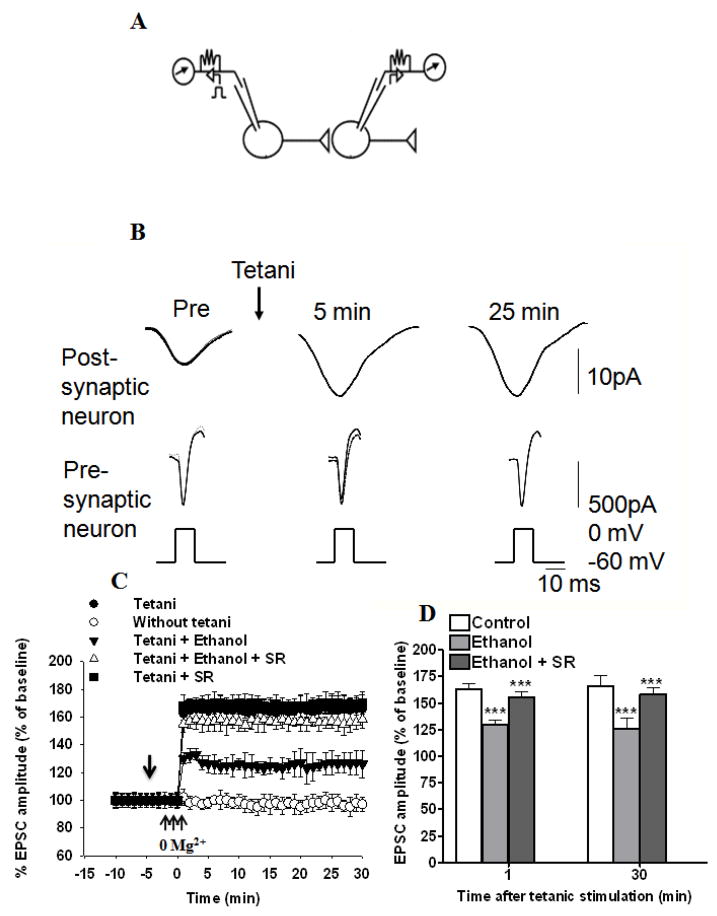
It has been demonstrated that long-lasting enhancement of EPSC amplitude can be reliably induced in monosynaptically connected pairs of cultured hippocampal neurons by high-frequency trains of depolarization (tetani) of the presynaptic neuron during temporary removal of Mg2+ from the bath solution (Ninan & Arancio 2004). Therefore, to examine the role of ECs in ethanol-induced inhibition of tetani evoked neurotransmitter release, we also studied potentiation of synaptic transmission between pairs of neurons in culture (Fig 8 A–D). To induce potentiation, high-frequency stimulation (three tetani of 50 Hz for 2 s at 20 s intervals during brief perfusion with an Mg2+-free medium) was given to a presynaptic neuron after recording a baseline for 10 min. This procedure produced an immediate potentiation (165% ± 9% and 159 % ± 7% at 1 and 30 min, respectively, after the tetanus) (Fig. 8C & D). Potentiation was stable after the tetani and showed statistical significance at each point. In interleaved experiments, we applied ethanol (50 mM) to a bath solution and applied tetani as before. Ethanol was added after recording the baseline (10 min) of 5 min duration; this did not alter the baseline recordings. Ethanol was continuously perfused throughout the experiments (35 min). Bath application of ethanol (50 mM) showed significant inhibition of the tetanus-induced enhancement of EPSC amplitude [126% ± 2% and 124% ± 3% at 1 (F1, 49 =25.23, p< 0.0001) and 30 min (F2,14 =29.80, p< 0.0001, two-way ANOVA), respectively, after the tetanus (Fig. 8 C & D)]. Bath application of SR141716A significantly reversed ethanol-induced inhibition of tetanus-induced enhancement of EPSC amplitude [155% ± 9 %, 158% ± 4.5 at 1 (F1, 14 =28.25, p< 0.0001) and 30 (F1, 43 =31.89, p< 0.0001, two-way ANOVA) min, respectively, after the tetanus]. Bath application of SR141716A (2 μM), along with tetani as before, did not show any significant effect on the tetanus-induced enhancement of EPSC amplitude (168% ± 6%, 170% ± 9% at 1 and 30 min, respectively, after the tetanus, t-test, p> 0.05) (Fig. 8D).
Fig. 8.
Ethanol-induced inhibition of long-term enhancement of EPSC amplitude (synaptic plasticity) in cultured hippocampal neurons is antagonized by bath perfusion of SR141617A. (A) Experimental design. (B) Sample traces are shown before (Pre) and 5 and 25 min after tetanic stimulation of the presynaptic neuron. Three successive traces are superimposed at each time point. (C) Average potentiation by tetanic stimulation (three tetani of 50 Hz for 2 s at 20 s intervals during brief perfusion with a Mg2+ free medium) of the presynaptic neuron (n=6), tetanic stimulation paired with ethanol (50 mM) (n=6), tetanic stimulation paired with ethanol (50 mM) and SR141716A (SR) (2 μM) (n=6), and tetanic stimulation paired with SR141716A (2 μM) (n=6). EPSCs were produced in the postsynaptic neuron by step depolarization, which elicited an inward current in the presynaptic neuron every 60 s. Leakage has been subtracted from the current in the presynaptic neuron. Ethanol was added after recording the baseline (10 min) of 5 min duration; this did not alter the baseline recordings. Ethanol was continuously perfused throughout the experiments (35 min). EPSC amplitudes were normalized to the average value during the 5 min before the application of ethanol in each experiment. [↓, Vehicle, ethanol, or SR141716A was added to the bath solution]. (D) Combined plots of the average EPSC amplitudes under control, ethanol, and ethanol + SR141716A treatment conditions. There was a significant overall difference between the groups, with the group that received the tetani being significantly different from the control group that did not receive tetani, as well as from the group with tetani + 50 mM ethanol. SR141716A antagonized the effect of ethanol on tetani-induced EPSC amplitudes. Average changes in EPSC amplitude in control (n=6), ethanol (n=6) and ethanol + SR141716A groups (n=6).
DISCUSSION
The results show a novel effect of acute ethanol on hippocampal neurons from neonatal mice. Acute ethanol enhances EC formation and reduces glutamate release in cultured hippocampal neurons. This finding is consistent with the previous observation that ethanol inhibits mEPSC frequency, probably by inhibiting glutamate release (Roberto et al. 2006) during early CNS development in the CA1 hippocampal region (Hendricson et al. 2004, Maldve et al. 2004, Mameli et al. 2005, Reynolds & Brien 1994), spinal cord (Ziskind-Conhaim et al. 2003), crayfish neuromuscular junction (Strawn & Cooper 2002), and central amygdala (Roberto et al. 2004). The present study identifies for the first time AEA and 2-AG as the ECs mediating ethanol’s effects on presynaptic glutamate release. The identification is based on the results of the present study in which we examined the steps leading to regulation of putative EC levels and their action on presynaptic CB1 receptors.
Acute ethanol enhanced the formation of AEA and 2-AG in hippocampal neurons and led to the inhibition of mEPSC frequency, which was reversed by addition of SR141716A in our experiments, pointing to a role of ECs as retrograde messengers. Addition of EGTA antagonized the effect of ethanol on AEA and 2-AG formation, suggesting the participation of calcium-dependent pathways (Basavarajappa 2007). Self-administration of ethanol in adult animals significantly increased dialysate 2-AG levels without a concomitant change in dialysate AEA levels in the nucleus accumbens (NAc) (Caille et al. 2007). Administration of a high dose of ethanol (4 g/kg, 520 mg %, 114 mM) in adult rats decreased AEA in the cerebellum, hippocampus, and ventral striatum (Ferrer et al. 2007), suggesting the sensitivity of this system to the concentration of ethanol and CNS development. Similar increases in 2-AG and AEA levels were observed during chronic ethanol treatment in cerebellar granular (CG) neurons derived from 7-day-old pups (Basavarajappa & Hungund 1999a, Basavarajappa et al. 2000, Basavarajappa et al. 2003). Taken altogether, these observations suggest that ethanol changes EC tone by regulating EC metabolism in hippocampal neurons.
Our electrophysiological data, in which ethanol suppresses mEPSC frequency and EPSC amplitude and demonstrates significant reversal by selective CB1 receptor antagonist, strongly demonstrate the predominant contribution of changes in ECs rather than changes in presynaptic CB1 receptors in the action of ethanol on glutamate release. Consistent with this observation, acute ethanol did not significantly change the levels of CB1 receptors in presynaptic pyramidal neurons. In contrast to acute ethanol, chronic ethanol treatment (Basavarajappa et al. 1998, Basavarajappa & Hungund 1999b, Ortiz et al. 2004) and ethanol withdrawal (Mitrirattanakul et al. 2007) decrease CB1 receptor levels in various animal models. Consistent with our observations, numerous studies have demonstrated the presence of CB1 receptors on the terminals of GABAergic interneurons (Katona et al. 1999) and glutamatergic terminals in various brain regions, including the hippocampus(Kawamura et al. 2006). Activation of these receptors inhibits depolarization-induced suppression of inhibition (DSI) and excitation (DSE) through the retrograde messenger activity of ECs (Katona et al. 1999, Kawamura et al. 2006, Ohno-Shosaku et al. 2002, Robbe et al. 2001, Robbe et al. 2002). Previous studies have reported a blockage of LTP in the CA1 region of hippocampal slices by a wide range of ethanol concentrations, with LTP inhibition observed at ethanol concentrations as low as 5 mM (partial inhibition) and as high as 100 mM (Blitzer et al. 1990, Randall et al. 1995, Schummers et al. 1997, Sinclair & Lo 1986, Sugiura et al. 1995a). In some studies, ethanol failed to block LTP at concentrations up to 50–60 mM (Sinclair & Lo 1986, Swartzwelder et al. 1995). In the present study, the block was about 50 % at 30 min ethanol exposure. Thus, it is possible that longer exposure (> 30 min) may be necessary to achieve a complete block, and we are currently investigating this possibility, as well as the contributions of other pathways (e.g., NMDA, voltage-gated calcium channels).
The existence of an EC membrane transporter (EMT) has been postulated, although its precise role and the protein (s) mediating this process remain to be investigated. The EMT appears to act via facilitated diffusion. Thus, transport could be possible in both directions depending on the EC concentration gradient (Glaser et al. 2003). Inhibition of EC uptake during acute ethanol exposure led to potentiation of ethanol-induced depression of the mEPSC frequency, suggesting the involvement of EMT in the uptake process rather than the release process in the current experimental conditions, a function that has previously been proposed by us (Basavarajappa et al. 2003) and others (Ronesi et al. 2004).
Fatty acid amidohydrolase is thought to be responsible for the in vivo breakdown of AEA (Kathuria et al. 2003, Lichtman et al. 2004). Mice lacking the FAAH gene have 15-fold higher levels of AEA in the brain and drink more ethanol (Basavarajappa et al. 2006, Blednov et al. 2006). Chronic ethanol treatment inhibits FAAH activity in the mice brain (Vinod et al. 2006). These observations suggest the important role of FAAH in the regulation of AEA levels during ethanol treatment. We found that selective FAAH inhibitor URB 597 significantly enhanced the ethanol-induced suppression of the mEPSC frequency, which was reversed by SR141716A in our experimental conditions. URB 597 alone had a significant effect on the mEPSC frequency that was not reversed by SR141716A. This suggests that URB 597 itself may have some effect on the releasing machinery, or AEA may have an additional effect through uncharacterized CB3 receptors (Breivogel et al. 2001, Di Marzo et al. 2000) and needs to be investigated in future studies.
Inhibition of MAGL, an enzyme responsible for the degradation of 2-AG, led to potentiation of the ethanol-induced depression of the mEPSC frequency, and was reversed by SR141716A in our experiments. This suggests that in addition to AEA, 2-AG also participates in the action of ethanol on mEPSCs in hippocampal neurons. In previous studies, 2-AG has been shown to mediate DSI and DSE in the hippocampus (Kim & Alger 2004, Makara et al. 2005, Straiker & Mackie 2005), and inhibition of MAGL prolonged the DSI in hippocampal slices and DSE in cultured hippocampal neurons (Makara et al. 2005).
Previous studies showed impairment of hippocampal long-term potentiation (LTP) after acute (Izumi et al. 2005, Randall et al. 1995)and chronic (Peris et al. 1997, Roberto et al. 2003) ethanol treatment. Earlier studies have shown that the predominant effect of EC-mediated CB1 receptor activation is restricting LTP (Slanina et al. 2005). SR141716A, in in vivo electrophysiological studies in adult anesthetized rats, fully antagonized the ethanol-stimulated firing rate of ventral tegmental area (VTA) DA neurons. Ethanol-induced suppression of the basolateral amygdala (BLA)-evoked NAc neuron spiking response was also antagonized by SR141716A. This was in fact mediated by ECs, because the FAAH inhibitor (URB 597) significantly suppressed ethanol-induced effects in the NAc that were antagonized by SR141716A (Perra et al. 2005). Recently, another study also demonstrated that the suppressive effect of ethanol on the electrical activity of putative glutamatergic BLA neurons projecting to the NAc was nearly completely prevented by the administration of SR141716A (Perra et al. 2008). Consistent with these observations, our double patch clamp recordings suggest that ethanol-induced ECs are in fact responsible for the ethanol-induced decrease in hippocampal synaptic plasticity. This is because the CB1 receptor antagonist SR141716A was able to reverse ethanol-induced inhibition of synaptic plasticity in hippocampal neurons. Thus, while acute ethanol treatment clearly affects many cellular pathways which regulate synaptic plasticity, the demonstrated alterations of the hippocampal EC system may represent a another major mechanism by which hippocampal-based synaptic functions (Acheson et al. 1998, Berry & Matthews 2004, Gulick & Gould 2007, Gulick & Gould 2008, Markwiese et al. 1998) are disrupted by acute ethanol treatment in hippocampal neurons.
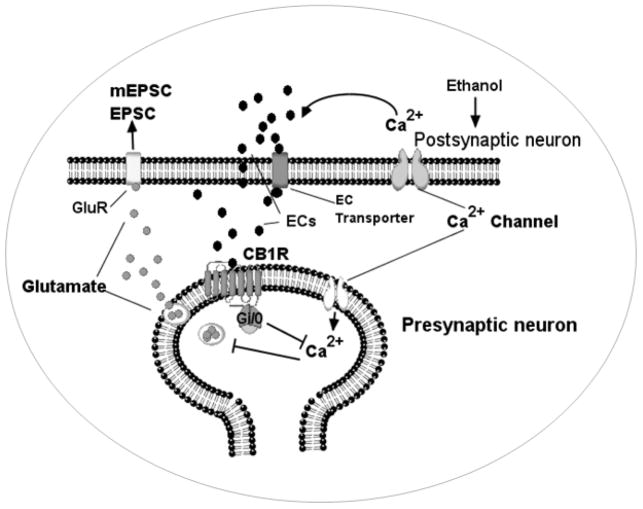
It has been shown that exposure to ethanol during the third trimester produces profound functional and structural alterations in the hippocampus (Galindo et al. 2005, Livy et al. 2003), and it has been proposed that these alterations may significantly contribute to the synaptic dysfunctions that are associated with FASD. Recent findings from neonatal rats suggest that ethanol may actually affect CA3 pyramidal neurons via inhibition of postsynaptic AMPARs and a decrease in glutamate release (Mameli et al. 2005). This study also suggests that ethanol effects on glutamate release could be occluded by directly blocking the N-type and P/Q-type voltage-dependent calcium channels (Mameli et al. 2005). In fact, exogenous cannabinoids inhibit glutamate release by activating CB1 receptor-mediated inhibition of N-type and P/Q-type calcium channels (Twitchell et al. 1997). However, there is evidence suggesting ethanol also affects glutamate release in other hippocampal regions. It was found that ethanol inhibits KCl-induced vesicular FMI-43 [N-(-3-trethylammoniumpropyl) -4-(-(dibutylamino) styryl) pyridinium dibromide] destaining in the CA1 stratum radiatum of P21-28 rats. This effect was occluded by blockers of N-type and P/Q-type calcium channels (Maldve et al. 2004). Collectively, these findings support the notion that ethanol inhibits glutamate release in CA1 pyramidal neurons via N-type and P/Q-type calcium channels. Yet, our findings suggest that ethanol may actually inhibit glutamate release in pyramidal neurons via the activation of an EC system that is negatively coupled to N-type and P/Q-type calcium channels (Fig. 9). Thus, we have identified a novel mechanism in hippocampal neurons that may have an important role in the pathophysiology of FASD. Future studies will be aimed at examining the changes in these EC events in the hippocampus using neonatal ethanol model.
Fig. 9.
A model to show the action of ethanol on excitatory neurotransmission involving ECs and CB1 receptors. The action of ethanol causes the generation of ECs (AEA and 2-AG). These ECs then activate the CB1 receptors (CB1R) at presynaptic terminals and suppress the release of glutamate by inhibiting N-type and P/Q-type calcium channels (Hoffman & Lupica 2000, Huang et al. 2001, Shen & Thayer 1998). GluR, glutamate receptors.
Acknowledgments
This work was supported by grants from NIH AA11031 (BSB) and New York State Psychiatric Institute. BSB would like to thank T. B. Cooper and Dr. B. L. Hungund for their support and encouragements.
References
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcoholism, clinical and experimental research. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS. Critical Enzymes Involved in Endocannabinoid Metabolism. Protein and Peptide letters. 2007;14:237–246. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic Ethanol Increases the Cannabinoid Receptor Agonist, Anandamide and its Precursor N-Arachidonyl phosphatidyl ethanolamine in SK-N-SH Cells. J Neurochem. 1999a;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S] GTPγS binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999b;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochemica Biophysica Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. NeuroReport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science (New York, NY,) 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Berry RB, Matthews DB. Acute ethanol administration selectively impairs spatial memory in C57BL/6J mice. Alcohol. 2004;32:9–18. doi: 10.1016/j.alcohol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, II, Walker D, Harris RA. Role of Endocannabinoids in Alcohol Consumption and Intoxication: Studies of Mice Lacking Fatty Acid Amide Hydrolase. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res. 1990;537:203–208. doi: 10.1016/0006-8993(90)90359-j. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein- coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Annual review of neuroscience. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcoholism, clinical and experimental research. 2000;24:706–715. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Ferrer B, Bermudez-Silva FJ, Bilbao A, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcoholism, clinical and experimental research. 2007;31:1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology. 2008;196:483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Sibbald JR, Morrisett RA. Ethanol alters the frequency, amplitude, and decay kinetics of Sr2+-supported, asynchronous NMDAR mEPSCs in rat hippocampal slices. J Neurophysiol. 2004;91:2568–2577. doi: 10.1152/jn.00997.2003. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532(Pt3):731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001a;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001b;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Maldve RE, Chen X, Zhang TA, Morrisett RA. Ethanol selectively inhibits enhanced vesicular release at excitatory synapses: real-time visualization in intact hippocampal slices. Alcoholism, clinical and experimental research. 2004;28:143–152. doi: 10.1097/01.ALC.0000106304.39174.AD. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcoholism, clinical and experimental research. 1998;22:416–421. [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2- monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Lopez-Valdes HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcoholism, clinical and experimental research. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Molnar E, Pickard L, Duckworth JK. Developmental changes in ionotropic glutamate receptors: lessons from hippocampal synapses. Neuroscientist. 2002;8:143–153. doi: 10.1177/107385840200800210. [DOI] [PubMed] [Google Scholar]
- Ninan I, Arancio O. Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron. 2004;42:129–141. doi: 10.1016/s0896-6273(04)00143-6. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminal. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Perez S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol and alcoholism. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- Peris J, Anderson KJ, Vickroy TW, King MA, Hunter BE, Walker DW. Neurochemical basis of disruption of hippocampal long term potentiation by chronic alcohol exposure. Front Biosci. 1997;2:d309–316. doi: 10.2741/a193. [DOI] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcoholism, clinical and experimental research. 2008;32:443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology. 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Randall RD, Lee SY, Meyer JH, Wittenberg GF, Gruol DL. Acute alcohol blocks neurosteroid modulation of synaptic transmission and long-term potentiation in the rat hippocampal slice. Brain Res. 1995;701:238–248. doi: 10.1016/0006-8993(95)01007-9. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, Brien JF. Effects of acute ethanol exposure on glutamate release in the hippocampus of the fetal and adult guinea pig. Alcohol. 1994;11:259–267. doi: 10.1016/0741-8329(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Brunelli M, Sanna PP, Gruol DL. The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. Eur J Neurosci. 2003;17:1646–1654. doi: 10.1046/j.1460-9568.2003.02614.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and Chronic Ethanol Alter Glutamatergic Transmission in Rat Central Amygdala: an In Vitro and In Vivo Analysis. Jl of Neuroscience. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, et al. Actions of acute and chronic ethanol on presynaptic terminals. Alcoholism, clinical and experimental research. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Bentz S, Browning MD. Ethanol’s inhibition of LTP may not be mediated solely via direct effects on the NMDA receptor. Alcoholism, clinical and experimental research. 1997;21:404–408. doi: 10.1111/j.1530-0277.1997.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- Sinclair JG, Lo GF. Ethanol blocks tetanic and calcium-induced long-term potentiation in the hippocampal slice. Gen Pharmacol. 1986;17:231–233. doi: 10.1016/0306-3623(86)90144-8. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long- term potentiation via CB1. Neuropharmacology. 2005;49:660–668. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Cooper RL. The effects of ethanol on pre-synaptic components of synaptic transmission in a model glutamatergic synapse: the crayfish neuromuscular junction. Comp Biochem Physiol C Toxicol Pharmacol. 2002;131:395–404. doi: 10.1016/s1532-0456(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shoyama Y, Saito H, Abe K. The effects of ethanol and crocin on the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Jpn J Pharmacol. 1995a;67:395–397. doi: 10.1254/jjp.67.395. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995b;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long- term potentiation by ethanol in immature versus mature hippocampus. Alcoholism, clinical and experimental research. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Veeranna Kaji T, Boland B, et al. Calpain mediates calcium-induced activation of the erk1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer’s disease. Am J Pathol. 2004;165:795–805. doi: 10.1016/S0002-9440(10)63342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The Cannabinoid CB1 Receptor Mediates Retrograde Signals for Depolarization-Induced Suppression of Inhibition in Cerebellar Purkinje Cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]