Abstract
Background/Aim
Adherence to an extracellular matrix (ECM) rescues hepatocytes from apoptosis, but how hepatocytes adhered to different ECM respond to apoptotic and cytoprotective stimuli is unknown.
Methods
Rat hepatocytes were plated on type 1 collagen (CI), laminin (LM) or polylysine (PL) and the amount of apoptosis induced by glycochenodeoxycholate (GCDC), deoxycholate (DCA), Fas ligand or serum withdrawal was determined by Hoechst staining. The response to cytoprotection by cAMP-guanine exchange factor (cAMP-GEF) activation was determined. Kinase activation was determined by immunoblotting with phosphospecific antibodies.
Results
Hepatocytes on LM and PL had more apoptosis in response to all apoptotic stimuli. GCDC increased c-jun-N-terminal kinase (JNK) phosphorylation 2-fold in hepatocytes on CI, but 15 and 30-fold in hepatocytes on PL or LM. SP-600125, a JNK inhibitor, prevented LM and PL potentiation of bile acid apoptosis. GCDC induced dephosphorylation of focal adhesion kinase (FAK) was prevented by cAMP-GEF activation. Cytochalasin B which decreased FAK phosphorylation prevented cAMP-GEF cytoprotection.
Conclusion
JNK activation augments apoptosis in hepatocytes plated on PL and LM. Decreased FAK phosphorylation as seen in cells treated with bile acids or attached to PL and LM promotes hepatocyte apoptosis.
Keywords: cAMP-GEF, exchange protein regulated by cAMP (EPAC), phosphoinositide 3-kinase (PI3K), bile acids, focal adhesion kinase, JNK, extracellular matrix, collagen, polylysine, laminin
Introduction
Hepatocytes require contact with extracellular matrix (ECM) to inhibit detachment-induced apoptosis (anoikis) (1–5). Although it is known that hepatocytes can be rescued from anoikis by re-attachment to a matrix, little is known about how hepatocytes respond to apoptotic stimuli when adhered to different ECM. Since profound changes in the composition of the ECM occur in liver disease (6–11), knowledge of how the ECM might modulate hepatocyte sensitivity to apoptosis could have application in the design of anti-apoptotic therapeutic strategies.
Hepatocyte adherence to the ECM is mediated by integrins. Integrins are heterodimeric cell surface proteins composed of unique α and β subunits that selectively bind to the ECM (12). Integrins serve as transmembrane linkers between their ECM ligands and the intracellular actin cytoskeleton. Although they lack intrinsic catalytic activity, integrins transduce signals through activation of integrin associated proteins which collectively assemble into focal adhesions. One important member of this signaling complex is focal adhesion kinase (FAK), a non-receptor protein tyrosin kinase. FAK is activated by integrin clustering, mechanical linkage to actin through actin binding proteins and autophosphorylation at tyrosine 397 and is responsible for integrin mediated survival in many cell types (13,14). In hepatic and bile duct carcinoma cells decreased FAK phosphorylation has been associated with increased sensitivity to apoptosis (15–16). Although it is known that FAK is activated in hepatocytes attached to integrin engaging ECM (4), the role of FAK in the survival of normal hepatocytes has not been studied.
During cholestatic liver disease, serum and tissue bile acid concentrations increase and contribute to liver damage by causing hepatocyte apoptosis and necrosis (17,18). Bile acid induced apoptosis involves activation of the pro-apoptotic stress activated kinase, c-Jun N terminal kinase (17) and in some instances activation of p38 mitogen activated kinase kinase (p38 MAPK) (19, 20). Emerging evidence suggests that bile acids may also modulate FAK. In the isolated perfused liver, choleresis associated with infusion of the cytoprotective bile acid, tauroursodeoxycholate (TUDC), is associated with FAK activation and, in colonic epithelial cells, the hepatotoxic bile acid deoxycholate (DCA) dephosphorylates FAK (21,22). The role of FAK in bile acid mediated apoptosis in hepatocytes has not been investigated.
Increases in cAMP have a protective effect against bile acid induced hepatocyte apoptosis (23, 24). The cytoprotective effect of cAMP is not mediated by protein kinase A, but instead requires the activation of cAMP-guanine exchange factors (cAMP-GEF)’s signaling through a phosphoinositide -3- kinase (PI3K)/Akt survival pathway (25). Although the role of cAMP-GEF’s in liver physiology is only beginning to emerge, the major function of cAMP-GEF’s in non-hepatic cells is the control of cell-cell and cell-matrix interactions (26, 27). Thus, it was of interest to explore whether the cytoprotective effects of cAMP-GEF activation might be dependent on ECM mediated events such as the activation of FAK.
The goal of these studies was to determine if adherence to different ECM could modulate hepatocyte sensitivity to apoptosis or hepatic cytoprotection by cAMP-GEF’s.
Methods
Rat hepatocytes were isolated by collagenase perfusion as previously described (25). These studies were conducted in accordance with the institutional animal care and use committee and NIH guidelines. Hepatocytes were plated at equal cell density on tissue culture plastic or glass coverslips coated with type I collagen (CI) (10ug/cm2), type IV collagen (CIV) (10ug/cm2), fibronectin (FB) (5ug/cm2), laminin (LM) (5ug/cm2), (BD Biosciences, Bedford MA) or polylysine (PL) (10ug/cm2, Sigma–Aldrich, St. Louis MO). Polylysine was used as a control for non-integrin mediated attachment (4). Hepatocytes were plated for 1 hour in modified eagle’s medium (MEM, Sigma–Aldrich, St. Louis MO) supplemented with: insulin (100nM), penicillin (100U/ml), streptomycin (0.1mg/ml), and 10% dialyzed fetal calf serum. Media was changed to unsupplemented MEM for 3 hours before experimentation. Hepatocytes maintained overnight were placed in MEM with 5% FCS and FCS withdrawn 1 hr before initiating experiments.
Whole cell lysates were prepared from hepatocytes plated on different ECM in lysis buffer as previously described (25). For immunoblotting, 50–100 µg of sample, as determined by Lowry protein assay, was electrophoresed on SDS polyacrylamide gels, and transferred to nitrocellulose membranes. Activation of protein kinases was determined using phosphospecific antibodies for pAktSer473, c-Jun N-terminal kinase (pJNK Thr183Y185), p38 mitogen activated kinase (p38MAPKThr180/Y182) or p44/p42 mitogen activated kinase (pMAPKThr202/Tyr204) (Cell Signaling Technology, Beverly MA), or focal adhesion kinase (pFAKTyr397) (EMD Biosciences, San Diego, CA). Equal protein loading was verified with phosphorylation independent antibodies. Blots were developed with chemiluminescence, digitized and results quantified using computer software.
Hepatocyte size was quantified as the area of attachment to culture plates, and measured from photomicrographs of hepatocytes plated on cover slides coated with ECM, fixed and stained with Hoechst. Measurements were made in a minimum of 50 cells per experiment by digitally photographing cells under phase contrast to accentuate cell borders; the background was digitally removed, and cell area quantified using computer software (28). Concurrent photographs were taken of a size standard for calibration of measurements.
Apoptosis on the different ECM was assessed following a 2 hr treatment with 50 uM GCDC as previously reported (23,25), 4 hr treatment with 100 uM DCA or 50 ng/ml Fas ligand (Axxora, LLC, San Diego, CA), or 6 hrs of serum starvation after overnight incubation in the presence of FCS. Apoptosis was quantified by examination of Hoechst stained cells for nuclear features typical of apoptosis (nuclear fragmentation, condensation and/or marginalization) and expressed as percent apoptosis seen in 500 randomly counted cells. Biochemical confirmation of apoptosis was performed by immunoblotting for the 17/19kd cleaved fragment of activated caspase 3 (Cell Signaling Technology, Beverly, MA). In some studies, hepatocytes were pre–treated for 15 minutes with the JNK inhibitor, SP 600125 (5 uM), the p38 MAPK inhibitor SB 203580 (5 uM), the PI3K inhibitor, wortmannin (50 nM), (EMD Biosciences, San Diego CA), or with cytochalasin B (1 uM) (Sigma–Aldrich, MO) or for 30 minutes with a specific activator of cAMP-GEF’s (20 uM) (8-(4-chlorophenylthio)-2’-O-methyl cAMP, (CPT-2-Me-cAMP, Axxora, LLC, San Diego, CA) before bile acid challenge.
Radiolabeled bile acid, 14C–glycocholate and 3H–taurocholate (Perkin Elmer, MA), accumulation was quantified at 30 minutes in cells attached to different ECM as previously described (25).
Statistical analyses were performed with computer software using a standard α value of 0.05 as a determination of significance, all experiments were repeated a minimum of 3 times and are reported as mean +/− standard deviation.
Results
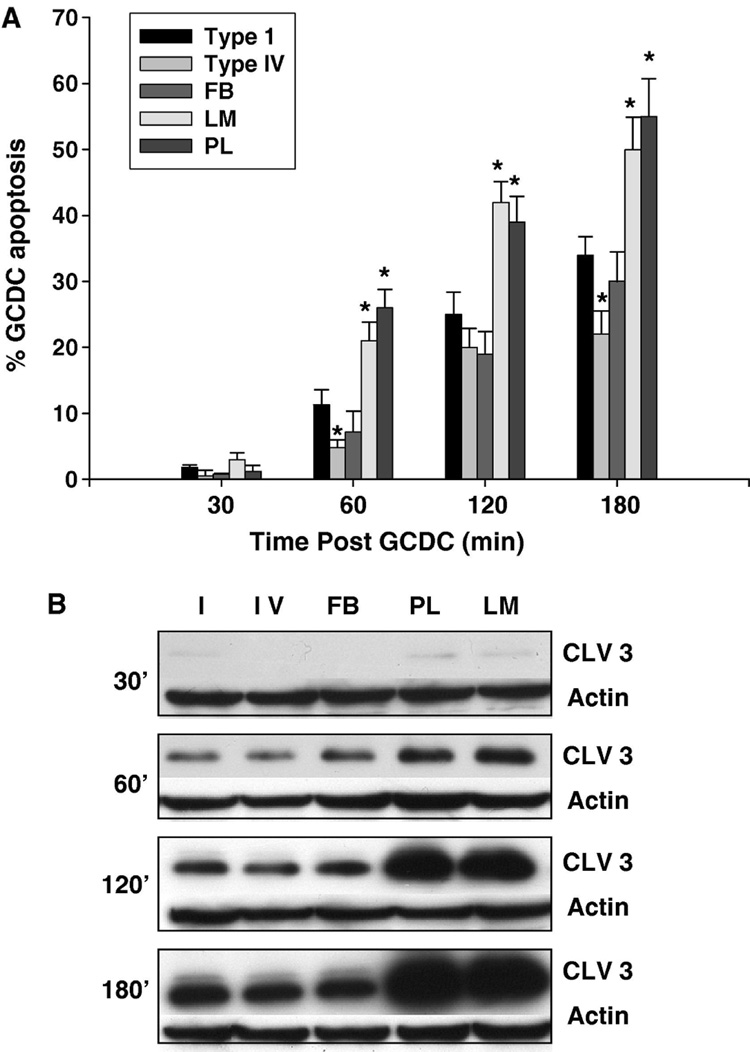
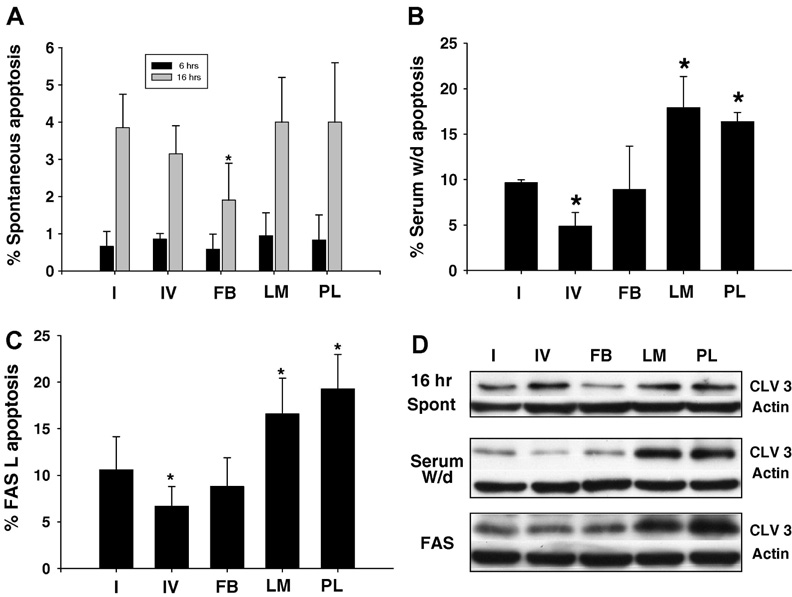
Hepatocytes showed ECM mediated differential sensitivity to GCDC induced apoptosis (Fig 1A,B). Hepatocytes attached to LM and PL demonstrated the greatest amount of apoptosis while those on CI and FB had similar levels of apoptosis. A small survival effect was seen in hepatocytes plated on CIV after 60 and 180 min of GCDC exposure. The differential sensitivity to apoptosis extended for up to 3 hrs after GCDC exposure and could be appreciated by both morphologic (evaluation of Hoechst staining) and biochemical (cleavage of caspase 3) assessment of apoptosis (Fig 1A,B). We examined whether this ECM mediated differential sensitivity was relevant to other models of hepatocyte apoptosis by evaluating the amount of apoptosis occurring spontaneously after 6 or 16 hrs in culture (Fig 2A,D), following 5 hrs of serum withdrawal after overnight culture in FCS (Fig 2B,D) and after treatment with Fas ligand (Fig 2C,D). There was a significant increase in apoptosis induced by all 3 stimuli in hepatocytes plated on LM and PL compared to those plated on CI (Fig 2). Hepatocytes on FB had a survival advantage after 16 hrs of culture and those plated on CIV had a survival advantage after serum withdrawal and Fas ligand challenge.
Figure 1. The extracellular matrix modulates hydrophobic bile acid induced rat hepatocyte apoptosis.
Primary rat hepatocytes were plated on Type 1 collagen (I), Type IV collagen (IV), fibronectin (FB), laminin (LM) or polylysine (PL) and the amount of apoptosis after exposure to 50 uM glycochenodeoxycholate (GCDC) determined at the indicated time points. Apoptosis was monitored morphologically using Hoechst staining (A) and biochemically by immunoblotting for the 17/19 kd cleavage fragment of caspase 3 (B). Cleaved 3 immunoblots were re-probed with actin to assure equal protein loading. The results represent the mean and standard deviation of at least 5 separate experiments. The * indicates the value is statistically different than the amount of apoptosis seen in hepatocytes plated on Type 1 collagen at that time point.
Figure 2. The extracellular matrix modulates spontaneous apoptosis and apoptosis induced by Fas ligand or serum withdrawal in rat hepatocytes.
Primary rat hepatocytes were plated on Type 1 collagen (I), Type IV collagen (IV) , fibronectin (FB), laminin (LM) or polylysine (PL) and the amount of apoptosis after 6 or 16 hrs in culture (A,D), 4 hrs after exposure to 50 ng/nl Fas ligand or 6 hrs following serum withdrawal after overnight incubation in serum was determined morphologically by Hoechst staining (A,B,C) or biochemically by immunoblotting for the 17/19 kD cleavage fragment of caspase 3 (D). Cleaved 3 immunoblots were re-probed with actin to assure equal protein loading. The results represent the mean and standard deviation of at least 3 separate experiments. The * indicates the value is statistically different than the amount of apoptosis seen in hepatocytes plated on Type 1 collagen.
In order to exert their toxic effects, bile acids must enter hepatocytes (29). To rule out an ECM effect on bile acid uptake, we measured accumulation of conjugated bile acids in hepatocytes plated on different matrices. The 30 minute accumulation of the radiolabeled bile acids, 14C–glycocholate and 3H–taurocholate was the same in hepatocytes regardless of ECM (Table 1).
Table 1. Extracellular matrix does not affect bile acid uptake in hepatocytes.
Hepatocytes plated on the indicated ECM were exposed to radiolabeled bile acids 14C–glycocholate and 3H–taurocholate for 30 minutes. Cellular uptake was quantified via liquid scintigraphy and expressed as nmol/mg protein. Results represent mean ± standard deviation of 3 separate experiments.
| Uptake nmol/mg protein | ||
|---|---|---|
| ECM | TC (50 uM) | GC (50 uM) |
| Type I Collagen | 19.0 (4.9) | 16.1 (1.9) |
| Type IV Collagen | 18.7 (4.2) | 16.2 (2.3) |
| Fibronectin | 19.3 (3.6) | 17.0 (1.6) |
| Laminin | 21.4 (4.3) | 19.1 (3.2) |
| Polylysine | 20.0 (3.8) | 18.1 (3.2) |
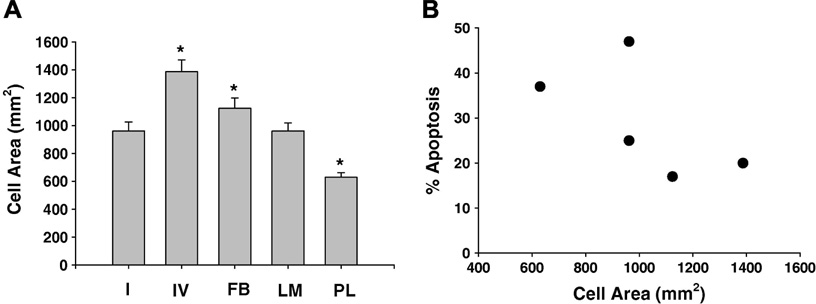
Integrin ligation leads to cell spreading which has been correlated with cell survival (30). Differential cell spreading occurs in hepatocytes attached to different ECM (2, 31), but the relationship between cell spreading and survival in hepatocytes is unknown. In our experiments, hepatocytes on CI, CIV and FB appeared larger assuming a more flattened spread morphology with increased cell-cell contact. We quantified cell spreading by measuring the area of at least 50 cells. Hepatocytes plated on CIV and FB were significantly larger, and those plated upon PL were significantly smaller than those on CI or LM (Fig 3A). There was, however, no significant linear correlation between cell spreading and susceptibility to apoptosis (Fig 3B).
Figure 3. The extracellular matrix modulates hepatocyte size.
A. The average cell area of hepatocytes plated for 4 hr on Type 1 (I) or Type IV (IV) collagen or fibronectin (FB), laminin (LM) or polylysine (PL) was determined. Cells were fixed and stained, and areas of at least 50 randomly selected cells were measured from phase contrast photomicrographs as described in the methods. Results represent mean and standard deviation of at least 3 separate experiments. The * indicates the values are significantly different from hepatocytes plated on I (p<0.05). 3B. The amount of apoptosis induced by 2 hr treatment of hepatocytes with 50 uM GCDC was compared to average hepatocyte cell area on different ECM. Results represent mean and standard deviation of at least 3 separate experiments. Apoptosis was quantified as % of 500 randomly counted Hoechst stained cell cells
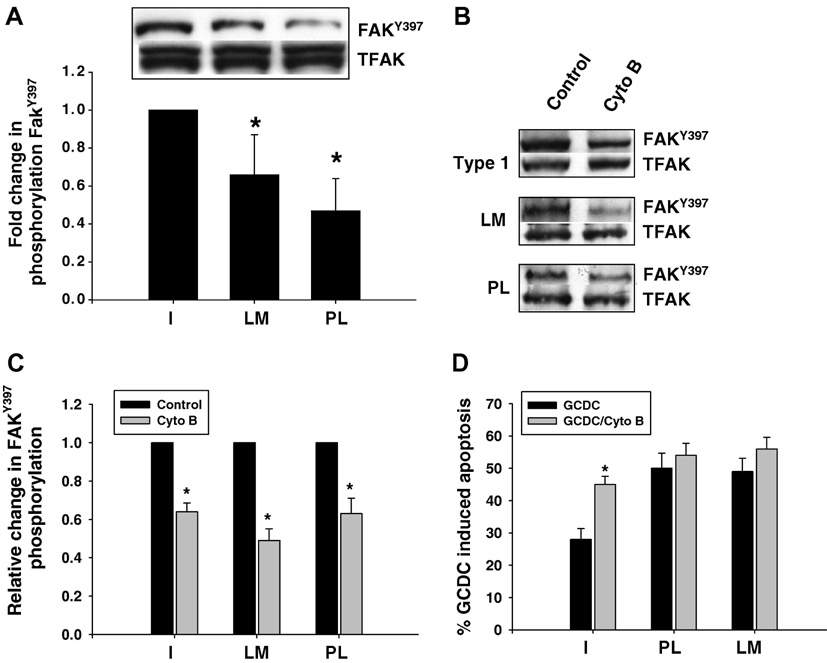
ECM mediated activation of FAK controls cell survival in non-hepatic cells (13, 14). We examined FAK activation in hepatocytes adhered to different ECM by determining phosphorylation of FAKTyr397. There was no FAKTyr397 phosphorylation in hepatocytes in suspension culture just prior to plating (data not shown). Phosphorylation of FAKTyr397 was present in cells plated on all the ECM, but was significantly decreased in hepatocytes plated on LM and PL compared to CI (Fig 4A,B). In order to determine if FAKTyr 397 phosphorylation was necessary for the CI mediated survival effect, we used short incubations with 1uM of cytochalasin B to disrupt FAK activation. This concentration has been shown to inhibit FAK without affecting actin filament polymerization (32–35) Cytochalasin B had no effect on hepatocyte attachment, cell size or cell viability, but significantly decreased FAK Tyr397 phosphorylation on all 3 matrices by 40 to 50% (Fig 4C). Cytochalasin B pre-treatment resulted in significantly more GCDC induced apoptosis in hepatocytes plated on CI (Fig 4D). There were only minor increases in apoptosis in hepatocytes adhered to LM and PL, perhaps due to the fact that apoptosis was already maximally stimulated on these matrices (Fig 4D). The net effect of inhibition of FAK phosphorylation was to ameliorate the ECM mediated differential sensitivity to GCDC induced apoptosis.
Figure 4. The role of FAK in GCDC induced apoptosis in rat hepatocytes.
Rat hepatocytes were plated on Type 1 collagen (Type 1), laminin (LM) or polylysine (PL) and the amount of FAKTyr397 phosphorylation determined by immunoblotting with phosphospecific antibodies 6 hrs after plating (4A). Cultures plated on the different ECM were treated with 1 uM of cytochalasin B (Cyto B) for 15 minutes, and the amount of FAKTyr397 phosphorylation and total FAK determined (4B,C) by immunoblotting. Representative immunoblots are shown in 4B and the quantification of 3 separate experiments in 4C where the amount of phosphorylated FAK in cyto B treated hepatocytes is compared to the amount in untreated cells plated on the same ECM. D. Hepatocytes were treated with 1 uM cytochalasin B for 15 min and then the amount of apoptosis induced by glycochenodeoxycholate (GCDC) determined 2 hr later by morphologic evaluation of Hoechst stained cells. The results are the mean and standard deviation of at least 3 separate experiments. The * indicates the value is statistically different than the amount of apoptosis in untreated hepatocytes adhered to Type 1 collagen.
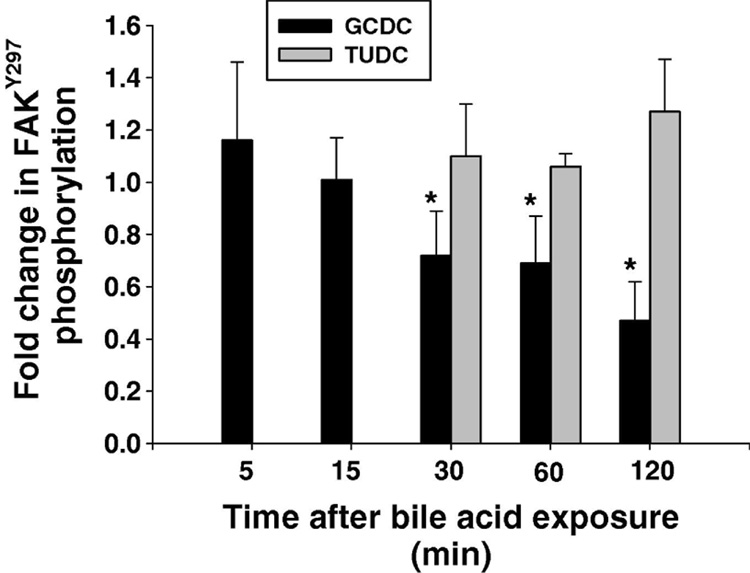
Bile acids can modulate FAK dephosphorylation in colonic epithelial cells (22). We thus examined the effect of GCDC on FAK phosphorylation in hepatocytes plated on CI. Figure 4A shows the time course for phosphorylation of FAK Tyr397 after the addition of GCDC or the non-toxic bile acid, TUDC. While TUDC has no effect on FAK phosphorylation, GCDC treatment resulted in a significant 30 to 60% decrease in FAKTyr397 phosphorylation at 30, 60 and 120 minutes, (Fig 5).
Figure 5. Effect of bile acids on FAK phosphorylation.
Hepatocytes plated on to Type 1 collagen were treated with 50 uM tauroursodeoxycholate (TUDC) or 50 uM glycochenodeoxycholate (GCDC) and the amount of FAKTyr397 phosphorylation at the indicated time points determined by immunoblotting. The results represent the mean and standard deviation of 5 separate experiments. The * indicates the value is statistically different than in control untreated hepatocytes.
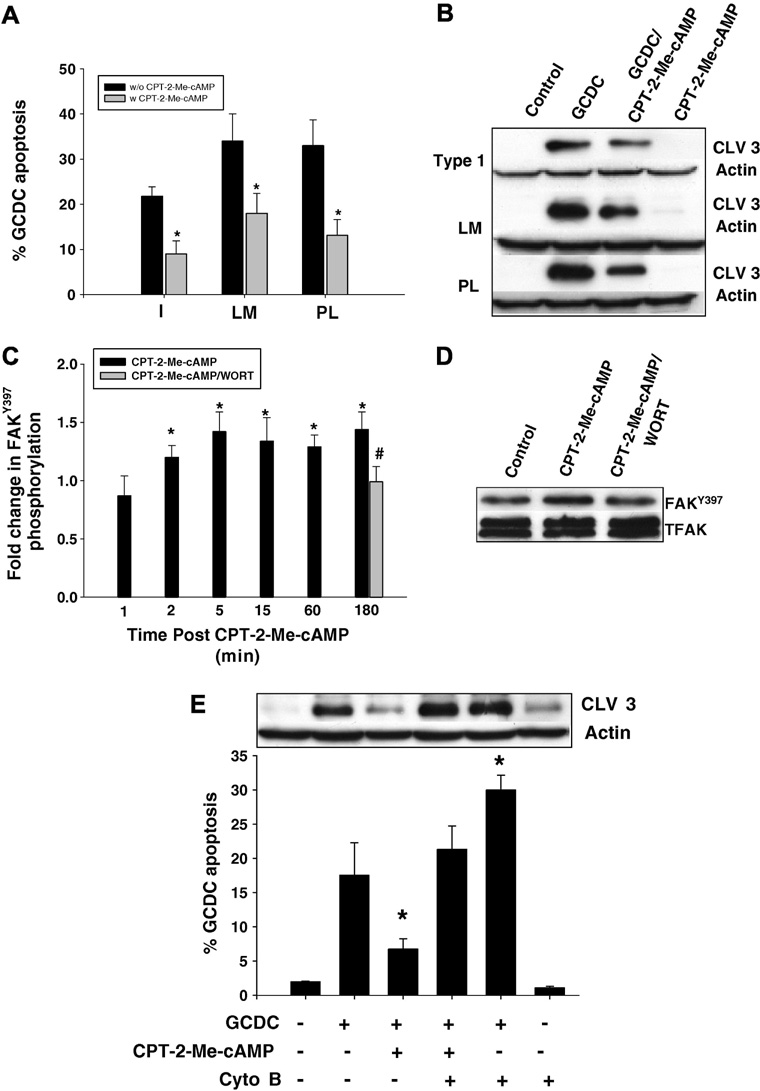
In hepatocytes adhered to CI, cAMP protects against bile acid induced apoptosis by a cAMP-GEF mediated PI3K dependent pathway (25). Here we extend these observations to show that treatment with the specific cAMP-GEF activator, CPT-2-Me-cAMP, promotes survival from GCDC induced apoptosis in hepatocytes adhered to PL and LM. (Fig 6A,B). Since a major function of cAMP-GEF’s in other cells is to modulate cell-matrix interactions, we determined whether cAMP-GEF cytoprotection might involve FAK. Treatment of hepatocytes plated on CI with a CPT-2-Me-cAMP caused a small, sustained increase in FakTyr397 phosphorylation from 5 to 180 min (Fig 6C). Similar increases in cAMP-GEF mediated FakTyr397 phosphorylation occurred in hepatocytes plated on LM and PL (data not shown). Pretreatment with the PI3K inhibitor, wortmannin, prevented this phosphorylation event. Thus cAMP-GEF activation in hepatocytes is associated with PI3K dependent phosphorylation of FAK.
Figure 6. The cytoprotective effect of CPT-2-Me-cAMP is ECM independent but dependent on PI3K mediated FAK phosphorylation.
Hepatocytes were plated on Type 1 collagen (I), laminin (LM) or polylysine (PL). Some cultures were pre-treated with 20 uM CPT-2-Me-cAMP for 20 minutes, and subsequently the amount of apoptosis after 2 hr treatment with 50 uM glycochenodeoxycholate (GCDC) was determined by morphologic evaluation of Hoechst stained cells (A) and by immunoblotting for the 17/19 kD cleavage product of caspase 3 (B). The * indicates the value is statistically different than that seen in the presence of GCDC alone. C. Hepatocytes plated on Type 1 collagen were treated with 20 uM CPT-2-Me-cAMP for the time indicated +/− pretreatment for 15 min with 50 nM wortmannin (WORT) and then the amount of FAKTyr397 determined by immunoblotting. Representative immunblots for the 180 min time point are shown in D. The * indicates the value is statistically different than seen in control cultures and the # indicate the value is different from that seen with CPT-2-Me-cAMP. E. Hepatocytes plated on Type 1 collagen were treated with 1 uM cytochalasin B (Cyto B), 20 uM CPT-2-Me-cAMP or sequentially with Cyto B followed by CPT-2-Me-cAMP and then apoptosis induced by 2 hr treatment with GCDC and quantified by morphologic evaluation of Hoechst stained cells or biochemically by immunoblotting for the 17/19kD cleavage fragment of caspase 3. The * indicated the value is significantly different from that seen in GCDC treated hepatocytes. The results in all the figures represent the mean and standard deviation of 4 experiments.
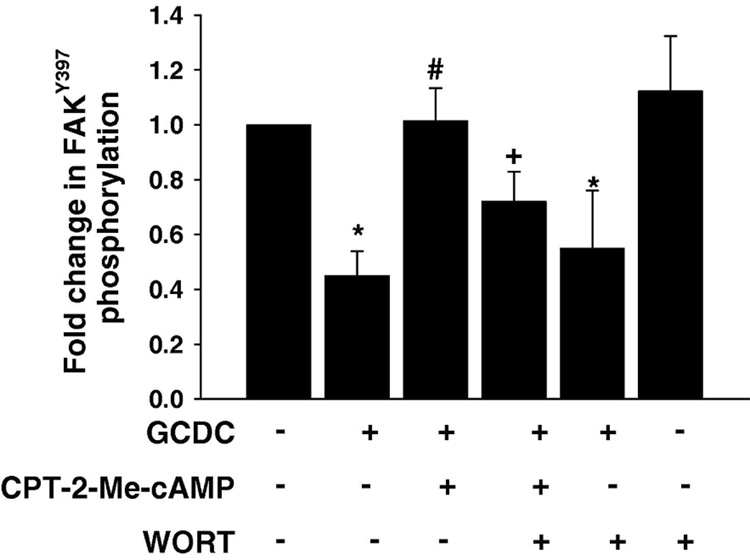
We explored the possibility that cAMP-GEF activation of FAK was involved in its cytoprotective effect against bile acid induced apoptosis Pretreatment with cytochalasin B prevented the cytoprotective effect of cAMP-GEF activation in bile acid induced apoptosis (Fig 6E). Then we determined if pre treatment with the cAMP-GEF activator had any effect on GCDC induced dephosphorylation of FAK. As seen in Fig 7 pretreatment with CPT-2-ME-cAMP restored normal levels of FAK phosphorylation in GCDC treated hepatocytes. When hepatocytes were pre-treated with wortmannin the ability of CPT-2-Me-cAMP to restore FAK phosphorylation in GCDC treated cells was partially blocked (Fig 7). These results are consistent with a cAMP-GEF/PI3K/FAK cytoprotective pathway in hepatocytes.
Figure 7. CPT-2-Me-cAMP mediated protection from GCDC induced FAK dephosphorylation is partially PI3K dependent.
Hepatocytes plated on Type 1 collagen were treated with 50 nM wortmannin, 20 uM CPT-2-Me-cAMP or sequentially with 50 nM wortmannin and 20 uM CPT-2-Me-cAMP and then for 2 hrs with GCDC. The amount of FAKTyr397 was determined by immunoblotting. The results of 4 separate experiments were quantified and the mean and the standard deviation presented graphically. The * indicated the value is statistically different than that seen in control (untreated) hepatocytes, the # indicates the value is significantly different from that seen with GCDC treatment alone and the + indicates the value is significantly different from that seen with combined CPT-2-Me-cAMP and GCDC treatment.
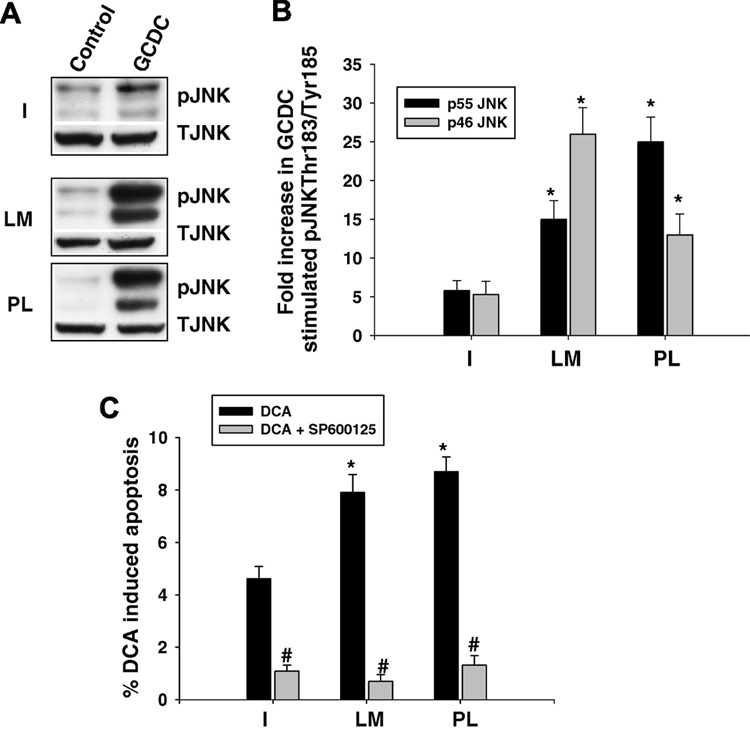
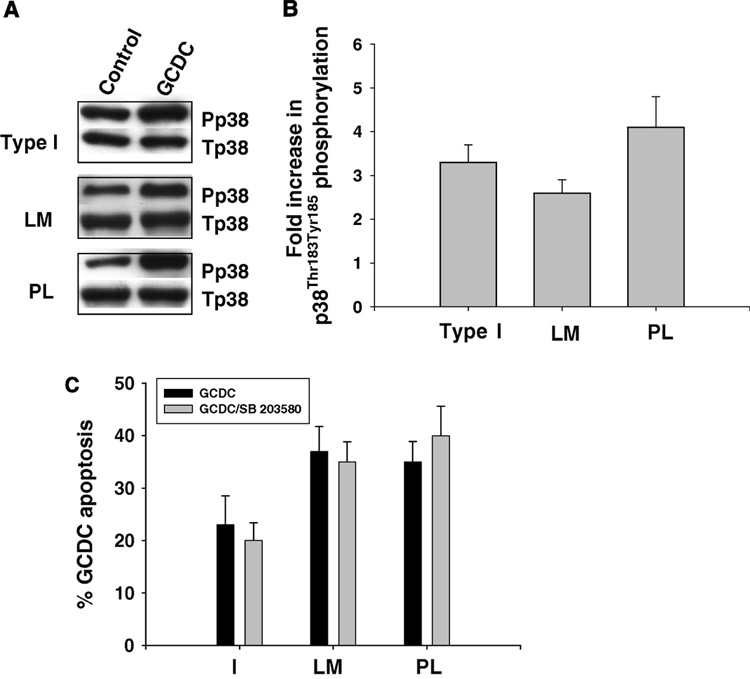
Since bile acid apoptotic signaling involves activation of the stress activated kinases, JNK and p38 MAPK (17,19,20), we examined whether adherence to different ECM influenced bile acid phosphorylation of these kinases. Hepatocytes plated on CI had a 4–5 fold increase in JNK phosphorylation in response to GCDC, while hepatocytes adhered to LM or PL had a 15 to 30 fold increase in GCDC induced JNK phosphorylation (Fig 8 A, B). In order to determine if enhanced JNK activation played a pro-apoptotic role in bile acid induced apoptosis, we evaluated the effect of the JNK inhibitor, SP 600125, on apoptosis induced by DCA. The JNK inhibitor protected against DCA induced apoptosis on all the ECM (Fig 8C). We could not evaluate the effect of the SP 600125 on GCDC induced apoptosis since the inhibitor at the concentration used, 5 uM, inhibited conjugated bile acid uptake (data not shown). GCDC phosphorylated p38 MAPK equally on CI, PL and LM (Fig 9A,B). Since p38 MAPK signaling has been implicated in both pro- (19,20) and anti-apoptotic bile acid signaling (29,36), we wished to clarify the role of p38 MAPK in GCDC induced apoptosis in our system. Inhibition of p38 MAPK with SB203580, had no effect on GCDC induced apoptosis in hepatocytes plated on C1, PL or LM (Fig 9 C).
Figure 8. Effect of ECM on bile acid induced phosphorylation of JNK.
Hepatocytes were plated on Type 1 collagen, laminin (LM) or polylysine (PL) and the amount of JNK phosphorylation determined by immunoblotting. Representative immunoblots are in A and B is the quantification of 3 separate experiments. The * indicates the value is significantly different than that seen in GCDC treated hepatocytes plated on Type I collagen. C. Hepatocytes were treated with 100 uM deoxycholate (DCA) for 4 hrs +/− pretreatment for 15 min with 5 uM of SP 600125 and the amount of apoptosis determined by morphologic evaluation of Hoescht stained cells. The results are the mean and standard deviation of 3 separate experiments and the * indicates the value is significantly different than that seen in hepatocytes treated with DCA alone.
Figure 9. Effect of ECM on bile acid induced phosphorylation of p38 MAPK.
Hepatocytes were plated on Type 1 collagen (I), laminin (LM) or polylysine (PL) and the amount of p38 MAPK phosphorylation determined by immunoblotting 6 hours later. Representative immunoblots are shown in A and B is the quantification (mean and standard deviation) of 3 separate experiments. C. Hepatocyte adhered to I, LM or PL were treated with 50 uM glycochenodeoxycholate (GCDC) for 2 hrs +/− 15 minute pretreatment with 5 uM SB 203580 and the amount of apoptosis determined morphologically by evaluation of Hoechst stained cells. The results are the mean and standard deviation of 3 separate experiments.
Bile acid induced apoptosis has been linked to activation of the Fas and TRAIL death receptors, secondary to JNK dependent increased plasma membrane expression of these receptors and subsequent ligand independent activation (17). Thus, we examined whether JNK activation in hepatocytes on LM and PL might be associated with increased TRAIL DR5 or Fas receptor expression. Immunoblotting for TRAIL DR5 and Fas receptor protein in whole cell lysates from hepatocytes plated on LM, PL or C1 for 6 hours did not reveal any difference in protein expression (data not shown).
Discussion
The principal findings of this study relate to the ability of the ECM to control hepatocyte survival. In particular the study shows: 1.) hepatocytes adhered to PL and LM are more sensitive to apoptotic stimuli than hepatocytes on CI, 2.) in hydrophobic bile acid induced apoptosis this differential sensitivity is associated with augmented JNK and decreased FAK phosphorylation and 3.) cAMP-GEF mediated cytoprotection from bile acid induced apoptosis is independent of ECM but requires PI3K dependent phosphorylation of FAK.
We have demonstrated that hepatocytes adhered to LM have increased sensitivity to apoptosis. In the normal liver, hepatocytes are primarily in contact with CI, CIV and FB (2,7,11). During acute and chronic liver disease, the composition of the ECM is altered with increases in all ECM constituents (6). Of relevance to these studies, increased laminin deposition has been documented in cholestatic liver disease (8–11). Bile duct ligated rats and human patients with neonatal cholestasis, biliary atresia and primary biliary cirrhosis have increased laminin deposition that is primarily localized in the space of disse and contributes to capillarization of the sinusoids (8,9,10). Cholestatic hepatocytes also acquire expression of the alpha 6 integrin which is a receptor for laminin (11). Laminin-integrin signaling also triggers Mallory body formation in vitro (37). Thus in cholestatic liver disorders, hepatocytes may more commonly adhere to laminin and our results suggest that this binding may sensitize hepatocytes to apoptosis.
We show that the cytotoxic bile acid, GCDC, but not the cytoprotective bile acid TUDC, dephosphorylates FAK and that correction of this phosphorylation deficit protects against apoptosis. This suggests a role for FAK in modulating apoptosis accompanying cholestatic liver disease. Previous studies have substantiated a role for FAK in hepatobiliary cancer cell survival. FAK inhibition sensitizes hepatocellular carcinoma cells to TNF alpha mediated apoptosis (14) and is associated with imatinib mesylate induced apoptosis in cholangiocarcinoma cells (15). Furthermore, enhanced FAK expression in human hepatocellular carcinoma has been linked to decreased survival time (38). Collectively these studies indicate an important role for FAK in hepatocyte survival.
The mechanism(s) whereby hydrophobic bile acids interfere with FAK phosphorylation is unknown. Normally FAK activation requires binding of integrins to the ECM, mechanistic linkage to the actin cytoskeleton, and autophosphorylation at tyrosine 397 (13,14). In colonic epithelial cells, DCA activates the tyrosine phosphatase, ShP2, leading to FAK dephosphorylation at Tyr 576 and 925 (22). In separate studies in a colon cancer cell line, DCA altered plasma membrane lipid composition leading to increases in membrane cholesterol and decreases in membrane fluidity which resulted in tyrosine phosphorylation of the epidermal growth factor receptor (39). Presumably other membrane associated proteins could also be modulated by these membrane perturbations. Bile acids also interfere with cytokeratins and influence actin polymertization in hepatocytes (40–42) and thus might inhibit FAK phosphorylation by preventing the interaction of actin binding proteins with FAK. Further studies are needed to characterize how bile acids modulate FAK in hepatocytes.
We demonstrate for the first time that cAMP-GEF activation in hepatocytes results in phosphorylation of FAK. This is in accord with the role of cAMP-GEF’s to promote integrin-mediated and non-integrin mediated cell adhesion (26,27). We also present evidence that cAMP-GEF phosphorylation of FAK is necessary for its cytoprotective effect in bile acid induced apoptosis and that, at least in part, this survival effect is associated with prevention of hydrophobic bile acid induced dephosphorylation of FAK. In non-hepatic cells, FAK activation can prevent apoptosis through 2 main pathways: PI3K signaling and PI3K independent inhibition of p53 (13,14). We have previously demonstrated that cAMP-GEF can activate PI3K and that the cytoprotective effect of cAMP-GEF in bile acid induced apoptosis is PI3K dependent (25). FAK signaling can be either upstream or downstream of PI3K (13,14, 43, 44). In our experiments, cAMP-GEF mediated FAK phosphorylation is PI3K dependent placing FAK downstream of PI3K. Collectively our results suggest the presence of a cAMP-GEF/PI3K/FAK survival pathway in hepatocytes.
Our work does not exclude the possibility of additional cAMP-GEF/FAK survival pathways in hepatocytes, as we have identified PI3K independent cAMP-GEF mediated cytoprotection in hepatocytes (25). Since recent evidence suggests that p53 signaling is important in bile acid induced apoptosis and that the cytoprotective effect of TUDC is mediated, in part, by increasing interaction of p53 with its repressor Mdm-2 (45,46), it is possible that a FAK/p53 survival pathway is present in hepatocytes. This is an area of ongoing exploration in our lab.
Bile acid mediated JNK activation is pro-apoptotic in hepatocytes (17,19,20). In the current study, hepatocytes plated on LM and PL had profound increases in JNK phosphorylation in response to GCDC compared to hepatocytes plated on Type 1 collagen, and chemical inhibition of JNK activation prevented bile acid induced apoptosis on all the matrices. These findings suggest that the enhanced JNK activation may contribute to the ECM differential sensitivity to apoptosis.
In conclusion, our studies suggest that hepatocytes adhered to integrins that engage collagens and FB have increased tolerance to apoptosis while those adhered to LM may have increased sensitivity to apoptosis. The ECM can modulate hepatocyte apoptosis by promoting differential activation of JNK and FAK. In addition, non-ECM mediated mechanisms of increasing FAK phosphorylation, such as treatment with cAMP-GEF activators, promote survival independent of ECM. Integrins are accessible drug targets and integrin targeted drugs are in development as anti-inflammatory, anti-thrombotic and anti-angiogenesis therapy (48, 49) and have been employed in the liver to suppress growth in hepatocellular carcinoma cells (50) and to inhibit stellate cell migration and activation (51). In addition, low molecular weight inhibitors of FAK are in pre-clinical development as chemotherapeutic agents (52). Thus, knowledge of the role of integrins/ECM in hepatic physiology will be critical to the biological application of these compounds.
Acknowledgement
The advice and editorial assistance of Dr. M. Sawkat Anwer and excellent technical help of Holly Jameson were valuable in conducting the experimental work and preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smets FN, Yongming C, Ling-Jia W, Soriano H. Loss of cell anchorage triggers apoptosis (anoikis) in primary mouse hepatocytes. Mol Gen and Metab. 2002;75:344–352. doi: 10.1016/S1096-7192(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 2.Pinkse GGM, Jiawan-Lalai R, Bruijn J, deHeer E. RGD peptides confer survival to hepatocytes via the b1-integrin-ILK-pAkt pathway. J Hepatol. 2005;42:87–93. doi: 10.1016/j.jhep.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Pinske GGM, Voorhoeve MP, Noteborn M, Terpstra OT, Bruijn JA, de Heer E. Hepatocyte survival depends on α 1 integrin mediated attachment of hepatocytes to hepatic extracellular matrix. Liver Inter. 2004;24:218–226. doi: 10.1111/j.1478-3231.2004.0914.x. [DOI] [PubMed] [Google Scholar]
- 4.Hosiba T, Nagahara H, Cho C-S, Tagawa Y-I, Akaike T. Primary hepatocyte survival on non-integrin recognizable matrices without the activation of Akt. Biomaterials. 2007;28:1093–1104. doi: 10.1016/j.biomaterials.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Qiao L, Farrel G. The effects of cell density, attachment substratum and dexamethasone on spontaneous apoptosis of rat hepatocytes in primary culture. In Vitro Cell Dev Biol. 1999;35:417–424. doi: 10.1007/s11626-999-0117-2. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Hernandez A. The hepatocellular matrix II: Electron immunohistochemical studies in rats with CCl4 induced cirrhosis. Lab Invest. 1985;53:166–186. [PubMed] [Google Scholar]
- 7.Martinez-Hernandez A. The hepatic extracellular matrix I: Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984;51:57–74. [PubMed] [Google Scholar]
- 8.Zeitlin L, Resnick MS, Konikoff F, Schuppan D, Bujanover Y, Lerner A, et al. Divergent patterns of extracellular matrix protein expression in neonatal versus adult liver fibrosis. Pediatr Pathol Mol Med. 2003;22:349–362. doi: 10.1080/pdp.22.4.349.362. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto S, Yamamoto K, Nagano T, Okamoto R, Ibuki N, Tagashira M, et al. Immunohistochemical study on phenotypical changes of hepatocytes in liver disease with reference to extracellular matrix composition. Liver. 1999;19:32–38. doi: 10.1111/j.1478-3231.1999.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 10.Desmouliere A, Darby I, Costa AM, Raccurt M, Tuchweber B, Sommer P, et al. Extracellular matrix deposition, lysyl oxidase expression and myofibroplastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76:765–778. [PubMed] [Google Scholar]
- 11.Volpes R, van den Oord JJ, Desmet VJ. Distribution of the VLA family of integrins in normal and pathologic liver tissue. Gastroenterology. 1991;101:200–206. doi: 10.1016/0016-5085(91)90478-4. [DOI] [PubMed] [Google Scholar]
- 12.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 14.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Chiorean MV, Guicciardi ME, Yoon JH, Bronk SF, Kaufmanns SH, Gores GJ. Imatinib mesylate induces apoptosis in human cholangiocarcinoma cells. Liver Int. 2004;24:687–695. doi: 10.1111/j.1478-3231.2004.0984.x. [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Wang L, Jin J, Zha X. Focal adhesion kinase affects the sensitivity of human hepatocellular carcinoma cell line SMMC-7721 to tumor necrosis factor a/cycloheximide induced apoptosis by regulating protein kinase B levels. Eur J Biochem. 2001;268:4513–4519. doi: 10.1046/j.1432-1327.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi H, Gores GJ. Bile acid regulation of hepatic physiology IV: Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734–G738. doi: 10.1152/ajpgi.00491.2002. [DOI] [PubMed] [Google Scholar]
- 18.Schoemaker MH, Gommans WM, Conde de la Rosa L, Homan M, Klok P, Trautwein C, et al. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–161. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell C, Park MA, Zhang G, Han SI, Harada H, Franklin RA, et al. 17-Allylamino-17-demethoxygeldanamycin enhances the lethality of deoxycholic acid in primary rodent hepatocytes and established cell lines. Mol Cancer Ther. 2007;6:618–632. doi: 10.1158/1535-7163.MCT-06-0532. [DOI] [PubMed] [Google Scholar]
- 20.Grambihler A, Higuchi H, Bronk SF, Gores GJ. cFLIP-L inhibits p38 MAPK activation: an additional anti-apoptotic mechanism in bile acid-mediated apoptosis. J Biol Chem. 2001;278:26831–26837. doi: 10.1074/jbc.M303229200. [DOI] [PubMed] [Google Scholar]
- 21.Haussinger D, Kurz AK, Wettstein M, Graf D, Vom Dahl S, Schliess F. Involvement of integrins and Src in tauroursodeoxycholate induced and swelling induced choleresis. Gastroenterology. 2003;124:1476–1487. doi: 10.1016/s0016-5085(03)00274-9. [DOI] [PubMed] [Google Scholar]
- 22.Khare S, Holgren C, Samarel AM. Deoxycholic acid differentially regulates focal adhesion kinase phosphorylation: role of tyrosine phosphatase ShP2. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1100–G1112. doi: 10.1152/ajpgi.00008.2006. [DOI] [PubMed] [Google Scholar]
- 23.Webster CRL, Usechek P, Anwer MS. cAMP inhibits bile acid induced apoptosis by blocking caspase activation and cytochrome C release. Am J Physiol Gastrointest Liver Physiol. 2002;283:G727–G738. doi: 10.1152/ajpgi.00410.2001. [DOI] [PubMed] [Google Scholar]
- 24.Graf D, Reinehr R, Kurz AK, Fischer R, Haussinger D. Inhibition of taurolithocholate 3-sulfate induced apoptosis by cyclic AMP in rat hepatocytes involves protein kinase A-dependent and independent mechanisms. Archives of Biochem Biophys. 2003;425:34–42. doi: 10.1016/s0003-9861(03)00224-8. [DOI] [PubMed] [Google Scholar]
- 25.Cullen K, McCool J, Anwer MS, Webster CRL. Activation of cAMP-guanine exchange factor (cAMP-GEF) confers protein kinase A independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G334–G343. doi: 10.1152/ajpgi.00517.2003. [DOI] [PubMed] [Google Scholar]
- 26.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen L, Mooney D, Vacanti J, Ingber D. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Chem. 1994;5:967–975. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, et al. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563–1573. doi: 10.1002/hep.20246. [DOI] [PubMed] [Google Scholar]
- 30.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 31.Bissell DM, Stamatoglou SC, Nermut MV, Hughes C. Interactions of rat hepatocytes with type IV collagen, fibronectin and laminin matrices. Eur J Cell Biol. 1986;40:72–78. [PubMed] [Google Scholar]
- 32.Wary KK, Dans M, Mariotti A, Giancotti FG. Biochemical analysis of integrin-mediated Shc signaling. Methods Mol Biol. 1999;129:35–49. doi: 10.1385/1-59259-249-X:35. [DOI] [PubMed] [Google Scholar]
- 33.Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, Tsuruo T, et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol. 2007;212:717–728. doi: 10.1002/jcp.21096. [DOI] [PubMed] [Google Scholar]
- 34.Harnois C, Demers MJ, Bouchard V, Vallee K, Gagne D, Fujita N, et al. Human intestinal epithelial crypt cell survival and death: Complex modulations of Bcl-2 homologs by Fak, PI3-K/Akt-1, MEK/Erk, and p38 signaling pathways. J Cell Physiol. 2004;198:209–222. doi: 10.1002/jcp.10399. [DOI] [PubMed] [Google Scholar]
- 35.Barberis L, Wary KK, Fiucci G, Liu F, Hirsch F, Brancaccio M, et al. Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J Biol Chem. 2000;275:36532–36540. doi: 10.1074/jbc.M002487200. [DOI] [PubMed] [Google Scholar]
- 36.Hirano F, Haneda M, Makino I. Chenodeoxycholic acid and taurochenodexycholic acid induce anti-apoptotic cIAP-1 expression in human hepatocytes. J Gastroenterol Hepatol. 2006;21:1807–1813. doi: 10.1111/j.1440-1746.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Nan L, Bardag-Gorce F, Li J, French BA, Wilson LT, et al. The role of laminin-integrin signaling in triggering MB formation. An in vivo and in vitro study. Exp Mol Pathol. 2005;79:1–8. doi: 10.1016/j.yexmp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, et al. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol. 2004;41:104–111. doi: 10.1016/j.jhep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006;281:14948–14960. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 40.Fickert P, Trauner M, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H. Cytokeratins as targets for bile acid induced toxicity. Am J Pathol. 2002;160:491–499. doi: 10.1016/S0002-9440(10)64868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuchweber B, Roy S, Desroches S, Yousef IM, Gicquad C, Weber AM, et al. Effects of bile acids on actin polymerization in vitro. Life Sci. 1990;47:1299–1307. doi: 10.1016/0024-3205(90)90193-u. [DOI] [PubMed] [Google Scholar]
- 42.Thibault N, Maurice M, Maratrat M, Cordier A, Feldman G, Ballet F. Effect of tauroursodeoxycholate on actin filament alteration induced by cholestatic agents. A study in isolated hepatocyte couplets. J Hepatol. 1993;19:367–376. doi: 10.1016/s0168-8278(05)80544-6. [DOI] [PubMed] [Google Scholar]
- 43.Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007;21:1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- 44.Montiel M, de la Blanca EP, Jimenez E. Angiotensin II induces focal adhesion kinase/paxillin phosphorylation and cell migration in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;25(327):971–978. doi: 10.1016/j.bbrc.2004.12.110. [DOI] [PubMed] [Google Scholar]
- 45.Amaral JD, Castro RE, Solá S, Steer CJ, Rodrigues CM. p53 is a key molecular target of ursodeoxycholic acid in regulating apoptosis. J Biol Chem. 2007;282:34250–34259. doi: 10.1074/jbc.M704075200. [DOI] [PubMed] [Google Scholar]
- 46.Castro RE, Amaral JD, Solá S, Kren BT, Steer CJ, Rodrigues CM. Differential regulation of cyclin D1 and cell death by bile acids in primary rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G327–G334. doi: 10.1152/ajpgi.00093.2007. [DOI] [PubMed] [Google Scholar]
- 48.Shaker AM. Anti-integrin as novel drug-discovery targets: potential therapeutic and diagnostic implications. Curr Opin Chem Biol. 2002;6:534–554. doi: 10.1016/s1367-5931(02)00350-2. [DOI] [PubMed] [Google Scholar]
- 49.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discovery. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Tan H, Dong X, Xu Z, Shi C, Han X, et al. Antisense integrin alphaV and beta3 gene therapy suppresses subcutaneously implanted hepatocellular carcinomas. Dig Liver Dis. 2007;39:557–565. doi: 10.1016/j.dld.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 51.Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J Hepatol. 2007;46:878–887. doi: 10.1016/j.jhep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Shi Q, Hjelmeland AB, Keir ST, Song L, Wickman S, Jackson D, et al. A novel low molecular weight inhibitor of focal adhesion kinase. TAE226, inhibits glioma growth Mol Carcinog. 2007;46:488–496. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]