Abstract
A popular theory is that the cerebellum functions as a timer for clocking motor events (e.g., initiation, termination). Consistent with this idea, cerebellar patients have been reported to show greater deficits during hand movements that repeatedly start and stop (i.e., discontinuous movements) compared with continuous hand movements. Yet, this finding could potentially be explained by an alternate theory in which the cerebellum acts as an internal model of limb mechanics. We tested whether a timing or internal model hypothesis best explains results from a circle-drawing task, where individuals trace a circle with the hand at a desired tempo. We first attempted to replicate prior results showing greater impairment for discontinuous versus continuous circling movements, and then asked whether we could improve patient performance by reducing demands in each domain. First, we slowed the movement down to reduce the need to predict and compensate for limb dynamics. Second, we supplied external timing information to reduce the need for an internal event timer. Results showed that we did not replicate the previous findings—cerebellar patients were impaired in both discontinuous and continuous movements. Slowing the movement improved cerebellar performance to near control values. The addition of an external visual timing signal paradoxically worsened timing deficits rather than mitigating them. One interpretation of these combined results is that the cerebellum is indeed functioning as an internal model and is needed to make appropriate predictions for movement initiation and termination.
INTRODUCTION
It is appealing to think that the cerebellum performs a single function, given its very regular anatomical structure. One prominent hypothesis is that it acts as a central timing device to clock events, such as movement initiation and termination, on millisecond to second timescales (Gibson et al. 1994; Ivry and Spencer 2004; Welsh et al. 1995). In support of this idea, a recent study showed that cerebellar subjects were impaired at making discontinuous movements, but made continuous movements normally, during a circling movement of the hand and arm (Spencer et al. 2003). The deficit in discontinuous movements (repeated starts and stops) was increased timing variability, which was taken as evidence for an impairment of a cerebellar “explicit timing” function. Continuous movement timing, which was spared, was interpreted as an emergent property independent of cerebellar control (Spencer et al. 2003).
These findings may also be compatible with the hypothesis that the cerebellum functions as an internal model of body mechanics, including kinematics and dynamics. Individuals with cerebellar damage have deficits consistent with this type of function, such as poor prediction and compensation for interaction torques (i.e., mechanical torques at a given joint caused by motion of connected joints) during reaching movements (Bastian et al. 1996, 2000; Topka et al. 1998). The cerebellum could act as two different types of internal models: an “inverse model” used to calculate the motor commands required to achieve a desired kinematic goal or a “forward model” that predicts upcoming body “states” (e.g., position, velocity) using an efferent copy of the motor commands that were issued and multiple sources of sensory information. Although there is no consensus on which type of model it represents, either could be especially important for predicting the correct motor commands for starts and stops during discontinuous movements (Miall et al. 1993; Paulin 1997; Wolpert and Miall 1996). Further, these mechanisms allow the nervous system to operate in a predictive mode for fast and accurate movements, rather than reacting to time-delayed sensory signals.
To explore these hypotheses, we studied a continuous/discontinuous circle-tracing task, previously used to support the timing hypothesis (Spencer et al. 2003). We first attempted to replicate findings from prior work (Spencer et al. 2003). Then, in experiment 1, we examined whether slowing the movement speed would alter patients’ performance. We reasoned that this simplifies the dynamics demands placed on the subject since the movements have lower velocities and accelerations. We expected that cerebellar subjects would improve at the slower speed under the internal model hypothesis, but would not under the timing hypothesis.
In experiment 2, we asked whether a constant external timing cue could improve motor performance in patients with cerebellar damage. Here we expected that cerebellar subjects would improve under the timing hypothesis since they would not be required to generate an internal timing signal, but would not under the internal model hypothesis. For this condition, we provided such timing cues in three different ways: a constant visual dot moving on the circle below the subject's finger, a pacing dot moving next to their hand, or a trained investigator tracing a circle at the desired pace.
METHODS
Subject
Eight patients with cerebellar damage and eight controls matched for age, gender, and handedness participated in the study. Subject demographics, including the International Cooperative Ataxia Rating Scale (ICARS) scores, are provided in Table 1. The ICARS is a scale that rates severity of cerebellar signs in four areas: gait and balance, limb movements, oculomotor control, and speech. Subjects had magnetic resonance imaging (MRI) and/or genetic tests confirming cerebellar involvement. MRIs from subjects with degenerative diseases showed cerebellar atrophy and sometimes pontine atrophy, with no other abnormalities. MRIs from subjects with focal lesions from stroke were checked to ensure minimal or no brain stem involvement. The individual with stroke in the posterior inferior cerebellar artery (PICA) distribution had minimal damage completely confined to the cerebellum (i.e., not the full PICA territory). The subject with the anterior inferior cerebellar artery stroke also showed a lesion confined to the cerebellum with minimal or no pontine involvement. All subjects were screened to ensure that they did not have extracerebellar signs that could affect this test including: spasticity, weakness, sensory loss, extrapyramidal signs, and neglect. One subject (CBL-4) had mild sensory loss on the feet, but normal sensory function of the hands. All subjects provided their consent before the experiment started. These procedures were approved by the Institutional Review Board of the Johns Hopkins University.
TABLE 1.
Cerebellar subject information
| Subject | Age | Gender | Lesion Information |
ICARS |
||
|---|---|---|---|---|---|---|
| Limb (52 max) | Oculomotor (6 max) | Total (100 max) | ||||
| CBL-1 | 64 | M | LPICA stroke | 1 | 1 | 5 |
| CBL-2 | 55 | M | LAICA stroke | 5 | 2 | 14 |
| CBL-3 | 47 | F | Childhood cerebellitis | 24 | 6 | 49 |
| CBL-4 | 62 | F | SCA6 | 30 | 5 | 66 |
| CBL-5 | 34 | M | SCA8 | 15 | 3 | 40 |
| CBL-6 | 63 | F | SCA6 | 20 | 3 | 37 |
| CBL-7 | 53 | F | Idiopathic cerebellar degeneration | 12 | 4 | 22 |
| CBL-8 | 52 | F | SCA6 | 7 | 5 | 17 |
PICA, posterior inferior cerebellar artery; AICA, anterior inferior cerebellar artery; SCA6, spinocerebellar ataxia type 6; SCA8, spinocerebellar ataxia type 8; ICARS, International Cooperative Ataxia Rating Scale, which comprises four subscales: gait and posture, limb kinetic, speech, and oculomotor. Breakouts of the ICARS limb and oculomotor subscores are also provided.
Procedures
Subjects used the index finger to trace a 6-cm-diameter circle displayed on a liquid crystal display computer screen embedded in a clear plastic surface. We asked the bilateral lesion patients to move their dominant hand and unilateral lesion patients to move their hand affected by the lesion. Control subjects were matched for hand used. The goal was to reproduce a specific period (e.g., every 550 ms). The arm was unconstrained and could rest on the surface. Movement was predominantly at the elbow and wrist. The experimenter explained and demonstrated the movement before each condition. Subjects performed two practice trials of each movement type, followed by three to five test trials. Trials were blocked by condition, but the order of the conditions was counterbalanced across subjects.
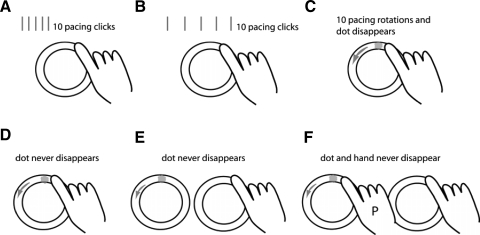
Figure 1 shows a schematic of the testing conditions that were studied. In experiment 1, we used an auditory tone (metronome click) for pacing. In the fast auditory condition (Fig. 1A), subjects were asked to synchronize their movement to 10 pacing tones (550-ms period) and then continue at their remembered pace for 20 s (Spencer et al. 2003). We then changed the beat period to slow the movement to an extent that would simplify dynamics, but should not affect timing variability (Gibbon et al. 1997). The pace on the slow auditory condition (Fig. 1B) was a 1,500-ms period and we recorded 30 s of data to have enough cycles for analysis.
FIG. 1.
Schematic of the different conditions tested. A: auditory fast condition where 10 pacing metronome clicks are given at a 550-ms period, and then subjects continue at a remembered pace. B: auditory slow condition where 10 pacing clicks are given at a 1,500-ms period, and subjects continue at a remembered pace. C: visual fast condition where a pacing dot revolves 10 times around the circle at a period of 550 ms and then subjects continue at a remembered pace. D: visual fast condition with constant pacing dot at 550-ms period (i.e., the dot never disappears). E: visual fast imitation condition where the pacing dot revolves on an adjacent circle at a 550-ms period and never disappears. F: visual fast human imitation condition where pacing at a 550-ms period is done by a trained person (P) on an adjacent circle with a pacing dot.
In experiment 2, we switched to a visual stimulus because we wanted to provide timing and position cues (Fig. 1C). The pacing stimulus was a white dot moving around the circle at a fast pace (550-ms period). We first studied a visual fast condition that was identical to the auditory fast condition (the pacing dot was on for 10 cycles) and then subjects continued at their remembered pace for 20 s. This was done to ensure that the visual stimuli yielded similar results to the auditory stimuli when doing the remembered pacing task.
We then studied three conditions where the visual pacing stimulus was provided throughout the entire trial. For the visually paced condition (Fig. 1D), the visual dot constantly moved on the circle below the finger for the entire trial time at the fast speed (550-ms period). The visual imitation condition (Fig. 1E) was similar except that subjects watched the pacing dot move on a circle drawn next to their hand (rather than on the circle that they were tracing). During the human imitation condition (Fig. 1F), a trained investigator traced a circle next to the subject's hand at the fast pace (550-ms period). We thought that such human hand motion would provide more information than the abstract dot motion (e.g., hand posture) for subjects to imitate.
For all testing conditions, subjects moved under continuous and discontinuous instructions (Spencer et al. 2003). For continuous movements, subjects traced the circle without stopping while attempting to hit the top of the circle synchronously with the beat. For discontinuous movements, subjects traced a single circle, attempting completion at the time of a beat, pausing at the top of the circle until the next beat, and so on. This was the procedure used in previous work (Spencer et al. 2003; Zelaznik et al. 2002). The instructions emphasized temporal consistency instead of spatial accuracy throughout the tests. In other words, we instructed subjects not to trace the dot but use the dot as a reference to make the movements as consistent as possible. The experimenter emphasized the instructions between trials but not during the trials.
Data analysis
Three-dimensional kinematic data were collected from the index fingertip, index finger metacarpal head, wrist, elbow, shoulder, and sternum at 100 Hz (Optotrak, Northern Digital). We also collected the position of the pacing dot at 1,000 Hz. Kinematic data were low-pass filtered at 10 Hz using a second-order Butterworth filter. Index finger position and velocity were used for these analyses. For all conditions, we did not analyze the first two cycles and last cycle so that we captured subjects’ steady-state performance only.
The position data were detrended before any analysis. A custom MATLAB program was used to mark each circle. For the continuous circle drawing, the start and end of each movement segment were marked when the position in the y-axis was positive and the position in the x-axis passed the zero (equivalent to the top of the circle). For the discontinuous circle drawing, movement starts were the time when finger velocity rose >5% of the maximum peak velocity; movement stops were when the velocity dropped <5% of the peak velocity. In addition, we visually inspected all start and stop positions to ensure that the analysis chose the appropriate point in the time series.
The main measures of interest were the coefficient of variation (CV) of cycle time (within a trial), the CV of the radius of the circle (within a cycle), and the mean movement time per cycle. CV was calculated as (SD/mean) × 100.
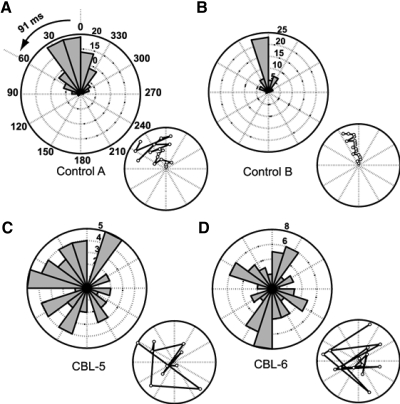
An additional analysis was done on the three conditions where a visual pacing stimulus was left on the entire time. We determined where the stimulus was at the time of movement onset for each cycle in the discontinuous condition. Polar histograms depicting this relationship were generated (e.g., Fig. 4A). If the visual cue positions at movement onset were closely clustered within the first 60° past the top point of the circle (i.e., within 91 ms of time), this suggested that subjects were using a predictive strategy because normal visual reaction time is >150 ms. Reactive or random strategies would be indicated by the stimulus being beyond 60° or in a uniform distribution around the circle, respectively.
FIG. 4.
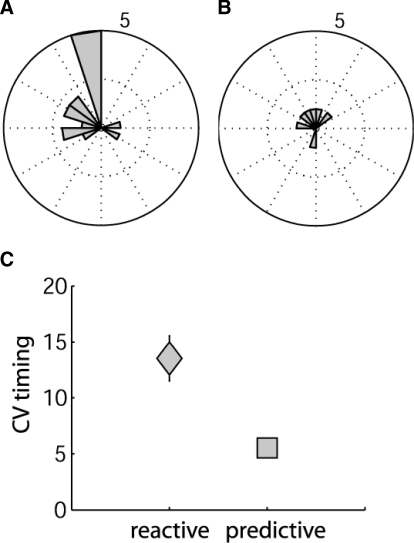
Polar histograms of pacing dot location at movement start. A–D: individual example subject data, with all trials in the discontinuous, constant paced condition shown. Bar magnitude represents number of movement cycles. A and B: 2 control examples that had significant preferred directions using the Rayleigh test (z scores: 59.6 and 37.4) and used predictive strategies (i.e., started while the dot was within the first 60°). C and D: 2 cerebellar examples with no preferred direction (z scores: 2.96 and 0.25). Inset plots show the pacing dot location at movement start from cycle to cycle in a single trial. Open points represent the dot position from each cycle with the first cycle at the center and subsequent cycles radiating out. A spiral pattern would occur if subjects were continually lagging behind on each subsequent cycle. This was typically not the case.
Statistical analysis
Effects of group (patients vs. controls, between-subjects factor) and movement type (continuous vs. discontinuous, within-subjects factor) for each testing condition (within-subjects factor) were assessed using repeated-measures ANOVA. Rayleigh tests of the strength (i.e., length) of the mean vector were used to determine whether polar histograms had a tendency for directionality (i.e., a nonuniform distribution). Significant directionality would indicate that a given subject was consistently starting the movement at a similar point in the stimulus cycle; this was challenging for the fast-paced movements, even for controls. For subjects that showed significant directionality, the mean vector direction was used to determine where in the circle the stimulus was at movement onset. Mean vector directions were used to characterize control versus cerebellar group behavior as being predictive or reactive.
To determine whether the subjects who used the predictive strategy had better performance (i.e., lower temporal variability), the mean CV of timing was compared for predictive versus reactive or random cases using a Student's t-test. This was done for pooled control and cerebellar subjects in the three discontinuous constant visually paced fast conditions.
RESULTS
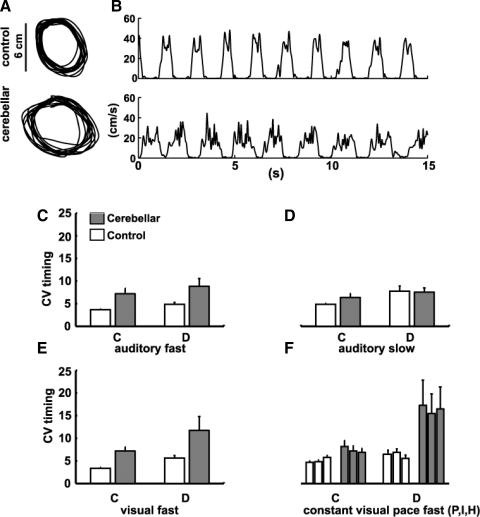
Figure 2, A and B shows representative examples of circle paths and velocities for control and cerebellar subjects making discontinuous movements. Our cerebellar group showed greater temporal variability than that of controls in the fast auditory condition [group effect: F(1,14) = 7.7, P < 0.05] and discontinuous movements were more variable than continuous movements [condition effect: F(1,14) = 4.5, P = 0.05; Fig. 2C]. However, in contrast to Spencer et al. (2003), we did not see a group × condition interaction [F(1,14) = 0.1, P = 0.72] that would indicate a differential cerebellar impairment across continuous versus discontinuous movements. Cerebellar subjects also had longer mean cycle times (Table 2) and higher spatial variability in the fast auditory condition [F(1,14) = 6.2, P = 0.03; Fig. 3A]. In the slower speed condition, however, there were no group differences for temporal or spatial variability (all P > 0.30; Figs. 2D and 3B). This is because the cerebellar group slightly improved and the control group slightly worsened performance at the slower speed. It is possible that the control group worsened because their movements were slower than the desired 1,500-ms period (Table 1). In sum, there was a difference in cerebellar and control performance in the fast, but not slow, auditory condition.
FIG. 2.
Temporal variability. A: example circle paths from the auditory slow (1,500-ms period) discontinuous condition. B: finger velocities for the same subjects. C–F: mean coefficient of variation (CV) of the circle time. C: fast auditory condition, remembered tempo: There was a significant group effect (P < 0.05) with cerebellar subjects more variable; and effect of movement type, with discontinuous movements more variable (P < 0.05). There was no group × movement interaction. D: slow auditory condition, remembered tempo: no group differences but an effect of movement type, with discontinuous movements more variable (P < 0.05). E: fast visual condition, remembered tempo: There was a significant group effect (P < 0.05) with cerebellar subjects more variable than controls and no group × movement interaction (P = 0.50). F: constant paced conditions (from left to right): constant visual pacing (P), imitation (I), and human imitation (H). There were no differences between these conditions (P = 0.89). There was a group effect (P = 0.01) with cerebellar subjects more variable, a movement type effect with discontinuous movements more variable (P = 0.01), and a group × movement type interaction, with cerebellar subjects more variable than controls on the discontinuous vs. continuous movement (P = 0.04).
TABLE 2.
Mean cycle time (SE) in milliseconds
|
Control |
Cerebellar
|
||||
|---|---|---|---|---|---|
| Continuous | Discontinuous | Continuous | Discontinuous | ||
| Auditory Fast | 521 (12) | 818 (39) | 729 (78) | 1,009 (116) | G*, C**, G × C ns |
| Auditory Slow | 1,533 (21) | 1,848 (120) | 1,357 (81) | 1,483 (141) | G*, C*, G × C ns |
| Visual Fast | 492 (13) | 733 (53) | 695 (79) | 1,054 (115) | G*, C**, G × C ns |
| Visual Paced | 512 (7) | 701 (36) | 618 (67) | 942 (79) | G*, C**, G × C ns |
| Visual Imitation | 555 (13) | 719 (27) | 667 (48) | 976 (124) | G*, C**, G × C ns |
| Visual Human | 569 (21) | 739 (26) | 678 (57) | 1,069 (151) | G*, C**, G × C ns |
G, group effect (patients vs. controls); C, movement type (continuous vs. discontinuous); G × C, group × movement type interaction.
P ≤ 0.05;
P < 0.01.
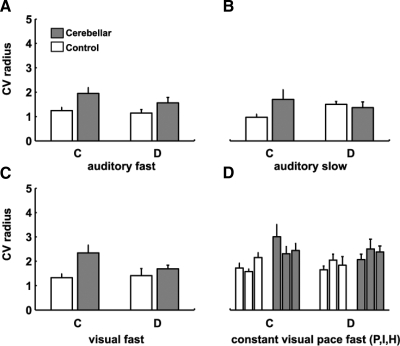
FIG. 3.
Spatial variability. Mean CV of the circle radius, reflecting spatial variability. A: fast auditory condition: there was a significant group effect (P = 0.05) with cerebellar subjects more variable than controls. B: slow auditory condition: no significant differences. C: fast visual condition: there was a significant group effect (P = 0.01) with cerebellar subjects more variable than controls. D: constant paced conditions (from left to right): constant visual pacing (P), imitation (I), and human imitation (H). There was a significant group effect (P = 0.001) with cerebellar subjects more variable than controls.
The fast visual condition was designed to ensure that visual pacing gave results similar to auditory pacing. We found that the 10-cycle presentation of the visual cue led to similar results during the remembered phase of the task; cerebellar subjects showed the same pattern of deficits in both spatial and temporal variability as they did in the fast auditory condition (Figs. 2E and 3C, Table 2). Figure 2E shows that there was a group effect for timing variability [F(1,14) = 9.7, P = 0.01], but no group × condition interaction [F(1,14) = 0.48, P = 0.50]. Figure 3C shows that there was a group effect for spatial variability [F(1,14) = 6.3, P = 0.03], with cerebellar subjects showing more variability than controls.
In experiment 2, we tested whether a constant cue for finger timing and position (a visual dot moving around the circle; Fig. 1D) would improve performance. Leaving the visual cue on for the entire task period should simplify or eliminate timing demands, without altering limb dynamics per se. Paradoxically, this produced a further worsening (i.e., increase) in timing variability for the cerebellar subjects, for discontinuous but not continuous movements [Fig. 2F, group × condition interaction: F(1,14) = 4.97, P = 0.04]. Worsening in the discontinuous conditions was comparable whether the constant visual cue was a dot displayed on the circle being traced, a dot moving around a separate circle, or a human hand performing the same task [effect of the pacing stimulus: F(2,28) = 0.20, P = 0.90]. As in the fast auditory and visual conditions, movements in the paced conditions were similarly slower (longer mean cycle time; Table 2) and spatial variability higher for cerebellar subjects relative to controls [Fig. 3, F(1,14) = 6.1, P = 0.03]. The finding that a constant pacing signal further degraded cerebellar subject performance in the discontinuous condition is inconsistent with both the pure timing and inverse dynamics model hypotheses.
Further analysis revealed that one specific difficulty for cerebellar subjects was in synchronizing movement initiation times with the visual cue. Figure 4, A and B shows two control examples where the visual cue positions at movement initiation were tightly clustered at or just past (usually within 100 ms of) the top of the circle. Given the normal visual reaction time delay of ≥150 ms, this cannot be based on reaction to the arrival of the cue at the top and must, instead, be based on predictive synchronization of movement initiation with the evolving cue position. Figure 4, C and D shows representative example cerebellar subjects who showed no significant tendency for direction. The wide distribution was not explained by individuals lagging behind cycle by cycle, thus producing progressively increasing lag times (Fig. 4, insets).
In general, there were more controls than cerebellar subjects who showed directionality in any of the three discontinuous movement conditions with constant pacing (8 subjects × 3 constant paced conditions possible). Controls showed directionality for 15/24 cases and cerebellar subjects showed directionality for only 6/24 cases. Figure 5, A and B shows polar histograms of the distribution of these control and cerebellar cases, respectively. Note that 9 control cases showed strongly predictive behavior versus 4 cerebellar cases. Figure 5C shows that there seemed to be some advantage to using predictive behavior as opposed to reactive or nondirectional strategies (t = 2.4, P = 0.02). Timing variability was significantly lower (better) for predictive performance when considering data pooled from both groups. This effect was mostly due to the cerebellar group, although a trend was present in controls, as described in the legend for Fig. 5C.
FIG. 5.
Cases showing directionality. Polar plot bar magnitudes represent the number of cases showing directionality by the Rayleigh test (i.e., 8 subjects moving in any of the 3 paced conditions for discontinuous movements; 24 possible cases). A: controls showed directionality 15/24 times, with 9 cases that were classified as predictive. B: cerebellar subjects showed directionality 6/24 times, with 4 cases that were classified as predictive. C: mean CV of timing for pooled control and cerebellar subjects who showed reactive (gray square) vs. predictive (gray triangle) behavior. Predictive behavior was less variable in timing than reactive behavior (t = 2.4, P = 0.02; note: reactive here includes nondirectional trials). This effect was also present when the groups were considered in isolation, although much more robustly for the cerebellar group (controls: reactive CV = 6.7 ± 0.6; predictive CV = 5.6 ± 0.7 vs. cerebellars: reactive CV = 18.6 ± 3.0; predictive CV = 5.4 ± 0.5).
The presence of a visual pacing signal reduces timing requirements, but may potentially add different complexities to the task. The pacing signal adds a visual requirement and, possibly, an oculomotor component (note: the circle diameter is small enough that eye movements are not required to see the pacing signal). It is known that coordination of an arm movement can worsen when coupled with an eye movement in individuals with cerebellar damage (Beppu et al. 1987; van Donkelaar and Lee 1994). Here, we reasoned that if oculomotor deficits were the cause of the problem then one might expect to see comparable worsening in both the continuous and discontinuous movements that were constantly paced. This was not the case.
Last, there was no correlation between our variability measures with the subareas of ICARS (gait and balance, limb movements, oculomotor control, and speech). Thus our study suggests that the high movement variability in the select discontinuous conditions was not due to tremor or dysmetria in our patient population.
DISCUSSION
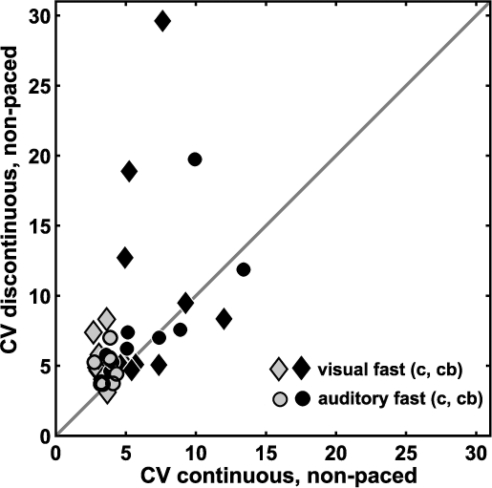
We were unable to replicate the previous finding of impaired discrete movement timing and normal continuous movement timing in individuals with cerebellar damage (Spencer et al. 2003). Our protocol was based on theirs and it is not entirely clear why our cerebellar group results differed. We saw an overall impairment in both discontinuous and continuous movements, although individual subjects varied in extent. Figure 6 shows individual control and cerebellar subject timing variability from the nonpaced discontinuous and continuous conditions most analogous to Spencer et al. (2003). Controls showed similar to slightly higher variability on discontinuous versus continuous movements. Some cerebellar subjects fell into the control range; most showed higher variability on both types of movements, with some slightly more variable on continuous movements (i.e., below diagonal line) and others more variable on discontinuous movements (i.e., above diagonal line).
FIG. 6.
Individual subject timing variability for discontinuous vs. continuous movements. Cerebellar (black) and control (gray) subjects are shown for 2 movement conditions that did not have continuous pacing: auditory fast (circles) and visual fast (diamonds). The dark gray line represents where CV discontinuous = CV continuous. Controls and cerebellar subjects fall along the gray line, showing that the timing variability in discontinuous movements is comparable to that in continuous movements. However, most cerebellar subjects showed elevated timing variability in both types of movement compared with controls.
The data presented by Spencer et al. (2003) differed from ours in two ways. First, they studied a larger sample of patients and reported separate results from people with unilateral cerebellar lesions versus bilateral degenerative diseases. Three of their degenerative patients had SCA3 (Machado–Joseph disease), which is not purely a cerebellar disease, making it more difficult to compare our degenerative patients to theirs. Nonetheless, we checked whether our results would differ if we separated the two subjects with unilateral lesions from the six that had bilateral degeneration. In the fast auditory condition (i.e., same condition studied by Spencer et al.), mean CV of timing from our six degenerative subjects was abnormal for both movement types: continuous versus discontinuous movements were (mean ± SD) 6.9 ± 3.9 and 9.4 ± 5.6, respectively. Likewise for our two lesioned subjects: 8.1 ± 1.1 and 7.3 ± 0.4. All are higher than our controls, which were 3.7 ± 0.5 and 4.8 ± 1.2. No cerebellar subgroup showed the patterns seen by Spencer et al. (2003).
Second, they used the cerebellar subjects’ contralesional (unaffected) hand as the control hand for the unilateral patients they tested. For six of seven of those patients, the contralesional hand was their left, nondominant hand, which might behave differently than the dominant hand. It has also been shown that unilateral cerebellar damage can cause some changes in the control of the contralesional hand (Fisher et al. 2006). For these reasons, perhaps comparing the ipsilesional ataxic hand with the contralesional hand is not ideal. Figure 3C of Spencer et al. (2003) shows data suggesting that this could be the case. Note that their unilateral subjects showed mean CV values of about 6% for both hands in the fast continuous condition. Our unilateral cerebellar subjects showed mean CV values of 8.1% but our controls showed CV values of only 3.7%. Thus if they had used matched controls instead of the patients’ other hand, they may have seen a similar deficit in the continuous condition. However, we cannot say definitively whether this affected the results.
In sum, we cannot definitively resolve the differences in the two studies. However, we do clearly demonstrate impairments in both discontinuous and continuous movements for many cerebellar subjects compared with controls.
Internal models or timing?
We found that slowing the desired movement time made cerebellar timing variability comparable to controls. Slowing the movement may have made the movement “simpler” by reducing dynamics demands and could therefore be interpreted as supporting the pure dynamics hypothesis. Yet, an inverse dynamics model alone may not account for all of our data.
An interesting finding was that a constant visual cue dramatically worsened cerebellar performance compared with controls. Others have shown that cerebellar subjects’ hand -tracking movements can be worsened when the eyes also track the target (Beppu et al. 1987; van Donkelaar and Lee 1994). It is striking, however, that our cerebellar subjects showed much greater worsening on the discontinuous compared with continuous movement condition when a visual cue was present. This was true no matter how the constant visual cue was presented—on the circle being traced, on a circle next to the traced circle, or by another person. Thus the added coordination demand of eyes and hand alone cannot fully explain our results.
One hypothesis that might account for all these findings is that the cerebellum is acting more like a forward model, representing the ability to estimate stimulus and hand position in space as well as their integration (Bares et al. 2007; Hore et al. 2005; Miall et al. 1993; Paulin 1997). The pure timing hypothesis is inconsistent with group performance at the slower movement speed: cerebellar subjects slightly improved (reduced) timing variability and controls slightly worsened (increased) timing variability, making overall performance equivalent between groups. The timing hypothesis is also inconsistent with the degradation of performance in the discontinuous condition in the presence of a constant visual cue (which obviates the need for covert interval timing). Indeed, the inverse dynamics hypothesis also cannot explain the additional degradation produced by the continuous visual cue.
We suggest that the presence of the constant visual cue reduces timing demands, but increases the demand for sensorimotor prediction in the discontinuous condition, producing what appears to be a particularly difficult task condition for cerebellar subjects (Bares et al. 2007). This is because the fast-paced period (550 ms) is too short for subjects to simply react to the visual stimulus and maintain timing invariance. Indeed, control and cerebellar subjects often commented that they were unsure of how well they were doing in the fast conditions and that they had no specific reaction strategy. Therefore the fast movements should ideally use predictive (rather than reactive) information about how their movement will unfold, how the stimulus movement will unfold, and the interaction between the two. Given our findings and the fact that cerebellar subjects have difficulties with visual interception (Bares et al. 2007), we think that the cerebellum could normally be providing one or more of these predictions.
We speculate that, conversely, the slow movement condition in experiment 1 decreased the demand for sensorimotor prediction (during the initial pacing period) since a reactive strategy would suffice at the slower pace, resulting in the improved performance by cerebellar subjects. The cerebellum may be essential for general timing and for control of complex limb dynamics in many situations, but in the timed, repetitive movement tasks studied here and by Spencer et al. (2003), we conclude that a critical impairment of cerebellar subjects is sensorimotor prediction.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01-HD-040289 to A. J. Bastian and Neuroscience Summer Fellowship at the University of Maryland, College Park to J. Bo.
Acknowledgments
We thank J. Diedrichsen, E. Connor, and J. Bastian for helpful comments.
Present address of J. Bo: Division of Kinesiology, University of Michigan, Ann Arbor, MI 48109.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bares et al. 2007.Bares M, Lungu O, Liu T, Waechter T, Gomez CM, Ashe J. Impaired predictive motor timing in patients with cerebellar disorders. Exp Brain Res 180: 355–365, 2007. [DOI] [PubMed] [Google Scholar]
- Bastian et al. 1996.Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76: 492–509, 1996. [DOI] [PubMed] [Google Scholar]
- Bastian et al. 2000.Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol 83: 3019–3030, 2000. [DOI] [PubMed] [Google Scholar]
- Beppu et al. 1987.Beppu H, Nagaoka M, Tanaka R. Analysis of cerebellar motor disorders by visually-guided elbow tracking movement. 2. Contribution of the visual cues on slow ramp pursuit. Brain 110: 1–18, 1987. [DOI] [PubMed] [Google Scholar]
- Fisher et al. 2006.Fisher BE, Boyd L, Winstein CJ. Contralateral cerebellar damage impairs imperative planning but not updating of aimed arm movements in humans. Exp Brain Res 174: 453–466, 2006. [DOI] [PubMed] [Google Scholar]
- Gibbon et al. 1997.Gibbon J, Malapani C, Dale CL, Gallistel C. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol 7: 170–184, 1997. [DOI] [PubMed] [Google Scholar]
- Hore and Watts 2005.Hore J, Watts S. Timing finger opening in overarm throwing based on a spatial representation of hand path. J Neurophysiol 93: 3189–3199, 2005. [DOI] [PubMed] [Google Scholar]
- Ivry and Spencer 2004.Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol 14: 225–232, 2004. [DOI] [PubMed] [Google Scholar]
- Miall et al. 1993.Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav 25: 203–216, 1993. [DOI] [PubMed] [Google Scholar]
- Paulin 1997.Paulin MG Neural representations of moving systems. Int Rev Neurobiol 41: 515–533, 1997. [DOI] [PubMed] [Google Scholar]
- Spencer et al. 2003.Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 300: 1437–1439, 2003. [DOI] [PubMed] [Google Scholar]
- Thach 1998.Thach WT What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci 2: 331–338, 1998. [DOI] [PubMed] [Google Scholar]
- Topka et al. 1998.Topka H, Konczak J, Schneider K, Boose A, Dichgans J. Multijoint arm movements in cerebellar ataxia: abnormal control of movement dynamics. Exp Brain Res 119: 493–503, 1998. [DOI] [PubMed] [Google Scholar]
- Trouillas et al. 1997.Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 12: 145: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- van Donkelaar and Lee 1994.van Donkelaar P, Lee RG. Interactions between the eye and hand motor systems: disruptions due to cerebellar dysfunction. J Neurophysiol 72: 1674–1685, 1994. [DOI] [PubMed] [Google Scholar]
- Welsh et al. 1995.Welsh JP, Lang EJ, Suglahara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature 374: 453–457, 1995. [DOI] [PubMed] [Google Scholar]
- Wolpert and Miall 1996.Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw 9: 1265–1279, 1996. [DOI] [PubMed] [Google Scholar]
- Zelaznik et al. 2002.Zelaznik HN, Spencer RM, Ivry RB. Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. J Exp Psychol Hum Percept Perform 28: 575–588, 2002. [DOI] [PubMed] [Google Scholar]