Abstract
Increasing evidence suggests that deep brain stimulation (DBS), which is currently being used as a therapy for neurological diseases, may be effective in the treatment of psychiatric disorders as well. Here, we examined the influence of DBS of the nucleus accumbens shell on cocaine priming-induced reinstatement of drug seeking, an animal model of relapse. Rats were allowed to self-administer cocaine (0.25 mg, i.v.) 2 h daily for 21 d and then cocaine-seeking behavior was extinguished by replacing cocaine with saline. During the reinstatement phase, DBS was administered bilaterally to the nucleus accumbens shell through bipolar stainless steel electrodes. Biphasic symmetrical pulses were delivered at a frequency of 160 Hz and a current intensity of 150 μA. DBS began immediately after a priming injection of cocaine (0, 5, 10, or 20 mg/kg, i.p.) and continued throughout each 2 h reinstatement session. Results indicated that only the higher doses of cocaine (10 and 20 mg/kg) produced robust and reliable reinstatement of cocaine seeking. DBS of the nucleus accumbens shell significantly attenuated the reinstatement of drug seeking precipitated by these higher cocaine doses. Additional experiments indicated that this DBS effect was both anatomically and reinforcer specific. Thus, DBS of the dorsal striatum had no influence on cocaine reinstatement and DBS of the accumbens shell did not affect the reinstatement of food seeking. Together, these results suggest that DBS of the nucleus accumbens shell may be a potential therapeutic option in the treatment of severe cocaine addiction.
Keywords: deep brain stimulation, relapse, addiction, psychostimulant, striatum, accumbens shell
Introduction
After detoxification, the relapse rate among human cocaine addicts is discouragingly high (Carroll et al., 1994). Craving-induced relapse of drug-taking behavior is precipitated by three major factors: a stressful life event, an environmental stimulus previously associated with drug taking, or reexposure to the drug itself (Spealman et al., 1999; Goeders, 2002; Schmidt et al., 2005; See, 2005; Epstein et al., 2006). For example, laboratory studies indicate that cocaine administration precipitates craving (i.e., the urge to use drugs) among experienced human cocaine users (Jaffe et al., 1989). Cocaine craving-induced relapse of drug taking among human addicts is modeled in rodents and monkeys by administering a noncontingent priming drug injection to animals in which cocaine self-administration behavior has been extinguished. Using this paradigm, systemic or intravenous injections of relatively low doses of cocaine result in robust reinstatement of cocaine-seeking behavior (Spealman et al., 1999; Shalev et al., 2002; Schmidt et al., 2005). Although considerable progress has been made in identifying the neuronal circuitry and neurochemical mechanisms underlying cocaine craving, an effective therapeutic for cocaine addiction remains elusive. Evidence obtained using the reinstatement paradigm indicates that the nucleus accumbens shell is an important locus for cocaine priming-induced reinstatement of drug-seeking behavior (Anderson et al., 2003, 2006; Bachtell et al., 2005; Schmidt et al., 2006; Schmidt and Pierce, 2006). Therefore, disruption of neuronal activity within the nucleus accumbens shell would be expected to attenuate the reinstatement of cocaine-seeking behavior precipitated by a systemic-priming injection of cocaine.
Deep brain stimulation (DBS) of thalamic, subthalamic, or pedunculopontine nuclei is effective in the treatment of Parkinson's disease (Perlmutter and Mink, 2006). Despite the risks associated with major surgery, DBS also is being tested as a therapy for psychiatric disorders and a growing literature indicates promise for this therapeutic modality (Mayberg et al., 2005). Specifically, DBS of the nucleus accumbens, including the shell subregion, has been shown to be efficacious in the treatment of refractory major depression and Tourette's syndrome as well as obsessive-compulsive and anxiety disorders (Sturm et al., 2003; Kuhn et al., 2007b; Schlaepfer et al., 2008). In addition, a case study indicated that DBS of the nucleus accumbens substantially reduced alcohol consumption in an alcohol-dependent patient (Kuhn et al., 2007a). It seems reasonable, therefore, to propose DBS as a potential treatment in cases of extreme (i.e., life threatening) cocaine addiction. The preclinical experiments outlined here examined the influence of DBS of the nucleus accumbens shell on cocaine priming-induced reinstatement of drug seeking.
Materials and Methods
Animals and housing.
Male Sprague Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Farms. Animals were individually housed with food and water available ad libitum. A 12 h light/dark cycle was used with the lights on at 7:00 A.M. All experimental procedures were performed during the light cycle.
Materials.
All experiments used Med Associates instrumentation enclosed within ventilated, sound-attenuating chambers. The apparatus was equipped with response levers, stimulus lights, food pellet dispensers, and injection pumps for injecting drugs intravenously.
Surgery.
Before surgery, the rats were anesthetized by intraperitoneal injections of 80 mg/kg ketamine and 12 mg/kg xylazine. An indwelling SILASTIC catheter was placed into the right jugular vein (side opposite the heart) and sutured in place. The catheter was then threaded subcutaneously over the shoulder blade and was routed to a mesh backmount platform (CamCaths) that was sutured below the skin between the shoulder blades. Catheters were flushed daily with 0.3 ml of an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline. The catheters were sealed with plastic obturators when not in use.
After catheter implantation, the rats were mounted in a stereotaxic apparatus (Kopf Instruments), and bipolar stainless steel electrodes (Plastics One) were implanted into to the nucleus accumbens shell or dorsal striatum according to the following coordinates, relative to bregma (Paxinos and Watson, 1997): nucleus accumbens shell: +1.0 mm anteroposterior (A/P), ±3.0 mm mediolateral (M/L), −7.3 mm dorsoventral (D/V); dorsal striatum: +1.0 mm A/P, ±4.0 mm M/L, −5.0 mm D/V. The stereotaxic arms were set at 17° angles for both brain regions. Electrodes were cemented in place by affixing dental acrylic to three stainless steel screws fastened to the skull.
Cocaine self-administration, extinction, and reinstatement.
After a 7 d recovery period from surgery, the rats were placed in operant chambers and allowed to lever press for intravenous cocaine infusions (0.25 mg cocaine per 56 μl saline per infusion over 5 s). Rats initially were trained using a fixed ratio 1 (FR1) schedule of reinforcement. When the animals achieved stable responding with the FR1 schedule (i.e., <15% variation in response rates over 3 consecutive days), they were switched to an FR5 schedule. A 20 s timeout period during which responses had no scheduled consequences followed each cocaine infusion. The rats were limited to a maximum of 30 cocaine infusions per daily 2 h self-administration session.
After 21 d of cocaine self-administration total, the animals underwent an extinction phase during which cocaine was replaced with saline. Daily 2 h extinction sessions were conducted until responding was <15% of the response rate maintained by cocaine self-administration. After the extinction phase, priming-induced reinstatement of drug-seeking behavior was assessed via the intraperitoneal administration of 0 (0.9% saline), 5, 10, or 20 mg/kg cocaine. For the reinstatement sessions the FR5 schedule was used, but satisfaction of the response requirements for each component resulted in a saline rather than a cocaine infusion. Each reinstatement session was followed by extinction sessions until responding was <15% of the response rate maintained by cocaine self-administration. There were typically 1 to 2 extinction days between each reinstatement session.
Using this experimental design, the rats underwent a series of extinction and reinstatement sessions that lasted ∼16 d. During this period, extinction of the ability of cocaine to induce reinstatement is a concern. However, we have previously shown that reinstatement of cocaine seeking persists for at least 20 d after the initial extinction of cocaine self-administration (Park et al., 2002; Anderson et al., 2003). Moreover, because the drug treatments were counterbalanced across reinstatement days, in the current experiments we were able to assess the magnitude of reinstatement across sessions. Thus, reinstatement of cocaine seeking was assessed at the beginning, middle, and end of the reinstatement phase. All subjects demonstrated stable drug seeking throughout the reinstatement phase of these experiments.
Food self-administration, extinction, and reinstatement.
Rats were trained to self-administer 50 mg Noyes sucrose pellets (Research Diets) using the same procedures described above. After 2 weeks of daily 1 h food-reinforced operant sessions, rats underwent an extinction phase where responding no longer resulted in food delivery. After lever pressing decreased to 15% or less of the responding maintained by contingent food reinforcement, animals began reinstatement testing. Reinstatement of food seeking was promoted by the noncontingent administration (remotely by the experimenter) of one sucrose pellet every 2 min during the first 10 min of the reinstatement session. Each 1 h reinstatement session was followed by extinction sessions (typically only one or two) until responding was again <15% of the response rate maintained by food.
Deep brain stimulation.
In most DBS experiments (both clinical and preclinical), many parameters are fixed and uniform across studies. We used continuous alternating current with biphasic symmetrical square pulses (60 μs pulse width, 160 Hz frequency), parameters that are consistent with previous work in this field (Chang et al., 2003; Mayberg et al., 2005). Stimulation intensities, in contrast, are often varied within and between studies, usually in the range of 50–200 μA (Benazzouz and Hallett, 2000; Chang et al., 2003; Mayberg et al., 2005). Immediately before the start of a reinstatement session, 70–150 μA of current was delivered to the bipolar electrodes. The stimulation continued until the end of the reinstatement session.
Verification of electrode placements.
After the completion of all experiments, the animals were given an overdose of pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline followed by 10% formalin. The brain was removed and coronal sections (100 μm) were taken at the level of the nucleus accumbens with a Vibratome (Technical Products International). An investigator unaware of the animals' behavioral responses determined electrode placements as well as potential electrode-induced neuronal damage. Animals with electrode placements outside of the areas of interest, or with excessive mechanical damage, were excluded from subsequent data analysis.
Results
DBS of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking
In initial experiments, animals were administered 0, 70, 100, or 150 μA DBS bilaterally in the nucleus accumbens shell immediately after a priming injection of cocaine (0 or 10 mg/kg, i.p.) during the reinstatement phase. Results indicated that only the 150 μA current intensity significantly attenuated the reinstatement of cocaine seeking (data not shown). Therefore, this current intensity was used for all subsequent experiments. Notably, the behavioral hyperactivity produced by cocaine did not increase or decrease during DBS. No other abnormal behaviors of any kind were noted with cocaine plus DBS or saline pretreatment before DBS.
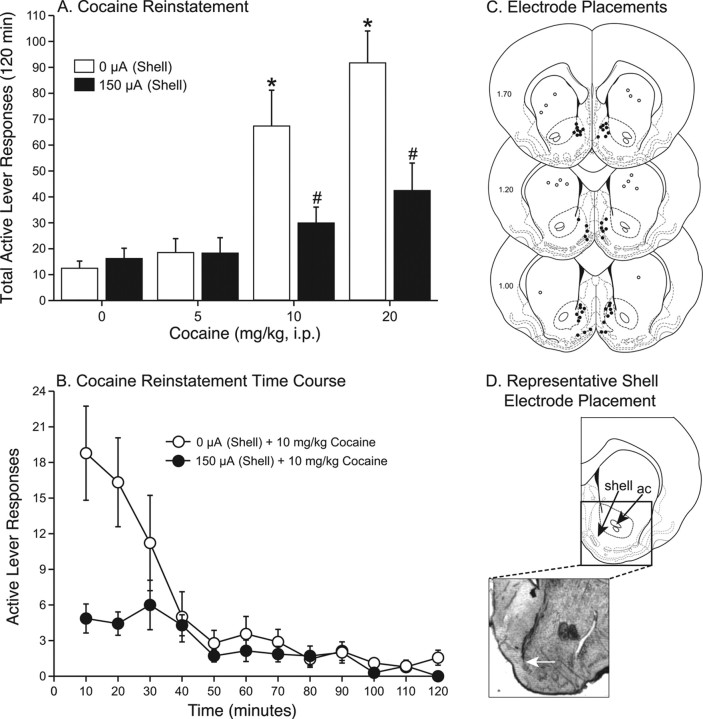
For the cocaine dose-response experiment summarized in Figure 1, A and B, the number of active lever presses on the last day of cocaine self-administration was 131.46 ± 6.35 (mean ± SEM). The extinction criterion was reached in 4.92 ± 0.49 (mean ± SEM) d. Active lever responding on the last day of extinction was 14.46 ± 1.03 (mean ± SEM).
Figure 1.
Deep-brain stimulation of the nucleus accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking in rats. A, DBS (0 or 150 μA) began immediately after intraperitoneal administration of 0, 5, 10, or 20 mg/kg cocaine and continued throughout the 2 h reinstatement session (n = 7–12). *Significant differences from the 0 μA/saline treatment. #Significant differences from 0 μA at that cocaine dose. B, The time courses of the effect of 0 (n = 9) or 150 (n = 7) μA DBS on the reinstatement of drug seeking precipitated by 10 mg/kg cocaine from A. C, Electrode placements in the nucleus accumbens shell (filled circles) and dorsal striatum (open circles) for all subjects. D, Representative electrode placement in the shell. The white arrow indicates the end of the electrode tract. ac, Anterior commissure.
The total active lever presses during the reinstatement phase were analyzed with a two-way ANOVA, which revealed significant main effects of cocaine dose (F (3,77) = 18.449; p <0.0001) and current intensity (F (1,77) = 11.322; p <0.0012) as well as a significant interaction between these factors (F (3,77) = 4.996; p <0.0032). Subsequent pairwise analyses (Fisher's LSD, p <0.05) showed that the total active responses after 10 and 20 mg/kg cocaine with no stimulation were significantly different from saline. In addition, DBS significantly attenuated the reinstatement of drug seeking precipitated by both of these higher doses of cocaine. There were 7–12 subjects per treatment. These data indicate that DBS of the nucleus accumbens shell produced a rightward (or downward) shift in the cocaine reinstatement dose-response curve (Fig. 1 A). Analysis of the total inactive lever presses during the reinstatement phase (data not shown) indicated no significant main effects of cocaine dose or current intensity and no significant interaction between these factors, which suggests that neither cocaine nor DBS produced nonspecific changes in lever pressing. Figure 1 B depicts the time courses of the active lever responses in the 0 and 150 μA plus 10 mg/kg cocaine treatments from Figure 1 A.
DBS of the dorsal striatum has no influence on the reinstatement of cocaine seeking
In order to assess the anatomical specificity of the accumbens shell DBS effect, DBS (0 or 150 μA) was administered in the dorsal striatum during the reinstatement of drug seeking induced by an intraperitoneal-priming injection of 10 mg/kg cocaine. The data were as follows (mean active lever responses ±SEM): 0 μA, 59.14 ± 10.26; 150 μA, 49.25 ± 11.17. These data were analyzed with a t test, which revealed no significant difference between treatments (t (14) = 0.130; p <0.8985). There were eight subjects per treatment.
The effect of DBS of the dorsal striatum during a reinstatement session in which a saline-priming injection was administered also was assessed. The data were as follows (mean active lever responses ±SEM): 0 μA, 14.63 ± 3.35; 150 μA, 12.75 ± 3.32. These data were analyzed with a paired t test, which revealed no significant effect (t (14) = 0.398; p <0.6969). There were eight subjects in this experiment.
DBS of the nucleus accumbens shell does not affect the reinstatement of food seeking
Several measures were used to evaluate potential nonspecific rate suppressing effects of DBS. The modular testing chambers were equipped with an inactive lever, responses on which often are used as a measure of nonspecific alterations in lever pressing during the reinstatement phase. Although DBS did not have a significant influence on responding on the inactive lever, the low number of inactive lever presses limits the utility of this measure to meaningfully assess potential rate suppressant effects. Therefore, we also assessed the effect of nucleus accumbens shell DBS (0 or 150 μA) on the reinstatement of food seeking. For this experiment, the number of active lever presses on the last day of food self-administration was 150 ± 0 (mean ± SEM), the maximal number of reinforcers per session per subject. The extinction criterion was reached in 5.3 ± 0.39 (mean ± SEM) d. The data were as follows (mean active lever responses ±SEM): 0 μA, 48.67 ± 10.57; 150 μA, 53 ± 9.24. These data were analyzed with a paired t test, which showed no significant treatment effect (t (15) = 0.305; p <0.7618). Four of the food reinstatement subjects had previous experience with cocaine self-administration, but this factor had no influence on food self-administration or the reinstatement of food seeking. There were eight or nine animals per treatment group.
The electrode placements in the nucleus accumbens shell and dorsal striatum for all experiments are shown in Figure 1 C. A photomicrograph of a representative electrode placement in the nucleus accumbens shell is shown in Figure 1 D. There were no instances of excessive mechanical damage induced by DBS.
Discussion
The current results indicate that (1) DBS of the nucleus accumbens shell attenuated the reinstatement of cocaine seeking; (2) DBS of the dorsal striatum had no influence on cocaine reinstatement; (3) DBS of the nucleus accumbens shell did not affect the reinstatement of food seeking; and (4) DBS of the accumbens shell in the absence of a cocaine-priming injection did not promote the reinstatement of cocaine seeking. Collectively, these results demonstrate that DBS of the nucleus accumbens shell attenuates the reinstatement of cocaine seeking in an anatomically and reinforcer-specific manner.
In studies of parkinsonism, depression, and anxiety disorders, clinical measurements are made during DBS and compared with measurements when the stimulation is off. The stimulation is constant and the effects are completely reversed when the stimulation is turned off. For example, the antidepressant effect of continuous DBS was immediate, bidirectional, and reversible (Schlaepfer et al., 2008). That is, when the stimulator was turned on, depression ratings immediately improved, and when it was turned off, depression ratings worsened (Schlaepfer et al., 2008). Similar to clinical studies, DBS was administered continuously during the reinstatement session in the current experiments.
The clinical effect of DBS at high frequencies is due at least in part to the suppression of neuronal activity via the activation of inhibitory interneurons and/or depolarization inactivation (Benabid et al., 1991; Blond et al., 1992). Consistent with the view that DBS produces its effects by reversible neuronal inactivation, intra-accumbal administration of an AMPA receptor antagonist, which blocks excitatory neurotransmission, attenuates cocaine priming-induced reinstatement of cocaine seeking (Cornish et al., 1999; Cornish and Kalivas, 2000; Park et al., 2002). In contrast, activation of the nucleus accumbens with an AMPA microinjection promotes the reinstatement of cocaine seeking (Cornish et al., 1999; Cornish and Kalivas, 2000). However, there also is evidence that DBS inactivates cell bodies but activates axons in the stimulated area (Vitek, 2002; McIntyre et al., 2004). Consistent with this notion, DBS of the nucleus accumbens core suppressed neuronal activity in the orbitofrontal cortex apparently via antidromic stimulation of cortico-accumbal afferents and the subsequent activation of inhibitory cortical interneurons (McCracken and Grace, 2007). Potential DBS-induced inactivation of accumbal afferents is particularly significant given mounting evidence that activation of excitatory medial prefrontal cortical and orbitofrontal cortical afferents to the nucleus accumbens promotes the reinstatement of cocaine seeking (Park et al., 2002; McFarland et al., 2003; Fuchs et al., 2004). Together, these results suggest that DBS of the nucleus accumbens shell attenuates cocaine seeking by inhibiting neuronal activity in accumbal output neurons and/or inactivation of accumbal afferents.
It is well known that animals will self-stimulate various brain areas, including the nucleus accumbens (Mogenson et al., 1979; West and Wise, 1988). The electrical impulses that rats learn to self stimulate are brief (usually lasting less than 1 s) and stimulate neuronal activity (Wise et al., 1992). Previous evidence indicates that only the initial onset of brain stimulation is reinforcing (Pollock and Kornetsky, 1990). In contrast, in the present experiments, high frequency DBS was administered continuously throughout the reinstatement session to suppress neuronal activity. It has been shown that noncontingent stimulation can be aversive, even when the stimulation is identical to the self-administered pattern (Steiner et al., 1969). Importantly, clinical studies report that prolonged and continuous DBS of the nucleus accumbens is neither reinforcing nor aversive (Sturm et al., 2003; Kuhn et al., 2007a; Okun et al., 2007; Schlaepfer et al., 2008). The present results support this clinical observation. DBS of the nucleus accumbens shell in the absence of a cocaine-priming injection failed to promote drug seeking, which suggests that DBS is unlikely to promote drug craving. It is also notable that DBS of the nucleus accumbens shell did not attenuate food-seeking behavior in rats, which indicates that DBS does not produce a generalized disruption of normal behavior. Although it is unclear exactly why DBS of the nucleus accumbens shell produced reinforcer specific effects in the current study, it is likely because the neuronal circuits subserving cocaine and food-seeking behaviors are at least partially segregated (Carelli et al., 2000; Horvath and Diano, 2004).
In conclusion, the present results indicated that DBS of the nucleus accumbens shell blunts the reinstatement of drug seeking produced by reexposure to cocaine. This effect was anatomically specific in that DBS of the dorsal striatum did not influence cocaine reinstatement. In addition, DBS of the accumbens shell failed to influence the reinstatement of food seeking, which indicates that the DBS effect was selective to cocaine and not attributable to a generalized disruption in motivated behavior. Together, these results indicate that DBS of the nucleus accumbens shell may represent a novel therapeutic modality for the treatment of severe cocaine addiction.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01 DA15214 and K02 DA18678 (R.C.P.) as well as The Gennaro Acampora Charity Trust (D.A.C.). K.R.F. was partially supported by a National Research Service Award (NRSA) from the NIH (F30 DA19304) as well as NIH Training Grant T32 GM008541-7. H.D.S. was also partially supported by an NRSA from the NIH (F31 DA16824). C.K. was partially supported by K05 DA00099 from the NIH. We thank Audrey Pierce-Bancroft for administrative assistance as well as Lisa Tozier and Steven Crosby for technical support.
References
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55:S13–S16. [PubMed] [Google Scholar]
- Blond S, Caparros-Lefebvre D, Parker F, Assaker R, Petit H, Guieu JD, Christiaens JL. Control of tremor and involuntary movement disorders by chronic stereotactic stimulation of the ventral intermediate thalamic nucleus. J Neurosurg. 1992;77:62–68. doi: 10.3171/jns.1992.77.1.0062. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 2003;983:174–184. doi: 10.1016/s0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S. The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci. 2004;5:662–667. doi: 10.1038/nrn1479. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, Sturm V. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007a;78:1152–1153. doi: 10.1136/jnnp.2006.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Mai JK, Huff W, Lee SH, Koulousakis A, Klosterkoetter J, Sturm V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007b;254:963–965. doi: 10.1007/s00415-006-0404-8. [DOI] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo . J Neurosci. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Takigawa M, Robertson A, Wu M. Self-stimulation of the nucleus accumbens and ventral tegmental area of Tsai attenuated by microinjections of spiroperidol into the nucleus accumbens. Brain Res. 1979;171:247–259. doi: 10.1016/0006-8993(79)90331-7. [DOI] [PubMed] [Google Scholar]
- Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, Knight W, Martin P, Goodman WK. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78:310–314. doi: 10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J, Kornetsky C. Pharmacologic evidence for nociception resulting from noncontingent “rewarding” brain stimulation. Physiol Behav. 1990;47:761–765. doi: 10.1016/0031-9384(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Systemic administration of a dopamine, but not a serotonin or norepinephrine, transporter inhibitor reinstates cocaine seeking in the rat. Behav Brain Res. 2006;175:189–194. doi: 10.1016/j.bbr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behavior in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Steiner SS, Beer B, Shaffer MM. Escape from self-produced rates of brain stimulation. Science. 1969;163:90–91. doi: 10.1126/science.163.3862.90. [DOI] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- West TE, Wise RA. Effects of naltrexone on nucleus accumbens, lateral hypothalamic and ventral tegmental self-stimulation rate-frequency functions. Brain Res. 1988;462:126–133. doi: 10.1016/0006-8993(88)90594-x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]