Abstract
Background
Endothelial function deteriorates after glucose ingestion. This may be attributed to hyperglycemia-induced oxidative stress. Acute endurance exercise might improve postprandial endothelial function by enhancing glucoregulation and reducing postprandial hyperglycemia.
Objective
To determine if endurance exercise performed 17h prior to high-sugar food ingestion attenuates postprandial impairment in endothelial function.
Design
Healthy men and women (n=13; age: 48±17y) were studied on 2 occasions: after ≥48h with no exercise (CON) and 17h after a 60-min bout of endurance exercise (EX). During each trial, brachial artery flow-mediated dilation (FMD) was used to assess endothelial function before and after the ingestion of a candy bar and soft drink. Glucose, insulin, and thiobarbiturate reactive substances (TBARS), as a marker of oxidative stress, were measured in blood obtained during each FMD measurement. Insulin sensitivity index (ISI) was calculated from the glucose and insulin data.
Results
FMD decreased significantly after food ingestion in both trials. However, prior exercise shifted the entire FMD curve upward (main treatment effect: p=0.0002), resulting in a greater area under the curve for FMD (774±122 vs. 607±122 % · min, p=0.01). Prior exercise shifted the glucose and insulin curves downward (main treatment effects: p=0.05 and p=0.0007, respectively) and increased ISI (10.8±0.7 vs. 9.2±0.7, p=0.01). TBARS did not differ between trials.
Conclusion
Postprandial endothelial function was improved by endurance exercise performed ~17 hours earlier. This effect was accompanied by exercise-induced improvements in insulin action and reductions in glycemia but did not correspond with reductions in oxidative stress, as assessed by TBARS.
Keywords: endothelial function, acute exercise, postprandial, hyperglycemia
INTRODUCTION
The development of impaired endothelial function is an early atherogenic event (1). Accordingly, poor brachial artery flow mediated dilation, which reflects impaired endothelial function, is an independent predictor of clinical cardiovascular events and death due to cardiovascular disease (2,3). While vascular function studies are typically performed on fasting subjects, endothelial function deteriorates acutely after glucose ingestion according to most studies (4–7), but not all (8,9). This effect may be attributed to hyperglycemia-induced oxidative stress (5,7). If poor endothelial function promotes atherogenesis and endothelial function is impaired during postprandial periods, it is conceivable that the long-term development of atherosclerosis might be partly attributed to repeated exposures to impaired endothelial function after food ingestion.
Regular physical activity is independently associated with a lower risk of death due to cardiovascular disease (10,11). Part of this effect may be attributed to exercise-induced improvements in endothelial function (12,13). In light of evidence that postprandial glycemia is reduced by exercise that was performed hours or days earlier (14) and that postprandial hyperglycemia likely contributes to postprandial impairments in endothelial function (4,6,15), it is conceivable that acute endurance exercise minimizes the postprandial impairment in endothelial function. If this proposition is true, this would support the notion that some of the cardioprotective benefits of exercise are attributable to improvements in postprandial endothelial function.
The purpose of the present study was to test the hypothesis that acute endurance exercise performed ~17 hr prior to high-sugar food ingestion attenuates postprandial impairment in endothelial function. To maximize the clinical relevance of our findings, we administered a candy bar and soft drink as “high-sugar food” rather than a glucose tolerance test beverage, as has been used in other studies. Furthermore, because most meals and snacks are not immediately preceded or followed by exercise, even for individuals who perform daily exercise, we sought to determine if acute exercise provides a vasoprotective effect that lasts for 16–19 hours after exercise cessation. A secondary purpose of our study was to gain insights into the mechanisms for exercise-induced improvements in endothelial function; therefore, we also tested the hypothesis that improvements in endothelial function, if present, are accompanied by improvements in glucoregulation and reductions in oxidative stress.
SUBJECTS AND METHODS
Subjects
Thirteen healthy men and women, aged 26–75 yr were recruited and gave their informed written consent to participate in the study, which was approved by the Human Research Protection Office at Washington University. Because chronic diabetes may cause secondary damage to the endothelium, volunteers with self-reported diabetes or those with “provisional diabetes,” as determined by using a 75-g oral glucose tolerance test (16), were excluded. Volunteers were not eligible for the study if they had any major chronic diseases or conditions that would interfere with exercise, in which exercise is contraindicated, or that would interfere with interpretation of results. Examples include self-reported or clinical evidence of coronary artery disease, significant obstructive airway disease, stroke, resting blood pressure ≥170 mmHg systolic or ≥100 mmHg diastolic, malignancy, orthopedic or musculoskeletal problems, and current smoking. No subjects were taking medications for diabetes, metabolic disease, or cardiovascular disease or other medications or dietary supplements that are known to affect glucose metabolism. During the 6 months prior to study enrollment, 4 of the participants were sedentary (exercise <2 d/wk) and 9 performed regular endurance exercise ≥2 d/wk. All of the participants who were screened and eligible completed the study.
Study Design
The study was conducted using a cross-over design, in which each subject underwent both the control and exercise treatments. The testing sequence was counterbalanced, such that half of the participants performed the control trial first and the others performed the exercise trial first. The participants reported to our laboratory on four occasions, once for screening, body composition, and maximal oxygen uptake assessments, once for a bout of endurance exercise, and twice for postprandial endothelial function testing. The bout of endurance exercise was performed in the afternoon/evening before one of the endothelial function testing sessions (exercise trial); the other endothelial function testing session was preceded by at least 48 hours of abstinence from all exercise (control trial). The control and exercise trials were separated by 10±4 days.
Body composition, height, and weight
Body fat percentage was assessed by using dual-energy x-ray absorptiometry with enhanced whole body analyses (Hologic QDR-1000/W, Waltham, MA; software version 5.73). Height and weight were measured with a wall-mounted stadiometer and balance beam scale, respectively, and were used to calculate body mass index (kg/m2).
Maximal oxygen uptake
Maximal oxygen uptake (VO2max) was determined by using indirect calorimetry (True Max 2400, ParvoMedics, Salt Lake City, UT) during a maximal graded treadmill exercise test as described previously (17). Heart rate was measured from electrocardiograms recorded during each stage of the test and maximal exercise.
Acute Endurance Exercise
For the exercise trial, a 60-minute bout of endurance exercise was performed between 3:00 and 7:00 pm, which was 16.8±0.3 hr prior to the commencement of endothelial function testing the next day. The exercise consisted of indoor, supervised endurance exercise on rowing and elliptical machines, a stationary cycle and/or a treadmill. The elliptical machine and cycle both included handles for arm exercise. The heart rate associated with 70% of VO2max was calculated for each participant using simple linear regression analyses of the heart rate and oxygen uptake data from the VO2max test. The participants were monitored and encouraged to maintain their heart rate within 5 beats/min of this value. The average heart rate, as measured using wristwatch-type monitors (S610, Polar Electro Oy, Kempele, Finland) during exercise was 142±5 beats/min or 80±1% of measured maximal heart rate, which is equivalent to approximately 63% of VO2max based on published standards (18).
High-Sugar Food
The food was selected to represent a “snack” that is commonly consumed by people in the United States and consisted of a 59 g (2.07 ounce) candy bar (Snickers, Mars Corporation, McLean VA) and a 591 mL (20 fluid ounce) lemon-lime flavored soft drink (Sprite, Coca-Cola Corporation, Atlanta GA). The candy bar contained the following ingredients: milk chocolate (sugar, cocoa butter, chocolate, lactose, skim milk, milk fat, soy lecithin, and artificial flavor), peanuts, corn syrup, sugar, skim milk, butter, milk fat, partially hydrogenated soybean oil, lactose, salt, egg whites, and artificial flavor. The soft drink contained carbonated water, high fructose corn syrup, citric acid, natural flavors, sodium citrate, and sodium benzoate. Nutrient information for the candy bar and soft drink combined was determined by using Nutrition Data System for Research (version 5.0, Nutrition Coordination Center, University of Minnesota, Minneapolis, MN) and was as follows: energy, 534 kcal; total fat, 14 g; saturated fat, 7.4 g; trans-fat, 0 g; total carbohydrate, 101 g; glucose, 28 g; fructose, 38 g; sucrose, 27 g; starch, 2 g; and protein, 5 g.
Preparation for Endothelial Function Tests
The participants were advised to refrain from taking nutritional supplements, medications, and alcohol during the day prior to endothelial function assessments and fasted for 12–14 hours before testing. Furthermore, for 1 day prior to the first study trial (i.e. control or exercise trial), the participants kept a diary of all foods consumed and were asked to replicate this diet during the second study trial. Upon arrival for testing, the participants were questioned to confirm that they complied with all preparation instructions. If any of the instructions were not followed, the testing was rescheduled.
Endothelial Function
Participants arrived at the laboratory at 10:30 am and rested quietly in the supine position for ≥20 minutes before any assessments began. Blood pressure was measured in the left arm with an automated oscillometric non-invasive monitor (Dinamap 1846SX, Critikon, Inc., Tampa, FL). Brachial artery flow mediated dilation (FMD) was measured twice before, and every 30 minutes for 2.5 hr after the consumption of the high-sugar food. The participant’s right arm was immobilized in a custom made device with the shoulder abducted at 70–90° and elbow fully extended. Ultrasound images were acquired using an ultrasound system (Agilent SONOS 5500, Andover, Massachusetts) equipped with an 11-3L linear array transducer. After the image was optimized the probe was secured using a stereotactic clamp (Noga Engineering, Ltd.) to maintain constant positioning over the artery. The images were fed to a separate computer for real time (30 ± 2 samples/sec) quantification of arterial diastolic diameter using Vascular Imaging Analysis software (VIA, version 9.60)(19,20). Baseline diameter was recorded for 2 minutes after which a pneumatic cuff (Hokanson E20 Rapid Cuff Inflator and AG101 Cuff Inflator Air Source, PMS Instruments, Ltd., Maidenhead, UK) on the right forearm was inflated to 200 mmHg to occlude blood flow. After 5 minutes of occlusion, the cuff was deflated and arterial diameter was recorded continuously for 5 minutes. FMD was calculated as the percent increase in diameter from baseline to peak diameter, where baseline diameter was the average diastolic diameter over the 2-minute baseline and peak diameter was recorded as the 10-second average of the highest diastolic diameter after cuff deflation. During some FMD assessments, a shift in baseline diameter was evident by comparing the pre-occlusion diameter with the diameter measured after the dilatory response had ended and the blood vessel was again constricted. In these cases, the end-of-test diameter was used as “baseline diameter,” provided that the diameter had decreased from peak and was stable.
Data from the two FMD measures performed before food ingestion were averaged to reflect fasting FMD. Area under the curve (AUC) was calculated with the fasting and all postprandial FMD data by using the trapezoidal rule (21).
Glucoregulation
Venous blood was drawn from an indwelling catheter in the participant’s left arm during each FMD assessment. Plasma was isolated and stored at − 20°C for later analysis. Glucose was analyzed using the glucose oxidase method (Micro-Stat GM7, Analox Instruments Inc., Lunenberg, MA) and insulin by using double-antibody radioimmunoassay (22). The samples were analyzed in duplicate and all samples for each subject were batch analyzed to eliminate inter-assay variability.
For both insulin and glucose, the two measures obtained before food ingestion were averaged to reflect fasting values and the AUCs were calculated from the fasting values and all postprandial values by using the trapezoidal rule (21). Furthermore, an index of insulin sensitivity (ISI) was calculated as follows: ISI = 10,000[(FPG × FPI) × (MPG × MPI)]0.5, where FPG is fasting plasma glucose (mg/dL), FPI is fasting plasma insulin (µU/mL), MPG is mean plasma glucose (mg/dL) from all time points (i.e. 0–150 min), and MPI is mean plasma insulin (µU/mL) from all time points. This method is based on that of Matsuda and DeFronzo (23). However, the original ISI method was developed for use with a 2-hr, 75-gram oral glucose tolerance test (23), whereas our test was longer (2.5 hr) and used a candy bar and soft drink. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting glucose (mmol/L) × fasting insulin (µU/mL) / 22.5 (24,25).
Oxidative stress
Serum was isolated from venous blood samples drawn during each FMD assessment and stored at −20°C for later batch analyses for thiobarbiturate reactive substances (TBARS) concentrations, as a marker of lipid peroxidation and oxidative stress. TBARS were measured in duplicate using a colorimetric assay (Cayman Chemical, Ann Arbor, MI). This assay is based on the formation of a malondialdehyde-thiobarbituric acid adduct in acidic conditions at 100°C. Concentration of the adduct was determined at 540 nm, using a Synergy HT Multi-Detection microplate reader (BioTek, Winooski, VT). The two fasting TBARS values were averaged and AUC was calculated from the fasting values and all postprandial values using the trapezoidal rule (21).
Statistical Analyses
Because we hypothesized that prior exercise attenuates or prevents postprandial reductions in endothelial function and postprandial increases in glucose, insulin, and TBARS, we analyzed these outcomes by using two-factor (treatment and time) ANOVAs with repeated measures. The treatment by time interaction from these analyses were interpreted to determine if the time-dependent changes in these outcomes were different between the control and exercise conditions. When the interaction term was not significant, main effects of treatment and test time were evaluated. A main effect of treatment was interpreted to mean that differences existed between the control and exercise condition at baseline and that the difference remained throughout the postprandial period. A main effect of time was interpreted to mean that differences existed among time points (for example, a difference between the fasting and 30 min values) and that this occurred regardless of the treatment condition. As secondary analyses, several outcomes that are represented by one data point (i.e. fasting values, AUCs, ISI, and HOMA-IR.) for each trial were analyzed by using one-factor ANOVAs with repeated measures to compare the exercise and control trial data. These tests were performed because fasting values, AUC, and indices of insulin action are commonly presented in this type of study but were also performed to assist in interpreting the results from the 2-way ANOVAs. The methods used for the calculation of fasting values, AUCs, ISI, and HOMA-IR are described above along with information about data acquisition. Residuals were examined for normality and homogeneity of variance. When necessary, data were transformed as follows to achieve normality before final data analyses were performed: insulin, HOMA-IR, and TBARS data were log transformed, FMD data were square root transformed, and baseline brachial artery diameter data were rank transformed. For clarity, the non-transformed means and error terms are presented; however, the p-values are based on the analyses of transformed data where transformations were used. Statistical significance was accepted at p≤0.05. Data are presented as means ± SEM unless noted otherwise. Analyses were conducted with SAS for Windows XP Pro (version 9.1, SAS Institute, Cary, NC).
RESULTS
Subjects
Characteristics of the participants are presented in Table 1. On average the participants were middle-aged with a mean BMI in the normal weight range and body fat percentages near the optimal level for both men and women. Mean VO2max was above average for middle-aged men and women (26). However, according to age- and sex-based normative data (26), some participants had high VO2max values and others had low values. Based on fasting and 120-min plasma glucose concentrations from a standard 75g oral glucose tolerance test (16), two participants had pre-diabetes and all others had normal glucose tolerance. Averages for systolic and diastolic blood pressures were within normal ranges and were not different between the control and exercise trials (Table 2).
Table 1.
Characteristics of the study participants.
| Sex, men/women | 5/8 |
| Age, yr | 48 ± 17 |
| Weight, kg | |
| Men | 81.8 ± 9.0 |
| Women | 66.5 ± 7.0 |
| BMI, kg/m2 | 24.0 ± 2.2 |
| Body fat, % | |
| Men | 16.8 ± 4.2 |
| Women | 29.2 ± 11.4 |
| VO2 max, L/min | |
| Men | 3.90 ± 0.86 |
| Women | 2.37 ± 0.79 |
| VO2 max, mL/kg/min | |
| Men | 47.6 ± 8.8 |
| Women | 36.4 ± 13.8 |
| Fasting glucose (mg/dL) | 91 ± 7 |
| 120-min glucose (mg/dL) | 96 ± 29 |
Data are means ± SD except for sex data, which are counts. BMI, body mass index; VO2 max, maximal oxygen uptake. Fasting and 120-min glucose concentration data were collected using a 75-g oral glucose tolerance test for diabetes screening (16).
Table 2.
Plasma glucose and insulin concentrations and flow mediated dilation of the brachial artery before and after ingestion of a candy bar and soft drink.
| Control Trial | Exercise Trial | P-value | |
|---|---|---|---|
| Fasting systolic BP, mmHg | 114 ± 4 | 115 ± 4 | 0.86 |
| Fasting diastolic BP, mmHg | 66 ± 2 | 66 ± 2 | 0.89 |
| Fasting glucose, mg/dL | 87 ± 2 | 84 ± 2 | 0.08 |
| Glucose AUC, mg/dL · min | 16828 ± 689 | 15858 ± 689 | 0.16 |
| Fasting insulin, µU/mL | 3.0 ± 0.3 | 2.6 ± 0.3 | 0.08 |
| Insulin AUC, µU/mL · min | 3898 ± 518 | 3158 ± 518 | 0.12 |
| Insulin sensitivity index | 9.2 ± 0.7 | 10.8 ± 0.7 | 0.01 |
| HOMA-IR | 0.64 ± 0.06 | 0.54 ± 0.06 | 0.04 |
| Fasting FMD, % | 4.7 ± 1.0 | 5.5 ± 1.0 | 0.31 |
| FMD AUC, % · min | 607 ± 122 | 774 ± 122 | 0.01 |
| Fasting TBARS, µmol/L | 13.0 ± 3.2 | 16.4 ± 3.2 | 0.40 |
| TBARS AUC, µmol/L · min | 1735 ± 218 | 1733 ± 218 | 0.82 |
Data are means ± SEM and represent 13 subjects who underwent both the control and exercise treatments. P-value reflects the significance for the comparison between the control and exercise conditions, which were performed using one-factor (treatment) ANOVAs with repeated measures. AUC, area under the curve; BP, blood pressure; FMD, flow mediated dilation of the brachial artery; HOMA-IR, homeostasis model assessment of insulin resistance. Insulin sensitivity index is unitless and was determined according to Matsuda and DeFronzo (23) using all data from the 150-minute test. HOMA-IR is a unitless index and was calculated according to Matthews et al. (24). Mean postprandial FMD was calculated using data from all postprandial measures.
Glucoregulatory function
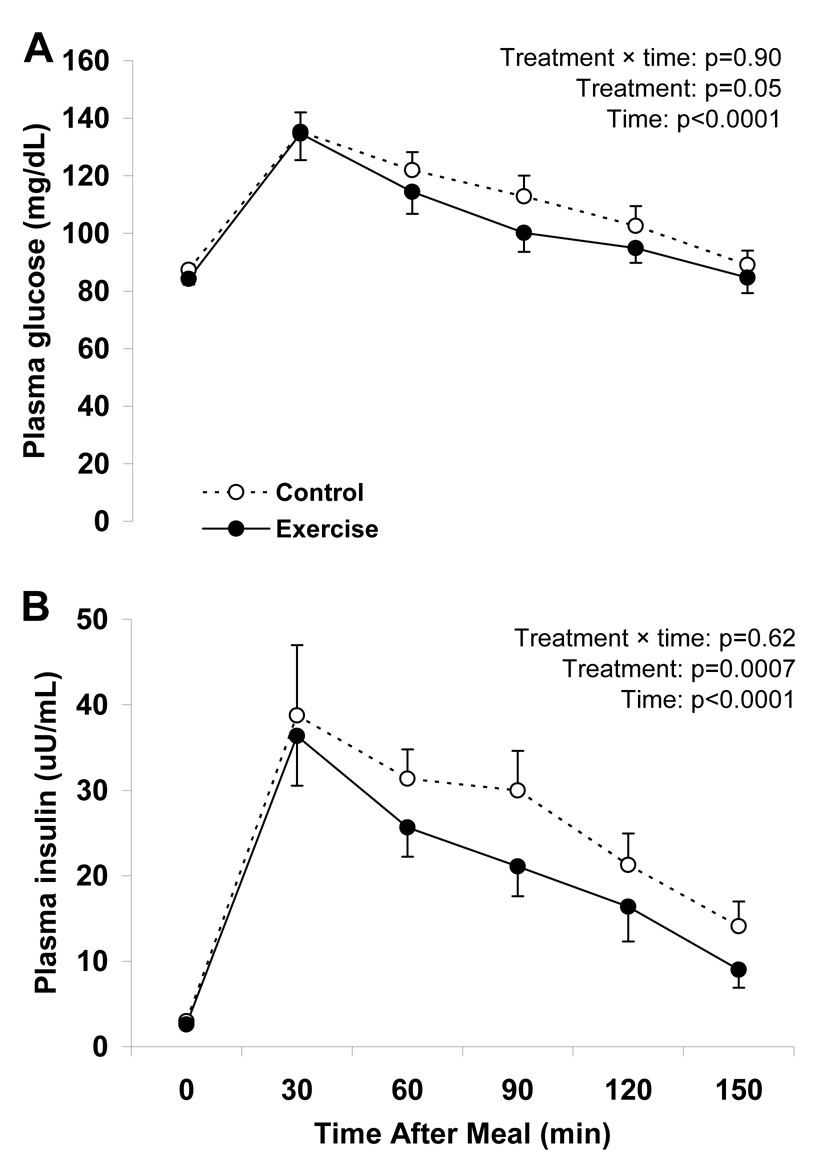
Plasma glucose and insulin concentrations increased after high-sugar food ingestion (Figure 1), and returned to baseline by the end of the test. For both glucose and insulin, the interaction between treatment (control vs. exercise) and test time was not significant but the main effect of treatment was significant. These results indicate that the shape of the glucose and insulin curves did not differ between the exercise and control trials; however the glucose and insulin curves were shifted downward in the exercise trial (Figure 1). As a secondary analysis to help in interpreting the glucose and insulin results, we also compared fasting glucose and insulin values and AUCs between the control and exercise trials. Although the fasting values for the plasma glucose and insulin concentrations and their AUCs were not significantly different between study trials (Table 2), there were weak tendencies for lower values in the exercise trial. ISI was significantly greater and HOMA-IR was significantly lower in the exercise trial (Table 2).
Figure 1.
Time dependent changes in plasma glucose (panel A) and insulin concentrations (panel B) in response to oral ingestion of a candy bar and soft drink ~17 h after a single bout of endurance exercise (Exercise) and after ≥ 48 h without exercise (Control). Data are means ± SEM and represent 13 subjects who underwent both the control and exercise treatments. Statistical analyses were performed using two-factor (treatment and time) ANOVAs with repeated measures.
Endothelial function
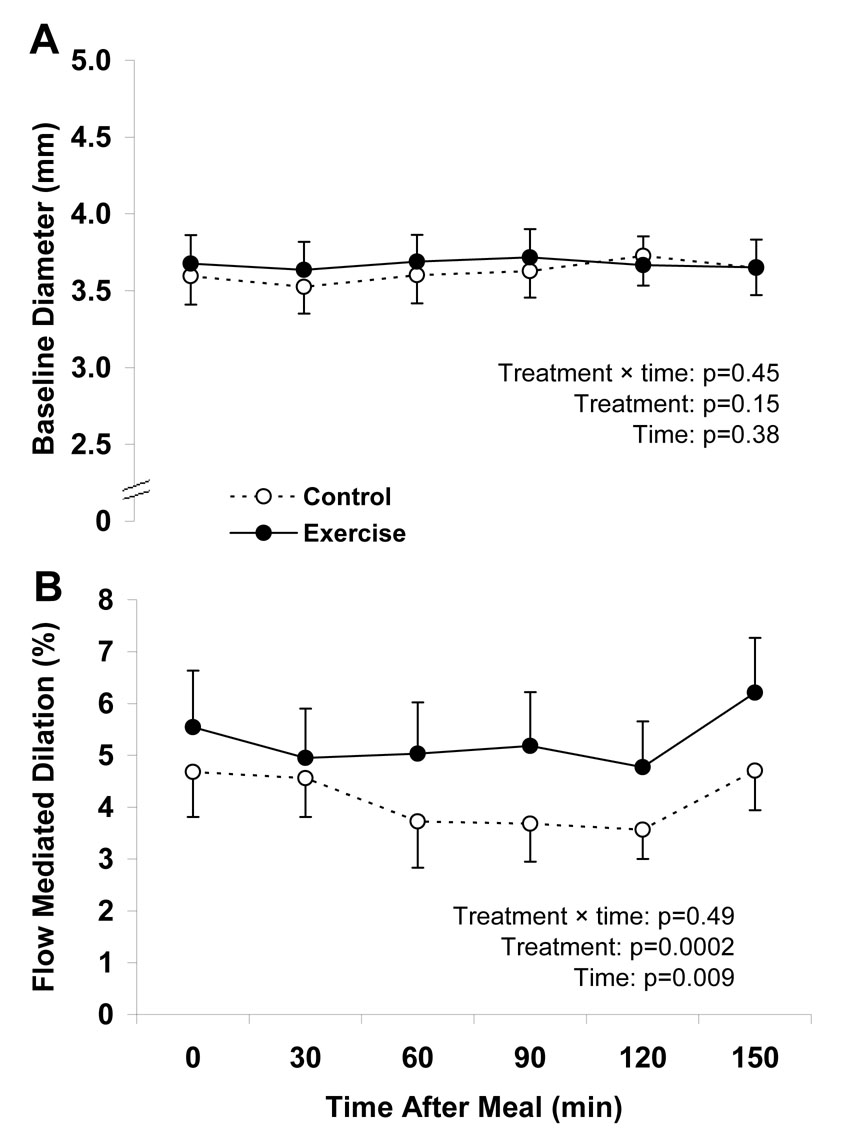
Baseline arterial diameter from the FMD tests did not change from fasting to the postprandial state in either the exercise or control trial and did not differ between trials (Figure 2). FMD decreased after ingestion of the high-sugar food, as indicated by a significant main effect of test time (Figure 2). There was no difference in the time-course for the postprandial decrease in FMD, as indicated by the non-significant treatment by time interaction. However, as indicated by the significant main effect of treatment, the entire FMD curve was shifted upward in the exercise trial. Secondary analyses were used to compare fasting and postprandial FMD to help in interpreting the FMD results. Fasting FMD did not differ between treatments. However, the FMD AUC was significantly greater in the exercise trial (Table 2).
Figure 2.
Time dependent changes in the diameter (panel A) and endothelial function (panel B) in the brachial artery in response to oral ingestion of a candy bar and soft drink ~17 h after a single bout of endurance exercise (Exercise) and after ≥ 48 h without exercise (Control). FMD, flow-mediated dilation. Data are means ± SEM and represent 13 subjects who underwent both the control and exercise treatments. Statistical analyses were performed using two-factor (treatment and time) ANOVAs with repeated measures.
Oxidative stress
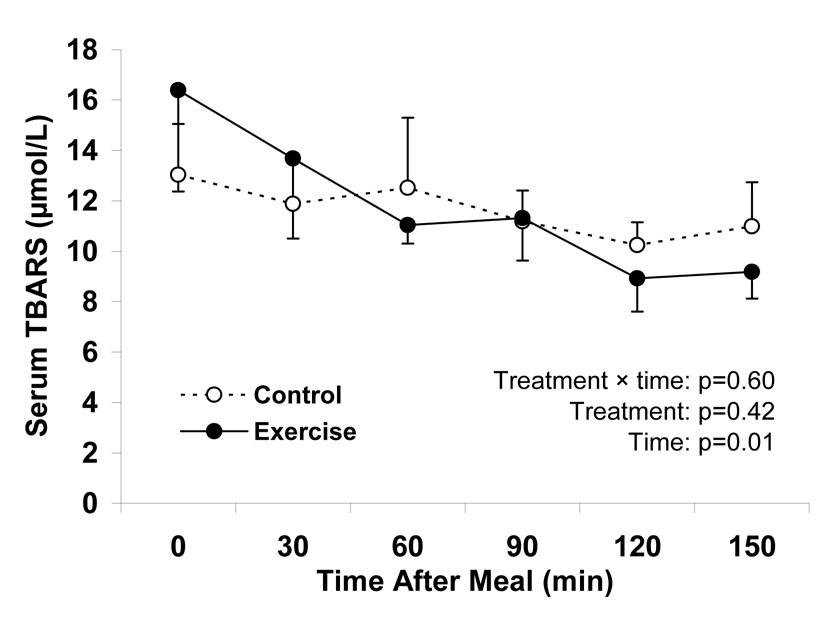
Serum TBARS concentrations decreased steadily after ingestion of the high-sugar food, as reflected by a significant test time effect (Figure 3). The time course for this decrease was not different between conditions, as indicated by the non-significant treatment by time interaction. Furthermore, acute exercise did not shift the curve upward or downward, as indicated by the non-significant main effect of treatment.
Figure 3.
Time dependent changes in oxidative stress in response to oral ingestion of a candy bar and soft drink ~17 h after a single bout of endurance exercise (Exercise) and after ≥ 48 h without exercise (Control). TBARS, thiobarbituric acid reactive substances. Data are means ± SEM and represent 13 subjects who underwent both the control and exercise treatments. Statistical analysis was performed using a two-factor (treatment and time) ANOVA with repeated measures.
DISCUSSION
Results from the present study suggest that a single bout of endurance exercise performed ~17 hours before high-sugar food consumption enhances postprandial endothelial function. This was evidenced by an upward shift in the entire FMD curve, such that the lowest postprandial FMD in the exercise condition was approximately equal to the greatest FMD value that occurred in the control condition. Furthermore, the area under the curve for FMD was approximately 28% greater in the exercise trial.
Results from the two-factor ANOVAs of FMD, glucose, and insulin suggest that fasting values started out different in the control and exercise trials and that the values remained different throughout the postprandial period (based on non-significant interactions paired with significant main treatment effects). However, the one-way ANOVAs of fasting values (Table 2) indicate no treatment effect on fasting values. We suspect that two things contribute to this apparent discrepancy for fasting values: (a) the treatment effect on fasting values is weaker than it is for postprandial values and that this difference between effects on fasting and postprandial values cannot be detected using a 2-factor ANOVA (interaction term) because it is very small, and (b) the treatment effects on fasting glucose and insulin values are non-significant due to a statistical power limitation for these analyses, and that with more statistical power, as may be present in the 2-factor ANOVA, the treatment effect would be significant. Our a priori decision was to interpret the results of the two-way ANOVAs for our main conclusions and the paired comparisons were performed as secondary analyses. However, in light of the discrepant results from these analytic approaches, we refrain from making strong comments and conclusions about treatment effects on fasting FMD, glucose, and insulin.
Only one other study assessed the effects of acute exercise on postprandial endothelial function (27). Zhu et al. showed that 45 min of treadmill exercise commenced immediately after ingestion of a 75 gram glucose beverage resulted in greater postprandial FMD values as compared to a control trial on the same subjects. Our study supports these findings and advances them in several respects. First, in our study, exercise was performed in the afternoon/evening prior to the postprandial vascular function tests, rather than during the postprandial period, and shows that exercise is vasoprotective after a meal/snack consumed many hours after exercise cessation. This difference is clinically relevant, as most people do not exercise immediately after consuming every meal. Secondly, we used a candy bar and soft drink, which represents a commonly consumed snack in the United States, rather than a glucose tolerance test beverage. While there is no strong reason to believe that the glucoregulatory and vascular effect of these “meals” differ, pure glucose is rarely consumed in “real world” settings. High sugar foods, such as those used in the present study, typically contain fructose, sucrose, and other sugars, as well as fat, protein, preservatives, and other ingredients, which could conceivably alter postprandial physiology. Finally, while Zhu et al. exclusively studied 19–26 year old men, our sample included men and women with a range of age, body weight, and habitual exercise habits, making the results more applicable to the general population.
We hypothesized that acute endurance exercise enhances endothelial function because hyperglycemia impairs endothelial function (4,6,15) and because acute endurance exercise enhances glucoregulation and reduces postprandial glycemia (14). Our findings support this hypothesis, as the exercise-induced improvement in postprandial endothelial function was accompanied by significant reductions in plasma glucose and insulin concentrations (significant treatment effects from 2-way ANOVAs), increases in insulin sensitivity index (calculated from fasting and postprandial glucose and insulin concentrations), and reductions in the HOMA-IR index of insulin resistance (calculated from fasting glucose and insulin concentrations).
It has been suggested that hyperglycemia impairs endothelial function by increasing oxidative stress because co-administration of antioxidant vitamins with glucose prevents the postprandial decline in FMD (5,7). Furthermore, oxidative stress has been shown to increase after ingestion of a 75-gram glucose beverage, as evidenced by postprandial increases in neutrophil superoxide anion formation (5) and plasma TBARS concentrations (6). However another study reported no change in plasma malondialdehyde or erythrocyte glutathione, glutathione peroxidase, or superoxide dismutase (7). In our study, serum TBARS concentration did not increase in the postprandial period, rather it decreased. This unexpected result suggests that the postprandial impairment in endothelial function is not necessarily caused by oxidative stress. While an explanation for the postprandial decrease in oxidative stress in our study is not clear, two ingredients in the soft drink that we used, citric acid and sodium citrate, may have increased the antioxidant capacity of blood. Although these compounds are not recognized as biologically important antioxidants, they have antioxidant properties for which they are used as preservatives in soft drinks (28).
We hypothesized that acute exercise enhances postprandial endothelial function by decreasing postprandial glycemia and thereby reducing postprandial oxidative stress. While exercise reduced postprandial glycemia, TBARS concentrations were unaffected, suggesting that reduced oxidative stress is not the mechanism for the observed improvements in postprandial endothelial function. An alternative mechanistic explanation is that exercise transiently induces endothelial nitric oxide synthase (eNOS), thereby leading to greater flow-mediated nitric oxide production, increased nitric oxide bioavailability, and greater dilation. While this explanation is speculative, it is noteworthy that acute endurance exercise transiently increases eNOS mRNA (29,30) and total NOS activity (31) in skeletal muscle. Furthermore, when cultured endothelial cells are exposed to acute shear stress of similar magnitude to that which occurs during exercise, eNOS mRNA and eNOS protein levels increase (32,33). However, to our knowledge, it is not known if acute exercise increases eNOS mRNA or protein levels in the endothelium of the intact vasculature.
Chronic endurance exercise training has been shown to improve fasting FMD in the blood vessels of exercised limbs but not in non-exercised limbs (34). We can not determine from our study if the beneficial effects of exercise on endothelial function are localized to the exercised limbs because we measured FMD in the brachial artery and our participants performed exercises that included upper body exercise. However, it is important to recognize that brachial artery FMD is clinically important because it correlates with coronary artery dilatory function (35). Since the heart is exercised during all types of endurance exercise, it would be expected that the coronary arteries benefit from acute endurance exercise, as did the brachial artery in our study.
We intentionally studied a heterogeneous sample which included men and women with a wide range of ages and physical activity levels so that the results would be applicable to the general population. However, a drawback to using such a heterogeneous sample is that it introduces the possibility that the significant findings are largely driven by a subset of the participants (for example, young participants may have benefited from exercise while older participants did not). While our sample size is too small to perform valid subgroup analyses or covariate analyses, it is noteworthy exercise improved the area under the curve for FMD and insulin sensitivity index in almost all subjects (11 of 13). Therefore, it appears as though acute exercise has beneficial effects on vascular function in a fairly heterogeneous population.
In exercise-related research, it is often argued that from a biological perspective, the exercised state is “normal” and the sedentary state is “abnormal” because during nearly all of the evolutionary past for humans/primates, large amounts of physical activity were required for survival. Therefore, an alternative interpretation of our findings is that abstinence from exercise for ≥48 hr results in “abnormal” or poor endothelial function. This perspective may be especially relevant in light of the fact that 69% of our subjects exercise at least twice weekly.
The results from this study have several implications. First, as has been previously shown in response to glucose beverage ingestion (4–7), a commonly consumed snack consisting of a candy bar and soft drink impairs vascular endothelial function for approximately 2 hours. This adverse effect occurs regardless of the presence or absence of prior exercise; although endothelial function during the entire fasting-to-postprandial period is better when preceded by acute endurance exercise. Although speculative, it is possible that routine exposure to this adverse effect may have long-term health consequences that are related to endothelial dysfunction, such as the development of atherosclerosis (1). This research also demonstrates two important benefits of endurance exercise. First, a single bout of exercise protects against the adverse effects of high-sugar food ingestion on endothelial function and this effect is evident ~17 hours after exercise. Furthermore, as has been shown by others (14), acute endurance exercise enhances glucoregulatory function.
CONCLUSION
Endothelial function after high-sugar food ingestion was improved by a single bout of endurance exercise performed ~17 hours earlier. This improvement was associated with exercise-induced improvements in glucoregulation but was not accompanied by reductions in oxidative stress.
ACKNOWLEDGEMENTS
EPW designed the study, analyzed and interpreted the data, drafted the manuscript, and is the corresponding author. HA participated in designing the study, revising the manuscript for intellectual content, and gave his approval of the final manuscript. DTV assisted in the interpretation of the data, provided critical intellectual feedback for the manuscript, and gave his final approval of the manuscript. EM assisted in the interpretation of the data, provided critical intellectual feedback for the manuscript, and gave his final approval of the manuscript. JOH participated in designing the study, revising the manuscript for intellectual content, and gave his approval of the final manuscript.
None of the authors have conflicts of interest related to any part of this study or manuscript.
Support: NIH grants RR00036 (General Clinical Research Center), AG00078, and AG028740 (Claude D. Pepper Older Americans Independence Center), and the University of Florida Institute on Aging.
REFERENCES
- 1.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin.Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 2.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 3.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll.Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 4.Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J.Vasc.Surg. 1998;28:687–694. doi: 10.1016/s0741-5214(98)70095-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee IK, Kim HS, Bae JH. Endothelial dysfunction: its relationship with acute hyperglycaemia and hyperlipidemia. Int.J.Clin.Pract.Suppl. 2002:59–64. [PubMed] [Google Scholar]
- 6.Kawano H, Motoyama T, Hirashima O, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J.Am.Coll.Cardiol. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 7.Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J.Am.Coll.Cardiol. 2000;36:2185–2191. doi: 10.1016/s0735-1097(00)00980-3. [DOI] [PubMed] [Google Scholar]
- 8.Dengel DR, Kelly AS, Steinberger J, Sinaiko AR. Effect of oral glucose loading on endothelial function in normal-weight and overweight children. Clin.Sci (Lond) 2007;112:493–498. doi: 10.1042/CS20060305. [DOI] [PubMed] [Google Scholar]
- 9.Siafarikas A, Watts K, Beye P, Jones TW, Davis EA, Green DJ. Lack of effect of oral glucose loading on conduit vessel endothelial function in healthy subjects. Clin.Sci (Lond) 2004;107:191–196. doi: 10.1042/CS20040004. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164:1092–1097. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 11.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 12.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson P, Montgomery HE, Mullen MJ, et al. Exercise training enhances endothelial function in young men. J Am Coll.Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 14.King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J.Appl.Physiol. 1995;78:17–22. doi: 10.1152/jappl.1995.78.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Wascher TC, Schmoelzer I, Wiegratz A, et al. Reduction of postchallenge hyperglycaemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur.J Clin.Invest. 2005;35:551–557. doi: 10.1111/j.1365-2362.2005.01550.x. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2005;28:S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 17.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. Baltimore: Williams & Wilkins; 2006. [DOI] [PubMed] [Google Scholar]
- 19.Newey VR, Nassiri DK. Online artery diameter measurement in ultrasound images using artificial neural networks. Ultrasound Med Biol. 2002;28:209–216. doi: 10.1016/s0301-5629(01)00505-1. [DOI] [PubMed] [Google Scholar]
- 20.Sidhu JS, Newey VR, Nassiri DK, Kaski JC. A rapid and reproducible on line automated technique to determine endothelial function. Heart. 2002;88:289–292. doi: 10.1136/heart.88.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 22.Morgan DR, Lazarow A. Immunoassay of insulin: two antibody system. Diabetes. 1963;12:115–126. [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. Baltimore: Williams & Wilkins; 1995. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Zhong C, Yu Y, Li K. Acute effects of hyperglycaemia with and without exercise on endothelial function in healthy young men. Eur.J Appl Physiol. 2007;99:585–591. doi: 10.1007/s00421-006-0378-3. [DOI] [PubMed] [Google Scholar]
- 28.Young JA. CLIP, Clinical Laboratory Information Profile for CAS No.: 77-92-9. Journal of Chemical Education. 2003;80:480. [Google Scholar]
- 29.Hellsten Y, Nielsen JJ, Lykkesfeldt J, et al. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic.Biol Med. 2007;43:353–361. doi: 10.1016/j.freeradbiomed.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol. 1999;277:E390–E394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- 32.Nishida K, Harrison DG, Navas JP, et al. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin.Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uematsu M, Ohara Y, Navas JP, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–C1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 34.Gokce N, Vita JA, Bader DS, et al. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002;90:124–127. doi: 10.1016/s0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- 35.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll.Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]