Abstract
Human CD133 (human prominin-1), a five transmembrane domain glycoprotein, was originally identified as a cell surface antigen present on CD34+ hematopoietic stem cells. Although the biological function of CD133 is not well understood, antibodies to CD133 epitopes have been widely used to purify hematopoietic stem and progenitor cells. The cancer stem cell (CSC) hypothesis postulates that a rare population of tumor cells possessing increased capacities for self-renewal and tumor initiation is responsible for maintaining the growth of neoplastic tissue. The expression of the CD133 epitopes, AC133 and AC141, has been shown to define a subpopulation of brain tumor cells with significantly increased capacity for tumor initiation in xenograft models. Following the discovery of the AC133/AC141+ population of brain tumor stem cells, the AC133 and AC141 epitopes have been extensively used as markers for purifying CSCs in other solid tumors. There are, however, several issues associated with the use of the AC133 and AC141 CD133 epitopes as markers for CSCs. The antibodies routinely used for purification of AC133 and AC141-positive cells target poorly characterized glycosylated epitopes of uncertain specificity. Discordant expression of the AC133 and AC141 epitopes has been observed, and the epitopes can be absent despite the presence of CD133 protein. In addition, CD133 expression has recently been shown to be modulated by oxygen levels. These factors, in combination with the uncertain biological role of CD133, suggest that the use of CD133 expression as a marker for CSCs should be critically evaluated in each new experimental system and highlight the need for additional CSC surface markers that are directly involved in maintaining CSC properties.
Keywords: Keywords CD133, Cancer stem cells, Monoclonal antibodies targeting AC133 and AC141 epitopes
Discovery of CD133 surface antigen: monoclonal antibodies AC133 and AC141
The CD133 surface antigen was originally discovered as the target of a monoclonal antibody, AC133, that was generated to bind the CD34+ population of hematopoietic stem cells [1]. To generate the AC133 antibody, mice were inoculated with CD34+ cells enriched from fetal liver, fetal and adult bone marrow, cord blood, peripheral blood, or mobilized leukapheresis, and one hybridoma that secreted an immunoglobulin G1 antibody (AC133) that specifically stained the CD34bright subset of a fetal liver preparation was isolated [1]. Subsequent fluorescence-activated cell sorting (FACS) analysis revealed that the human retinoblastoma cell line, WERI-Rb-1, expressed high levels of the AC133 antigen, and this cell line was used to immunize mice and generate additional AC133-like antibodies. The AC141 antibody was generated by this second immunization. The AC133 antigen was determined to be a glycosylated protein with an apparent molecular weight of 120 kDa [1]. FACS competition experiments indicated that AC133 and AC141 recognize spatially distinct epitopes [1] and immunoprecipitation experiments with tunicamycin-treated cells indicate that both AC133 [2] and AC141 (D.W. Buck, unpublished data) recognize glycosylated structures. To determine the target of the AC133 antibody, immunoaffinity-chromatography-purified AC133 antigen was subjected to digestion and sequencing. Because searches of protein and nucleotide databases at the time did not reveal any sequence similarity to known molecules, degenerate primers were designed based on the recovered protein sequence and used in low-stringency polymerase chain reaction to clone a 1.7-kb fragment containing a continuous open reading frame from a cDNA library [2]. The 5′ and 3′ ends of the putative AC133 cDNA were subsequently isolated, and primers that flanked the start and stop codons were designed and used to clone a 2.7-kb cDNA fragment. COS-7 cells transfected with the cDNA clone produced a product that was detected with the AC133 antibody by FACS and immunoprecipitation analysis. The AC141 antibody binds to a glycosylated CD133 epitope that is spatially distinct from the AC133 epitope [2].
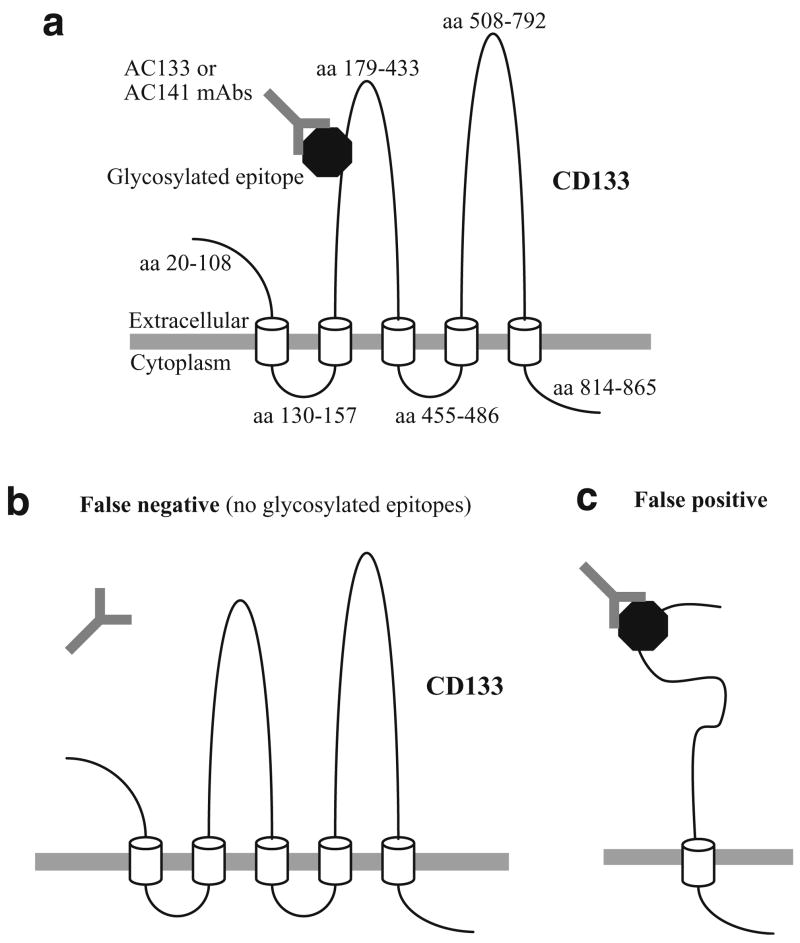
The cDNA of the AC133 antigen encodes a polypeptide of 865 amino acids (aa) with a predicted size of 97 kDa, which is now called CD133 [2]. The predicted structure consists of an 85 aa N-terminal extracellular domain, five transmembrane domains with two large extracellular loops containing eight potential N-linked glycosylation sites, and a 50 aa cytoplasmic tail (Fig. 1a). The CD133 protein shares approximately 60% homology with mouse prominin-1, a cell surface protein that localizes to microvilli on the apical surface of neuroepithelial stem cells [3].
Fig. 1.
Diagram of predicted topology of CD133 and issues concerning the use of AC133 and AC141 mAbs to monitor CD133 expression. a Predicted topology of the mature CD133 polypeptide is shown as well as binding of AC133 and AC141 mAbs to glycosylated epitopes on the extracellular region of CD133. The N-terminal signal peptide (aa 1–19) is not drawn. The epitope location shown in the diagram is hypothetical, as the exact location of the epitopes bound by AC133 and AC141 has not been described. However, the AC133 and AC141 mAbs are known to bind spatially distinct epitopes. Only one of the epitopes is shown. Cylinders in the diagram indicate transmembrane regions. b Potential for false negative data with AC133 and AC141 mAbs. If nonglycosylated CD133 is present, it will not be detected by the AC133 and AC141 mAbs. c Potential for false positive data with AC133 and AC141 mAbs. Given that the nature of the glycosylated AC133 and AC141 epitopes are poorly defined, it is formally possible that glycosylated epitopes on extracellular molecules other than CD133 could cross-react with the AC133 or AC141 mAbs
Although they are known to be glycosylated structures, the locations of the CD133 epitopes bound by the AC133 and AC141 monoclonal antibodies (mAbs; commercially available as CD133/1 and CD133/2 mAbs, respectively) have not been determined. Henceforth, we will refer to the epitopes bound by the AC133 and AC141 mAbs as the AC133 and AC141 epitopes, respectively. A summary of CD133-related nomenclature used in this review can be found in Table 1.
Table 1.
Explanation of CD133-related nomenclature used in this review
| Protein–epitope | mAbs | |
|---|---|---|
| CD133 | Human prominin-1, AC133 antigen | |
| AC133 | Epitope bound by the AC133 mAb | mAb that binds to the AC133 epitope |
| AC141 | Epitope bound by the AC141 mAb | mAb that binds to the AC141 epitope |
| CD133/1 | a.k.a. AC133 mAb | |
| CD133/2 | a.k.a. AC141 mAb |
CD133 epitopes as markers for stem and progenitor cell populations
Stem cells are defined by their ability to self-renew and undergo multilineage differentiation. In the original study that identified the CD133 surface antigen, AC133-epitope-expressing cells selected from bone marrow mononuclear cells were shown to engraft secondary recipients in a fetal sheep transplantation model, suggesting that this population has long-term self-renewal capabilities [1]. Subsequently, the AC133 and AC141 mAbs have been shown to be useful reagents for identifying and purifying various stem and progenitor cell populations. For example, vascular endothelial growth factor receptor-2-positive endothelial progenitors are AC133 epitope-positive and AC133 epitope expression is rapidly downregulated during maturation [4]. Downregulation of AC133 and AC141 epitope expression was also observed upon differentiation of the colon carcinoma cell line Caco-2 [5]. AC133+ AC141+ CD34− CD45− cells isolated by FACS from fresh human fetal brain tissue initiated neurosphere cultures, and the progeny of clonogenic cells could differentiate into both neurons and glial cells [6]. Upon transplantation into brains of immunodeficient neonatal mice, the sorted cells showed potent engraftment, proliferation, migration, and neural differentiation [6]. Additionally, AC133+ CD34+ CD3− human fetal liver cells [7] and skin-derived AC133+ cells [8] were shown to be capable of differentiating into astrocytes and neural cells. Finally, a small population of AC133 epitope-positive human prostate basal cells possess high in vitro proliferative potential and can reconstitute a fully differentiated prostatic epithelium in immunocompromised male nude mice [9]. Thus, expression of the AC133 and AC141 CD133 epitopes has proven to be a useful marker for defining and isolating various stem cell populations. The use of CD133 and the AC133 and AC141 epitopes as markers for adult stem cell populations has been reviewed in more detail elsewhere [10].
CD133 epitope expression defines brain tumor stem cells
It has long been known that not every tumor cell is a tumor-initiating cell. Millions of tumor cells are often required to transplant a new tumor from an existing one [11–13]. According to the cancer stem cell (CSC) hypothesis, tumors are formed and maintained by a rare population of undifferentiated cells that are characterized by their ability to self-renew and induce tumorigenesis [14–16]. By identifying, characterizing, and ultimately therapeutically targeting these CSC populations, it is hoped that more effective cancer treatments can be developed. CSCs were initially identified in several hematologic malignancies, e.g., acute myeloid leukemia [17]. The first example of cells with CSC-like properties within solid tumors came from experiments done with human breast cancer cells in which a subpopulation of cells that displayed increased tumorigenicity in a mouse model were isolated using cell surface markers [18]. Soon after this discovery, an AC133 epitope-positive subpopulation of cells with in vitro CSC-like properties (enhanced capacity for proliferation, self-renewal, and differentiation) was isolated from human brain tumors [19]. Later follow-up studies showed that the AC133 epitope-expressing subpopulation from human brain tumors has a dramatically enhanced capacity for initiating tumors in nonobese diabetic–severe combined immunodeficient mouse brains [20]. These AC133 epitope-positive brain tumor stem cells are resistant to chemotherapeutic agents [21] and radiation [22] and express higher levels of mRNA for the ABC transporter, BCRP1, the O6-methylguanine–DNA methyl-transferase, markers associated with neural precursors, and negative regulators of apoptosis [21].
The origin of AC133 and AC141 epitope-positive brain tumor stem cells is currently unknown [15]. Because the AC133 and AC141 epitopes are also expressed by normal neural stem cells, it is possible that they are the source of brain tumor stem cells. The AC141 epitope was shown to be present on ependymal cells in the adult human brain, although these cells are thought to be postmitotic [23]. Alternatively, it has been suggested that brain tumor stem cells could derive from AC133/AC141 epitope-positive cells not normally present in adult brains, or from AC133/AC141 epitope-negative neurogenic astrocytes which become AC133/AC141 epitope-positive during tumor development [23].
CD133 epitopes as CSC markers in other tumor types
Following the initial discovery of a subpopulation of AC133/AC141 epitope-positive glioblastoma cells with CSC properties [19, 20], the use of CD133 epitopes as markers for CSCs in other tumor types has been actively investigated. In vitro proliferation assays and in vivo tumor initiation experiments carried out with AC133/AC141 epitope-positive cells isolated from both primary tumors and cancer cell lines have provided evidence for the existence of CD133 epitope-expressing CSC populations in ependymoma [24], prostate cancer [25], colon cancer [26–28], lung cancer [29], hepatocellular carcinoma [30–33], laryngeal carcinoma [34], melanoma [35], ovarian cancer [36], and pancreatic cancer [37, 38]. A summary of investigations that have used CD133 epitope expression to isolate cells with CSC-like properties is shown in Table 2. Given the activity and rapid progress in this field, it is likely that more examples of CD133 epitope-expressing CSC populations will be reported.
Table 2.
Studies that have used CD133/1 and/or CD133/2 mAbs to isolate populations with CSC-like properties from solid tumors
| Tumor | Cell source | Antibody | Characterization | Reference |
|---|---|---|---|---|
| Brain tumor | Primary tumors | CD133/1 | In vitro | [19–21] |
| Cell lines | CD133/1 | In vitro | [22] | |
| Primary tumors, glioma xenografts | CD133/1 | In vitro and in vivo | [39] | |
| Primary tumors | CD133/2 | In vitro and in vivo | [24] | |
| Primary tumors | CD133/1 | In vitro and in vivo | [48, 49] | |
| CD133/2 | ||||
| Prostate cancer | Primary tumors | CD133/1 | In vitro | [25] |
| Colon cancer | Primary tumors | CD133/1 | In vitro and in vivo | [26] |
| Primary tumors | CD133/2 | In vivo | [27] | |
| CD133/1 | ||||
| Cell lines | CD133/1 | In vitro and in vivo | [28] | |
| Lung cancer | Primary tumors | CD133/1 | In vitro and in vivo | [29] |
| CD133/2 | ||||
| Hepatocellular carcinoma | Cell lines | CD133/2 | In vitro and in vivo | [30] |
| Cell lines | CD133/1 | In vitro and in vivo | [32] | |
| Cell lines | CD133/1 | In vitro | [31] | |
| Cell line | CD133/1 | In vitro and in vivo | [33] | |
| Laryngeal carcinoma | Cell line | CD133/1 | In vitro | [34] |
| Melanoma | Primary tumors, cell line | CD133/1 | In vitro and in vivo | [35] |
| Ovarian cancer | Primary tumors | CD133/1 | In vitro | [36] |
| CD133/2 | ||||
| Pancreatic cancer | Primary tumors | CD133/1 | In vitro and in vivo | [37] |
| CD133/2 | ||||
| Cell lines | CD133/1 | In vitro | [38] |
Signaling pathways involved in the CD133-epitope-positive CSC phenotype
Several signaling pathways have been shown to regulate the CD133 epitope-positive cancer stem cell phenotype. Upon irradiation, the DNA damage checkpoint is preferentially activated in AC133/AC141 epitope-positive glioma cells and this activation is dependent on the Chk1 and Chk2 checkpoint kinases [39]. Chemoresistance of AC133 epitope-positive hepatocellular carcinoma cells is dependent on increased expression of proteins involved in the Akt/PKB and Bcl-2 cell survival response [31]. In vitro treatment of glioblastoma cells with BMP4, a transforming growth factor-β family ligand that signals through Smad pathways, reduces the size of the AC133 epitope-positive population and prevents tumor initiation following in vivo transplantation [40]. Finally, the HEDGEHOG/GLI pathway was shown to regulate the proliferation, survival, self-renewal, and tumor-igenicity of glioma stem cell cultures [41]. It should be noted that although many of these signal transduction experiments were carried out with isolated AC133/AC141 epitope-positive cells, a direct involvement of CD133 in any of these pathways has not been demonstrated.
Issues concerning the use of AC133 and AC141 epitopes as markers for CSCs
Almost all CD133-related experiments carried out to date have made use of two mAbs AC133 and AC141 which were originally generated to aid in the purification and characterization of hematopoietic stem and progenitor cells and which subsequently led to the identification of the CD133 surface protein antigen [1]. Despite increasing reports of the successful use of the AC133 and AC141 mAbs to identify and purify CSC populations, there are several factors that need to be considered when interpreting these results (Fig. 1). First, there is very little information available about the molecular nature of the epitopes bound by the two antibodies. The AC133 and AC141 mAbs are widely reported to bind glycosylated epitopes on CD133, but the evidence supporting these claims is not well documented in the literature, consisting of unshown data [2] in the case of AC133 mAb and a brief reference to unpublished data [5] in the case of AC141 mAb. The exact location of the modified amino acid residues on CD133 targeted by AC133 and AC141 mAbs has not been described. Despite the fact that the AC133 and AC141 mAbs are commonly used interchangeably for analysis and cell purification, discordant expression of the AC133 and AC141 epitopes was observed in marrow precursor and peripheral blood stem cells from patients with myelodys-plastic syndrome and acute myelogenous leukemia [42]. Additionally, several studies have demonstrated that the AC133 and AC141 epitopes can be downregulated independently from the CD133 protein or mRNA [5, 43], and the tissue distribution of CD133 mRNA was found to be much more widespread than expression of the AC133 epitope [2]. An additional complicating factor is the presence of alternatively spliced CD133 isoforms. The human CD133 gene contains at least 37 exons spanning 152 kb on chromosome 4, and several alternatively spliced forms of CD133 have been described in both human [44, 45] and mouse [46]. For example, a CD133 isoform (termed AC133-2) lacking exon 4 results in a deletion of nine amino acids in the N-terminal extracellular domain [45]. Interestingly, AC133-2 was shown to be the predominant CD133 isoform expressed on hematopoietic stem cells and presumably recognized by the AC133 and AC141 mAbs [45]. Although currently unknown, alternatively spliced CD133 isoforms lacking the AC133 or AC141 epitopes could exist. Thus, given these complexities, it may be incorrect to call AC133 or AC141 epitope-negative cells “CD133-negative” without proper verification of CD133 protein or mRNA levels. Indeed, it is possible that the glycosylation status of CD133 may be a more specific marker of the CSC phenotype than CD133 protein levels. Another issue is that the AC141 mAb displays cross-reactivity with cytokeratin 18 [47], which could be a complicating factor with fixed cells or cells with damaged membranes such as those in immunohistochemistry studies. In addition, because the nature of the glycosylated epitopes have not been defined (i.e., sugar structure and peptide context), it is formally possible that the AC133 and/or AC141 mAbs recognize glycosylated epitopes on molecules other than CD133. Despite the uncertainty surrounding the target epitopes and specificity of the AC133 and AC141 mAbs, they have nonetheless been extremely useful reagents for CSC research. However, these factors should be kept in mind, particularly when analyzing the results of experiments carried out with “CD133-negative” cells purified with the AC133 and AC141 mAbs.
Anti-CD133 antibodies other than AC133 and AC141 that target unmodified extracellular CD133 epitopes are commercially available. For example, the rabbit polyclonal Abs H-284, mouse mAb 32AT1672, and rabbit mAb C24B9 were produced by immunizing with peptides corresponding to predicted extracellular regions of CD133 (Table 3). Although the use of these antibodies for isolation of CD133-positive cells has not been reported in the literature, they could be suitable for this application. By comparing the results of CSC isolation experiments done with anti-CD133 antibodies that target unmodified extra-cellular CD133 epitopes to the results obtained with the glycosylation-specific AC133 and AC141 mAbs, it should be possible to determine if there is a correlation between the glycosylation status of CD133 and the CSC phenotype.
Table 3.
Commercially available anti-CD133 antibodies recognizing nonglycosylated extracellular epitopes that could be suitable for CSC isolation
| Antibody | Company | Host | Immunogen | Vendor claimed applications |
|---|---|---|---|---|
| CD133 (H-284) | Santa Cruz Biotechnology | Rabbit polyclonal | aa 508–792 | W, IP, IHC |
| CD133 (32AT1672) | Abcam | Mouse monoclonal | aa 20–36 | W, E, F |
| CD133 (C24B9) | Cell Signaling Technology | Rabbit monoclonal | aa 562 region | W, IP, IHC, F |
W Western, E ELISA, IP immunoprecipitation, IHC immunohistochemistry, F flow cytometry
Oxygen levels modulate CD133 expression
It has been reported recently that CD133 expression is modulated by oxygen levels in vitro in glioma cultures. Lowering the oxygen tension (from 20% to 2–3%) during the culture of medulloblastoma and glioblastoma cells increased the expression of the AC133 and AC141 epitopes as well as CD133 mRNA levels [22, 48]. Because the oxygen tension in the brain is estimated to be 1–5% [49, 50] and is probably lower in most tumor tissues [51], these conditions may be more physiologically relevant than normal tissue culture conditions. Prolonged culture of tumor cell lines in the presence of 20% oxygen may therefore lead to a reduction in CD133 expression and could affect the tumor initiation potential of in vitro cultured cells reintroduced to a low oxygen tension in vivo environment. Thus, further studies will be needed to determine if oxygen tension is an important experimental variable in CSC experiments.
CD133-epitope-negative glioblastoma stem cells
Given the potential complex environmental factors affecting CD133 epitope expression in vitro, it is perhaps not surprising that there are conflicting reports in the literature regarding the existence of CD133 epitope-negative glioblastoma-derived cancer stem cells. In two similar studies, cell lines generated from glioblastomas fell into two distinct categories: neurosphere-like, nonadherent, AC133/AC141 epitope-positive cell lines, and adherent or semiadherent AC133/AC141 epitope-negative cell lines [52, 53]. Beier et al. reported that while tumor cells from the AC133/AC141 epitope-negative glioblastoma-derived cell lines have tumorigenicity similar to AC133/AC141 epitope-positive cell lines, AC133–AC141-epitope-negative cells purified from the AC133/AC141 epitope-positive cell lines were nontumorigenic [52]. The expression of CD133 mRNA was confirmed to be very low in the AC133/AC141 epitope-negative cell lines, indicating that expression of CD133 is not required for the CSC phenotype in these cell lines [52]. In this context, it should be noted that many brain tumor cell lines such as U87MG do not express CD133 epitopes [48] but nonetheless form tumors when grafted into immunodeficient mice [54]. In contrast, Gunther et al. [53] reported that AC133/AC141 epitope-negative glioblastoma-derived cell lines were less tumori-genic than AC133/AC141 epitope-positive cell lines. These discrepancies could be the result of experimental differences or reflect variations between individual glioblastoma-derived cell lines.
Based on these experiments, it can be speculated that there are at least two types of brain tumor CSCs that vary in their expression of CD133 and/or CD133 epitopes. The first type of CSC population is CD133 epitope-positive. In these cells, there may be a tight link between CD133 epitope expression and the CSC-like phenotype. According to this model, loss of CD133 epitope expression after prolonged periods in culture, possibly as a result of increased oxygen concentrations, would be predicted to be accompanied by a reduction in the CSC-like phenotype. Alternatively, CSC populations that are initially CD133 or CD133 epitope-dependent could adapt during prolonged culture and become CD133 or CD133 epitope-independent. Analysis of the tumorigenicity of CD133 epitope-positive glioblastoma-derived primary cell lines after prolonged culture in relation to the expression of CD133 and CD133 epitopes could test these possibilities.
The second type of glioblastoma-derived CSC population is CD133-negative. These cells are able to maintain the CSC phenotype without the function of CD133, as reported by Beier et al. [52]. Thus, markers other than CD133 will be required to define this population. Heterogeneity of CSC markers may be a general phenomenon applicable to glioblastoma as well as other solid tumors.
Future prospects
An obvious question that remains unanswered is whether CD133 or its glycosylation status plays a direct role in regulating the CSC phenotype. Although the AC133 and AC141 CD133 epitopes have been extensively used as markers, very little is known about the biological function of CD133. CD133 (human prominin-1) localizes to plasma membrane protrusions in various cell types where it interacts with membrane cholesterol and acts as a marker for a cholesterol-based lipid microdomain [55]. It has been speculated that these membrane microdomains may be enriched in components involved in maintaining stem cell properties, and their loss, perhaps through asymmetric cell division, might promote cell differentiation [56]. Although targeted knockdown experiments have not been performed, a single nucleotide deletion that results in a truncated form of CD133 causes retinal degeneration [57]. Thus far, however, a direct role for CD133 in maintaining the tumorigenic potential of CSCs remains to be defined. Because the anti-CD133 antibodies typically used (AC133 and AC141 mAbs) recognize undefined glycosylated epitopes, the possibility remains that the glycosylation status of CD133, rather than expression of the CD133 protein itself, can act as an indirect marker of the CSC phenotype. Future experiments designed to investigate the function of the CD133 molecule will be needed to clarify this issue. In particular, analysis of the tumor forming capacity of tumor-derived cell lines with AC133/AC141-positive CSC populations following targeted knockdown of CD133 expression would determine if CD133 expression is an absolute requirement for the tumorigenicity of these cell lines. Regardless, it would be desirable to discover additional CSC markers that function directly in maintaining the CSC phenotype.
Acknowledgments
We thank the National Institute of Health (R01 CA118919 to BL) and the US Army Medical Research and Material Command (W81XWH-05-1-0027 to BL) for financial support.
Abbreviations
- CSC
Cancer stem cell
- mAb
monoclonal antibody
- FACS
fluorescence-activated cell sorting
- aa
amino acids
References
- 1.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 2.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 3.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytypic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 5.Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 6.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao HN, Zhao J, Thomas RL, Parker GC, Lyman WD. Fetal human hematopoietic stem cells can differentiate sequentially into neural stem cells and then astrocytes in vitro. J Hematother Stem Cell Res. 2003;12:23–32. doi: 10.1089/152581603321210109. [DOI] [PubMed] [Google Scholar]
- 8.Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, Scalamogna M, Parati EA, Bresolin N, Torrente Y. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res. 2004;77:475–486. doi: 10.1002/jnr.20151. [DOI] [PubMed] [Google Scholar]
- 9.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 10.Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Lasky JL, 3rd, Wu H. Cancer stem cells. Pediatr Res. 2006;59:59R–64R. doi: 10.1203/01.pdr.0000203592.04530.06. [DOI] [PubMed] [Google Scholar]
- 12.Nakano I, Kornblum HI. Brain tumor stem cells. Pediatr Res. 2006;59:54R–58R. doi: 10.1203/01.pdr.0000203568.63482.f9. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 14.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 15.Dirks PB. Brain tumour stem cells: the undercurrents of human brain cancer and their relationship to neural stem cells. Philos Trans R Soc Lond B Biol Sci. 2008;363:139–152. doi: 10.1098/rstb.2006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dick JE, Lapidot T. Biology of normal and acute myeloid leukemia stem cells. Int J Hematol. 2005;82:389–396. doi: 10.1532/IJH97.05144. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 20.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133-cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Pfenninger CV, Roschupkina T, Hertwig F, Kottwitz D, Englund E, Bengzon J, Jacobsen SE, Nuber UA. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- 24.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 26.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 28.Ieta K, Tanaka F, Haraguchi N, Kita Y, Sakashita H, Mimori K, Matsumoto T, Inoue H, Kuwano H, Mori M. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol. 2008;15:638–648. doi: 10.1245/s10434-007-9605-3. [DOI] [PubMed] [Google Scholar]
- 29.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 30.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 31.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 32.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Wei X, Cheng L, Tian J, Jiang JJ. CD133, one of the markers of cancer stem cells in Hep-2 cell line. Laryngoscope. 2007;117:455–460. doi: 10.1097/01.mlg.0000251586.15299.35. [DOI] [PubMed] [Google Scholar]
- 35.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S, Scambia G. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2007 Sep 14; doi: 10.1111/j.1525–1438.2007.01056.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–97. [PubMed] [Google Scholar]
- 39.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 40.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 41.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green WB, Slovak ML, Chen IM, Pallavicini M, Hecht JL, Willman CL. Lack of IRF-1 expression in acute promyelocytic leukemia and in a subset of acute myeloid leukemias with del(5) (q31) Leukemia. 1999;13:1960–1971. doi: 10.1038/sj.leu.2401596. [DOI] [PubMed] [Google Scholar]
- 43.Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005;319:15–26. doi: 10.1007/s00441-004-1018-z. [DOI] [PubMed] [Google Scholar]
- 44.Shmelkov SV, Jun L, St Clair R, McGarrigle D, Derderian CA, Usenko JK, Costa C, Zhang F, Guo X, Rafii S. Alternative promoters regulate transcription of the gene that encodes stem cell surface protein AC133. Blood. 2004;103:2055–2061. doi: 10.1182/blood-2003-06-1881. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Flint A, Dvorin EL, Bischoff J. AC133-2, a novel isoform of human AC133 stem cell antigen. J Biol Chem. 2002;277:20711–20716. doi: 10.1074/jbc.M202349200. [DOI] [PubMed] [Google Scholar]
- 46.Fargeas CA, Joester A, Missol-Kolka E, Hellwig A, Huttner WB, Corbeil D. Identification of novel prominin-1/CD133 splice variants with alternative C-termini and their expression in epididymis and testis. J Cell Sci. 2004;117:4301–4311. doi: 10.1242/jcs.01315. [DOI] [PubMed] [Google Scholar]
- 47.Potgens AJ, Schmitz U, Kaufmann P, Frank HG. Monoclonal antibody CD133-2 (AC141) against hematopoietic stem cell antigen CD133 shows cross-reactivity with cytokeratin 18. J Histochem Cytochem. 2002;50:1131–1134. doi: 10.1177/002215540205000814. [DOI] [PubMed] [Google Scholar]
- 48.Platet N, Liu SY, Atifi ME, Oliver L, Vallette FM, Berger F, Wion D. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett. 2007;258:286–290. doi: 10.1016/j.canlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu LL, Wu LY, Yew DT, Fan M. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol Neurobiol. 2005;31:231–242. doi: 10.1385/MN:31:1-3:231. [DOI] [PubMed] [Google Scholar]
- 51.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 52.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 53.Gunther HS, Schmidt NO, Phillips HS, Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal M, Lamszus K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2007 Nov 26; doi: 10.1038/sj.onc.1210949. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Johns TG, Perera RM, Vernes SC, Vitali AA, Cao DX, Cavenee WK, Scott AM, Furnari FB. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13:1911–1925. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- 55.Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 56.Bauer N, Fonseca AV, Florek M, Freund D, Jaszai J, Bornhauser M, Fargeas CA, Corbeil D. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133) Cells Tissues Organs. 2007 Dec 21; doi: 10.1159/000112847. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, Kumaramanickavel G, John S, Nancarrow D, Roper K, Weigmann A, Huttner WB, Denton MJ. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9:27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]