Abstract
Enhancement of presynaptic Ca2+ signals is widely recognized as a potential mechanism for heterosynaptic potentiation of neurotransmitter release. Here we show that stimulation of a serotonergic interneuron increased spike-evoked Ca2+ in a manner consistent with its neuromodulatory effect on synaptic transmission. In the gastropod mollusk, Tritonia diomedea, stimulation of a serotonergic dorsal swim interneuron (DSI) at physiological rates heterosynaptically enhances the strength of output synapses made by another swim interneuron, C2, onto neurons in the pedal ganglion. Using intracellular electrophysiological recording combined with real-time confocal imaging of C2 (loaded with Oregon Green Bapta 1), it was determined that DSI stimulation increases the amplitude of spike-evoked Ca2+ signals in C2 without altering basal Ca2+ signals. This neuromodulatory action was restricted to distal neurites of C2 where synapses with pedal neurons are located. The effect of DSI stimulation on C2 spike-evoked Ca2+ signals resembled DSI heterosynaptic enhancement of C2 synapses in several measures: both decayed within 15 s, both were abolished by the serotonin receptor antagonist, methysergide, and both were independent of DSI's depolarizing actions on C2. A brief puff of serotonin could mimic the enhancement of spike-evoked Ca2+ signals in the distal neurites of C2, but larger puffs or bath-applied serotonin elicited nonphysiological effects. These results suggest that DSI heterosynaptic enhancement of C2 synaptic strength may be mediated by a local enhancement of spike-evoked Ca2+ signals in the distal neurites of C2.
INTRODUCTION
Although alteration of presynaptic Ca2+ signaling has been hypothesized to underlie heterosynaptic neuromodulation of synaptic strength (Blumenfeld et al. 1990; Eliot et al. 1993; Wu and Saggau 1997), to our knowledge, no studies have documented this phenomenon in response to stimulation of a neuromodulatory neuron in situ. This surprising absence in the literature is most likely due to the difficulty inherent in such experiments and the paucity of well-defined neuronal networks in which the actions of identified neuromodulatory interneurons can be assessed. Here we found that heterosynaptic facilitation of synaptic strength by a serotonergic interneuron is accompanied by an enhancement of spike-evoked Ca2+ signals in the modulated interneuron.
Previous studies in other systems have shown that serotonin can alter Ca2+ influx through voltage-gated Ca2+ channels. In cultured Aplysia sensory neurons, serotonin leads to spike-broadening, causing more Ca2+ influx per action potential (Eliot et al. 1993). Serotonin has also been shown to inhibit voltage-activated Ca2+ currents/transients (Bayliss et al. 1997; Diaz-Rios et al. 2007; Foehring 1996; Ladewig et al. 2004; Leonard et al. 2000; Rhee et al. 1996). The alteration of intracellular Ca2+ levels can have profound effects on neurotransmitter release and on neuronal excitability. For example, at parallel fiber to Purkinje cell synapses, the release of neurotransmitter has been shown to be exquisitely sensitive to increases in intracellular Ca2+ levels (Sabatini and Regehr 1997). Conversely, reduction of presynaptic Ca2+ influx can lead to inhibition of elicited neurotransmitter release (Wu and Saggau 1997). Further, a decrease in intracellular Ca2+ levels can reduce Ca2+-dependent K+ currents, resulting in an enhancement of neuronal excitability, as shown in spinal interneurons (Diaz-Rios et al. 2007; El Manira and Wallen 2000). Because intracellular Ca2+ levels can directly control neurotransmitter release and neuronal excitability, altering presynaptic Ca2+ levels may be a mechanism by which neuromodulatory neurons exert their effects.
In the mollusk, Tritonia diomedea (Katz 2007), the dorsal swim interneurons (DSIs, NeuronBank.org/Tri0001043) and cerebral neuron 2 (C2, NeuronBank.org/Tri0002380) are both members of the central pattern generator (CPG) for escape swimming (Getting et al. 1980). The serotonergic DSIs heterosynaptically modulate the strength of output synapses made by C2 in the pedal ganglion (Katz and Frost 1995a,b; Katz et al. 1994). The neuromodulatory effect on C2 synaptic strength caused by serotonin released from DSI is transient (Katz et al. 1994), presynaptic in nature, not mediated by synaptic depolarization of C2 (Katz and Frost 1995b), and involves G protein signaling (Clemens and Katz 2003). Here we sought to determine if synaptically released serotonin from DSI acts by modulating presynaptic Ca2+ signaling in C2.
We found that DSI stimulation transiently and locally enhanced spike-evoked Ca2+ signaling in C2. Properties of this neuromodulatory action such as its time course, spatial restriction, and sensitivity to the serotonin antagonist methysergide suggest that increased spike-evoked Ca2+ signaling in C2 may underlie the heterosynaptic potentiation of the C2 to pedal follower cell synapses by DSI.
Portions of this work have been previously presented in abstract form (Hill and Katz 2006).
METHODS
Experiments were performed on T. diomedea obtained from Living Elements (Delta, British Columbia, Canada). Animals were maintained in artificial recirculating, chilled (10°C) seawater. Dissection protocols were as described earlier (Getting et al. 1980; Hill and Katz 2007; Katz and Frost 1995a). The isolated brain, consisting of the fused cerebropleural ganglion and the pedal ganglia, was pinned to a silicone elastomer (Sylgard)-coated 35 mm Petri dish. The brain was chilled to 4–5°C, and then the fine connective sheath covering the ganglia was removed with fine forceps and scissors. After desheathing, the temperature was gradually raised to 10°C. The composition of the normal saline was (in mM): 420 NaCl, 10 KCl, 10 CaCl2, 50 MgCl2, 10 d-glucose, and 10 HEPES, pH 7.4.
Neurons were impaled with glass microelectrodes filled with 3 M KCl (12–20 MΩ resistance). C2 and DSI were identified on the basis of their soma location, appearance, action potential shape, and activity during a swim motor pattern (Getting 1983; Getting et al. 1980; Taghert and Willows 1978). Neurons were excluded from the study if they were damaged during the dissection or microelectrode impalement and had resting potentials outside of the normal range of −40 to −55 mV. In addition, all C2s and DSIs used in this study fired rhythmically during the swim motor pattern. As further indicators of the health of the neurons and preparation, all DSIs used in this study fired spontaneous action potentials at 1–2 Hz, and all C2s were silent at rest. The swim motor pattern was evoked by electrical stimulation of a body wall nerve, pedal nerve 2 or 3 (7–15 V, 2-ms pulses, 10 Hz for 1–1.5 s).
After identification, C2 was re-impaled with a microelectrode containing 2.5 mM Oregon Green Bapta 1 (OGB-1; Invitrogen, Carlsbad, CA) dissolved in dH2O. OGB-1 was iontophoresed using −1.0 to −7.0 nA, 500-ms pulses at 1 Hz for 10–40 min. After a successful injection, the soma always appeared pink to magenta in color. To allow for complete diffusion of the dye into distal regions of C2, the brain was then left for a few hours to overnight, constantly superfused with normal saline at 8–9°C. To image the distal neurites in the pedal ganglion, neuronal somata on the ventral surface of the pedal ganglion were removed with fine forceps. This had no apparent effect on the swim CPG because swim motor patterns recorded after removal of neuronal stomata on the ventral surface of the pedal ganglion appeared indistinguishable from motor patterns recorded with those cell bodies intact. After electrophysiological identification, DSI was re-impaled with a microelectrode containing Alexa 594 in 200 mM KCl (Invitrogen). Alexa 594 was iontophoresed using −1.0 to −7.0 nA, 500-ms pulses at 1 Hz for 5–20 min. After a successful injection, the soma of DSI always appeared purplish in color. Filling DSI with Alexa 594 allowed it to be unambiguously re-impaled on the Ca2+ imaging rig.
The Petri dish was then transferred to a fixed stage Zeiss Axioskop 2 microscope outfitted with a Zeiss LSM 510 confocal laser scanning system (Zeiss, Jena, Germany). C2 and DSI were re-impaled with fine tipped electrodes filled with 3 M KCl. For Ca2+ imaging, an Argon laser (488 nM) was used to excite OGB-1 and a band-pass filter (500–550 nm) was used to detect the emitted fluorescence. A ×5 air objective (Fluar, 0.25 N.A.) was used for imaging. After selecting a frame of interest, a time series of that frame was acquired [2.5–5 Hz (200–400 ms/per frame), 2.5 μs pixel dwell time, 512 × 512 or 1,024 × 1,024 pixel/frame]. A delay of 20–40 ms between frames was introduced to reduce photo-damage and phototoxicity.
To measure changes in Ca2+, regions of interest (ROIs) were selected, and the raw fluorescence data were saved as text files. Background fluorescence was corrected by averaging the values in two or more regions not stained by the Ca2+ indicator and subtracting those values from the ROIs of Ca2+ indicator-stained regions. The change in fluorescence (ΔF/Fo) was calculated with respect to the average baseline fluorescence (Fo; measured for 10 s) in each ROI. For quantification of Ca2+ signals, the integral of the Ca2+ signal (expressed as ΔF/F * seconds) for 2 s following the C2 spikes was calculated. Similar results were obtained with peak Ca2+ measurements, but the integral exhibited less random fluctuation. To examine the effects of DSI stimulation on C2 spike-evoked Ca2+ signals on a pixel-by-pixel basis, image analysis was performed with ImageJ (http://rsb.info.nih.gov/ij/). First, the SD of the “noise” from two unstimulated periods was calculated. The noise threshold was set at 3 times the SD of the noise. Only pixels that showed a Ca2+ signal greater than the noise threshold were accepted for analysis. The integral of the Ca2+ signals for 2 s following C2 spikes were calculated and presented as images.
For electrophysiological recordings, we used an AxoClamp 2B amplifier (Axon Instruments, Union City, CA) connected by a 1401micro AD converter [Cambridge Electronic Design (CED), Cambridge, UK] to a Windows XP computer running Spike2 software (CED). An output pulse from the AD converter was used to trigger the LSM imaging system, synchronizing the acquisition of imaging and electrophysiology data. C2 and DSI were stimulated to fire action potentials using brief intracellular current pulses (20 ms, 7–10 nA). Each pulse produced a single action potential as observed in the soma recording, in EPSPs in the follower neurons, and in extracellular recordings of time-locked action potentials on pedal nerves 5 and 6.
All experiments were carried out in high divalent cation saline solution (Hi Di) to reduce polysynaptic contributions. The composition of the Hi Di solution was (in mM) 285 NaCl, 10 KCl, 25 CaCl2, 125 MgCl2, 11 d-glucose, and 10 HEPES, pH 7.4. Hi Di saline has been used in all previous publications that studied the effects of DSI on C2 synaptic strength. Although it is possible that the increased Ca2+ concentration might alter the Ca2+ signals observed, it is necessary to conduct the experiments in such a manner. In normal saline, DSI stimulation at 10- to 20-Hz recruits additional neurons to fire, making it impossible to determine if the effects of DSI stimulation are direct.
To examine the morphology of these neurons, they were injected with Alexa 594 using −1.0 to −7.0 nA, 500-ms pulses at 1 Hz for 20–30 min. The dye was allowed to diffuse for 1–2 h, and then the brain was fixed in 4% paraformaldehyde in 0.1 M PBS for 1–2 h at 4°C. Following fixation, the brains were dehydrated in an ascending ethanol series, cleared in methyl salicylate, and mounted in Cytoseal 60 (Electron Microscopy Sciences, Washington, PA). Dye filled neurons were then scanned with the Zeiss LSM 510 confocal microscope using a ×10 air objective (Fluar, 0.5 N.A.).
Serotonin (5-hydroxytryptamine creatine sulfate, Sigma, St. Loius, MO) was dissolved in Hi Di saline just before use at a final concentration of 1 μM to 1 mM. Serotonin was either puffed using brief pressure pulses from a PicoSpritzer II (Parker General Valve, Fairfield, NJ) or bath-applied. For pressure puff experiments, 0.1% Fast Green (Sigma) was added to the serotonin solution to facilitate visualization of the serotonin puff. Between puffs of serotonin, the preparation was superfused with Hi Di saline solution to remove any remaining serotonin. Methysergide maleate (RBI, Natick, MA) was dissolved in dimethyl sulfoxide (DMSO, Sigma) to a concentration of 50 mM and then diluted in high-divalent cation saline solution to a final bath concentration of 50 μM, a concentration shown to be effective at blocking the neuromodulatory actions of DSI (Katz and Frost 1995a).
It should be noted that recordings of neurons are stable for many hours in this preparation and that repeated impalements of the neurons are routine. The neuromodulatory actions of the DSIs can be observed on subsequent days in the same preparation.
Statistical analyses were performed with SigmaStat (Jandel Scientific, San Rafael, CA). For analysis of repeated measures, we used a one-way repeated-measures ANOVA, a Friedman repeated-measures ANOVA on ranks, or a two-way repeated-measures ANOVA (with post hoc multiple pair-wise comparisons). P values of <0.05 were considered significant. Error bars represent mean ± SE.
RESULTS
DSI heterosynaptic facilitation of C2 to pedal neuron synapses occurs in distal neurites
As previously shown (Katz et al. 1994), DSI stimulation increased the amplitude of synaptic potentials evoked by C2 in postsynaptic pedal neurons (Fig. 1A). We recorded from a number of different pedal neurons that received excitatory synaptic inputs from C2. DSI stimulation increased the amplitude of C2-evoked EPSPs in all of its postsynaptic followers as previously reported (Katz and Frost 1995b).
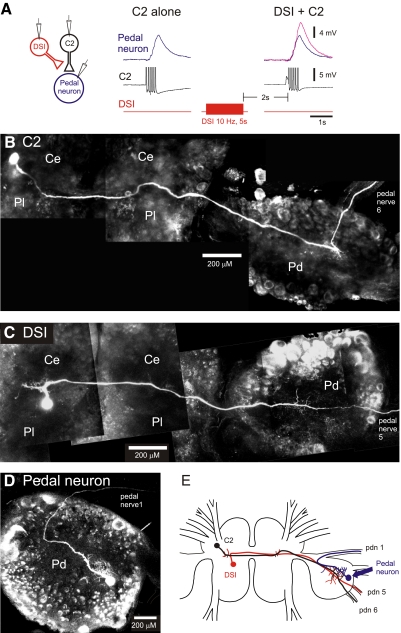
FIG. 1.
Dorsal swim interneuron (DSI) modulation of cerebral neuron 2 (C2) synaptic strength occurs in the pedal ganglion. A: DSI heterosynaptically increased the size of excitatory postsynaptic potentials (EPSPs) recorded in a pedal neuron in response to C2 action potentials. Intracellular electrodes were placed in C2, DSI, and a contralateral pedal neuron. Action potentials in C2 (5 spikes at 10 Hz) produced a summated monosynaptic EPSP in the pedal neuron. DSI stimulation increased the amplitude of the EPSP (magenta trace). C2 action potentials were truncated in this trace. B–D: composite confocal images of a C2 (B), a DSI (C), and a postsynaptic pedal neuron (D). Neurons were iontophoretically filled with Alexa 594. Both C2 and DSI have somata in the cerebral ganglia and axons that traverse to the contralateral pedal ganglion where they arborize before exiting through pedal nerve 5 (DSI) or pedal nerve 6 (C2). Note that the pedal ganglion was twisted to be ventral side up to facilitate imaging of DSI and C2 neurites. This altered the locations of the pedal nerves in the images. D: a typical pedal neuron that received excitatory synaptic input from C2. Its primary neurite arborized in the pedal ganglion before exiting via pedal nerve 1. E: a composite schematic of the morphology of these 3 neurons (DSI, C2, pedal neuron) showing the site of overlap of their axons in the pedal ganglion. Ce, cerebral ganglion; Pd, pedal ganglion; Pl, pleural ganglion.
Intracellular dye injections of C2, DSI, and postsynaptic pedal neurons showed that the only area of overlap of the three neurons was in the pedal ganglion, contralateral to the C2 soma (Fig. 1, B–E), making it the likely site of neuromodulation. The axons of C2 and DSI were in close proximity in both cerebral ganglia and in the contralateral pedal ganglion (Fig. 1, B, C, and E) as previously observed (Getting et al. 1980). Intracellular dye fills of six postsynaptic pedal neurons showed that in each case, the neurites of the pedal neuron were confined to the pedal ganglion except for an axon that projected out a pedal nerve (Fig. 1D). Therefore the only potential site of synaptic contact between C2 and postsynaptic pedal neurons is in the pedal ganglion contralateral to the C2 soma (Fig. 1E).
DSI stimulation enhanced spike-evoked Ca2+ signaling in distal neurites of C2
To test whether DSI potentiation of C2 synaptic strength might involve an enhancement of Ca2+ signals in C2, we filled C2 with a calcium indicator dye (OGB-1) and imaged its neurites while simultaneously recording and stimulating electrophysiologically with intracellular microelectrodes in the somata of both DSI and C2 (Figs. 2A and 3A). These experiments were carried out with the preparation bathed in high-divalent cation saline solution (Hi Di) to reduce polysynaptic contributions.
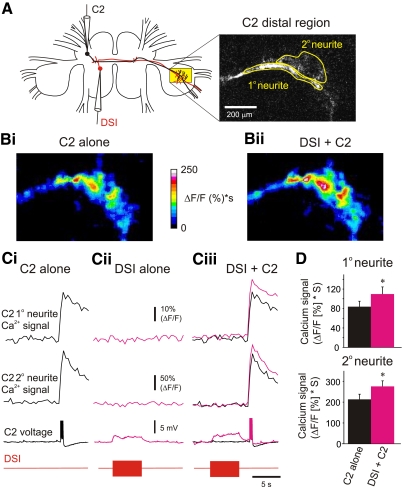
FIG. 2.
DSI stimulation enhanced spike-evoked Ca2+ signaling in the distal neurites of C2. A: experimental setup: imaging from the distal neurites of C2 with electrodes in the somata of C2 and DSI. The primary neurite and secondary neurites of C2 are circled in the fluorescence image of the pedal ganglion. B: the integral of the Ca2+ signal (expressed as ΔF/F*s for 2 s) in the distal neurites of C2 in response to 5 spikes at 10 Hz when C2 was stimulated alone (Bi) and when C2 was stimulated 2 s after a DSI spike train (10 Hz, 5 s; Bii). The signal was stronger when C2 was stimulated after DSI. C: time course of Ca2+ signals in the distal neurites of C2 caused by stimulation of C2 alone (Ci), DSI alone (Cii), or C2 stimulation following a DSI spike train (Ciii). Corresponding C2 and DSI voltage traces are shown below the C2 Ca2+ signals; C2's spikes were truncated to highlight the depolarization of C2 caused by the DSI spike train. C2 stimulation alone caused a brief Ca2+ transient in both the primary and secondary neurites of C2 (Ci). DSI stimulation alone depolarized C2 membrane potential but did not change the basal Ca2+ signal (Cii). When the C2 spikes were given 2 s after the end of the DSI spike train, the Ca2+ transients in both the primary and secondary neurites were larger (magenta traces) than those produced by C2 stimulation alone (black traces). D: in both primary and secondary neurites, DSI stimulation significantly enhanced spike-evoked Ca2+ signals [primary neurite: P < 0.001, Wilcoxon signed rank test (median before DSI stimulation = 77.94, median after DSI stimulation = 90.91), n = 19; secondary neurite: P < 0.001, paired t-test (mean before DSI stimulation = 213.11 ± 24.98, mean after DSI stimulation = 275.47 ± 26.54), n = 13].
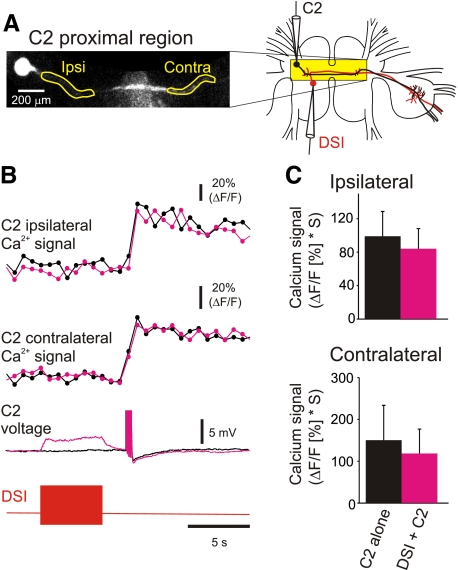
FIG. 3.
DSI stimulation did not affect spike-evoked Ca2+ signaling in the proximal neurites of C2. A: experimental setup: imaging from the proximal neurites of C2 with electrodes in the somata of C2 and DSI. The ipsi- and contralateral proximal neurites are circled in the fluorescence image. B: stimulation of a single DSI (bottom trace: 10 Hz, 5 s ending 2 s before C2 stimulation) depolarized C2 membrane potential but did not affect the spike-evoked Ca2+ signals in either the ipsilateral or contralateral neurites of C2 (black traces, C2 alone; magenta traces, C2 stimulation following the DSI spike train). C: in the proximal neurites of C2, DSI stimulation did not significantly affect spike-evoked Ca2+ signals [ipsilateral: P = 0.119, paired t-test (mean before DSI stimulaiton = 99.03 ± 29.65, mean after DSI stimulation = 84.08 ± 24.02), n = 8; contralateral: P = 0.156, Wilcoxon signed rank test (median before DSI stimulaiton = 85.05, median after DSI stimulation = 83.56), n = 6].
Changes in fluorescence were consistently observed when C2 was stimulated alone to fire five spikes at 10 Hz. These signals could be measured in distal neurites in the pedal ganglion (Fig. 2, Bi and Ci) as well as in proximal neurites in the cerebral ganglia (Fig. 3B). In the pedal ganglion, the spike-evoked Ca2+ signals were larger and more transient in the secondary neurites than in the primary neurite as we had previously observed using other stimuli (Hill and Katz 2007).
To test whether DSI stimulation affected basal Ca2+ signals, DSI was stimulated at 10 Hz for 5 s without C2 spiking (Fig. 2Cii). This did not produce a detectable change in the basal Ca2+ signal in either the primary or secondary distal neurites of C2 (9 distal primary and 6 secondary neurites) but did produce a modest depolarization of C2's membrane potential as recorded in the soma, consistent with the known monosynaptic connection between DSI and C2 (Getting 1981). Similarly, no change in Ca2+ was observed in proximal neurites in the cerebral ganglion when DSI was stimulated alone (8 ipsilateral and 6 contralateral samples). Therefore DSI stimulation alone did not detectably alter resting Ca2+ in C2.
To determine whether DSI stimulation affected C2 spike-evoked Ca2+ signals, C2 was stimulated 2 s after the end of the DSI spike train (Fig. 2, Bii and Ciii). This increased the amplitude of the C2 spike-evoked Ca2+ signals (Fig. 2B, i and ii). In both the primary and secondary distal neurites of C2, the spike-evoked Ca2+ signals were significantly larger following DSI stimulation (Fig. 2, Ciii and D). Thus DSI stimulation increased the amplitude of spike-evoked Ca2+ signals in the distal neurites of C2, where it makes synaptic contacts with its pedal follower neurons.
DSI did not produce a similar increase in C2 spike-evoked Ca2+ signals in the proximal neurites of C2 in the cerebral ganglia (Fig. 3). Although C2 has sparse secondary neurites in the cerebral ganglia (Getting et al. 1980), the branches were too fine for us to detect fluorescence signals in live preparations. We therefore examined spike-evoked Ca2+ signals in the primary neurites in the cerebral ganglia both ipsi- and contralateral to the C2 soma. Unlike in the distal neurites, DSI stimulation did not significantly alter the amplitude of spike-evoked Ca2+ signals in C2 proximal neurites either the ipsilateral or contralateral to the soma (Fig. 3, B and C). Thus the neuromodulatory effects of DSI on C2 spike-evoked Ca2+ signaling were restricted to the distal neurites of C2 in the contralateral pedal ganglion.
Effect of DSI on C2 spike-evoked Ca2+ signaling was transient
To examine the time course of the DSI action on C2 spike-evoked Ca2+ signals, we stimulated C2 at varying times with respect to the DSI spike train. For each trial in which C2 with a DSI spike train, there was a preceding trial in which C2 was stimulated alone (without DSI stimulation). The Ca2+ signals following DSI were compared with the Ca2+ signals caused by C2 stimulation alone. As a control, C2 was stimulated twice alone (no DSI stimulation), and the resultant Ca2+ signals were compared. DSI significantly enhanced C2 spike-evoked Ca2+ when C2 was stimulated 2 and 5 s after the end of the DSI train. The enhancement in C2 spike-evoked Ca2+ signals decreased monotonically with time after DSI stimulation with no significant enhancement 10 or 20 s after the DSI spike train (Fig. 4A).
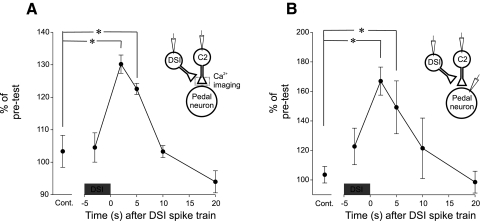
FIG. 4.
The time course of the DSI-evoked enhancement of C2 spike-evoked Ca2+ signals matched that of the DSI-evoked synaptic enhancement. A: calcium signals expressed as percentage pretest for control and at varying times with respect to a DSI spike train (10 Hz, 5 s). *, statistically significant differences from the control with a Friedman repeated-measures ANOVA on ranks [performed using normalized data, with the factor being the time between the end of the DSI spike train and the C2 spikes (−3, 2, 5, 10, 20 s) with post hoc pair-wise comparisons (Student-Newman-Keuls], n = 5. As a control, C2 stimulated twice with no DSI stimulation. B: the amplitude of C2-evoked EPSPs expressed as percentage of pretest for control and at varying times with respect to a DSI spike train (10Hz, 5s). *, statistically significant differences from the control using the same statistical methods as in A (n = 6).
When C2 was stimulated during the DSI spike train and thereby during the depolarization caused by DSI, there was no significant enhancement of spike-evoked Ca2+ signals (Fig. 4A). This suggests that the DSI synaptic depolarization of C2 itself is not responsible for the enhancement in the C2 spike-evoked Ca2+ signals and that the neuromodulatory effect of DSI on C2 spike-evoked Ca2+ takes >2 s to develop. Thus the effect of DSI on C2 spike-evoked Ca2+ was slowly rising and slowly falling with a time course of several seconds.
If the effect of DSI stimulation on C2 synaptic strength is mediated by the modulation of spike-evoked Ca2+, then the time course of the two effects should be similar. Using the same stimulation protocols for DSI and C2 and recording EPSPs in pedal postsynaptic neurons, we observed a similar slowly rising and slowly decaying effect on C2 synaptic strength (Fig. 4B). As with the Ca2+ signal, there was no significant enhancement in the amplitude of C2-evoked EPSPs during the DSI train, but the enhancement was significant 2 and 5 s after the end of the DSI train (compared with the control, Fig. 4B). The DSI enhancement of C2-evoked EPSPs decreased monotonically, with no significant enhancement 10 or 20 s after the DSI spike train. Thus the DSI modulation of C2 synaptic strength exhibited a time course similar to that of the DSI modulation of spike-evoked Ca2+ signaling, suggesting that the modulation of spike-evoked Ca2+ could underlie the enhanced synaptic strength.
Modulation of C2 spike-evoked Ca2+ signaling depended on DSI spike frequency
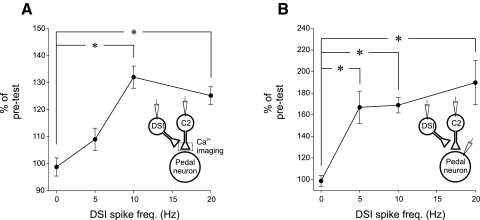
We next examined the relationship between the frequency of the DSI spike train and the extent of the modulation of C2 spike-evoked Ca2+ signaling by stimulating DSI to fire for 5 s at varying spike frequencies (5, 10, 20 Hz; Fig. 5A). For each trial, C2 was first stimulated alone (without DSI stimulation), and then C2 was stimulated 2 s after the DSI train. For a control, C2 was stimulated twice alone (DSI 0 Hz). DSI stimulation at 10 and 20 Hz significantly increased the amplitude of C2 spike-evoked Ca2+ signals (Fig. 5A). The modulatory effect of DSI seemed to saturate at 10 Hz; doubling the number of spikes and the frequency to 20 Hz did not increase the modulatory effect on the C2 spike-evoked Ca2+ signals (Fig. 5A). Thus the effect of DSI stimulation on C2 spike-evoked Ca2+ signaling is maximal at relatively low DSI spike frequencies well within the natural spike frequency range achieved during the swim motor pattern.
FIG. 5.
The effect of DSI spike frequency on C2 spike-evoked Ca2+ signaling and synaptic strength. A: calcium signals in C2 as a function of DSI spike frequency. DSI was stimulated for 5 s at various spike frequencies. Ca2+ signals are expressed as percentage pretest. *, significant differences from C2 stimulated with no DSI DSI stimulation (0 Hz) with a Friedman repeated-measures ANOVA on ranks (performed using normalized data, with the factor being the level of DSI stimulation) with post hoc pair-wise comparisons (Student-Newman-Keuls, n = 8). B: C2-evoked EPSPs in postsynaptic pedal neurons plotted as a function of DSI stimulation. *, significant differences with a 1-way repeated-measures ANOVA [performed using normalized data, with the factor being the level of DSI stimulation (0, 5, 10, 20 Hz) with post hoc pair-wise comparisons (Student-Newman-Keuls, F = 15.515, df between subjects = 7, df between treatments = 3, n = 8)].
To examine whether the modulation of C2 synaptic output showed a similar dependence on DSI spike frequency, we used the same protocol as above and measured the effects on the EPSPs recorded in postsynaptic pedal neurons. We found that stimulating DSI to fire at 5, 10, and 20 Hz all significantly increased the amplitude of C2-evoked EPSPs (Fig. 5B). This differs somewhat from the change in Ca2+ signals, which were not significantly different at 5-Hz DSI stimulation.
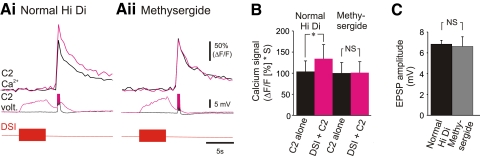
Methysergide blocked the enhancing effects of DSI on C2 spike-evoked Ca2+ signals
It was previously shown that the serotonin receptor antagonist methysergide blocks the DSI potentiation of C2 synaptic strength, but does not block DSI rapid synaptic actions, suggesting that they are mediated by different serotonin receptors (Katz and Frost 1995a). We tested whether methysergide would also block DSI modulation of C2 spike-evoked Ca2+ signals. Bath application of methysergide (50 μM) did not alter the baseline amplitude of C2 spike-evoked Ca2+ signals when C2 was stimulated alone, but it blocked the enhancement of C2 spike-evoked Ca2+ signals by DSI (Fig. 6, A and B). Further, methysergide did not affect the DSI-evoked depolarization of C2 (Fig. 6C). These data suggest that the DSI enhancement of C2 spike-evoked Ca2+ is mediated by serotonin released from DSI acting through metabotropic serotonin receptors.
FIG. 6.
Methysergide blocked DSI enhancement of C2 spike-evoked Ca2+ signals. A: a DSI spike train (10 Hz, 5 s) enhanced C2 spike-evoked Ca2+ signals in the distal neurites (Ai; black trace, C2 alone; magenta trace, C2 stimulation 2 s following the DSI spike train). C2 was stimulated to fire 5 spikes at 10 Hz. Corresponding C2 and DSI voltage traces are shown below the C2 Ca2+ signals; spikes were truncated to highlight the depolarization of C2 caused by the DSI spike train. In the presence of the serotonin receptor antagonist methysergide (50 μM), DSI stimulation no longer enhanced C2 spike-evoked Ca2+ signals (Aii; black trace, C2 stimulation alone; magenta trace, C2 stimulation following the DSI spike train). However, methysergide did not block the DSI-evoked depolarization. B: averaged data showing that in normal Hi Di saline the C2 spike-evoked Ca2+ signals were significantly larger following the DSI spike train; however, in methysergide, there was no significant difference between the Ca2+ signals evoked by C2 stimulation alone or following the DSI spike train {2-way repeated-measures ANOVA (performed using the raw data, with one factor being whether or not DSI was stimulated, and the other factor being Hi Di versus methysergide in Hi Di) with post hoc pair-wise comparisons [Student-Newman-Keuls, F = 8.075 (DSI stimulation. × methysergide), DF between subjects = 4, n = 5]}. Methysergide did not significantly affect the amplitude of the Ca2+ signals evoked by C2 stimulation alone. C: methysergide did not significantly affect the amplitude of the DSI-evoked depolarization of C2 [P = 0.82, paired t-test (mean amplitude in normal Hi Di = 6.84 ± 0.37 mV, mean amplitude in methysergide = 6.62 ± 0.92 mV), n = 5].
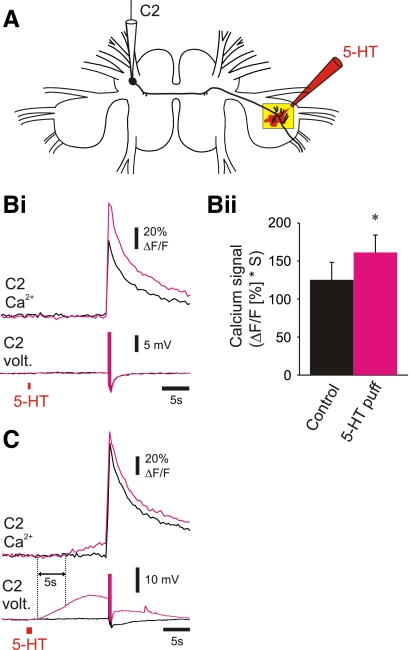
Pressure-applied puffs of 5-HT partially mimicked the effect of DSI stimulation
We found that brief puffs of 5-HT could mimic the effects of DSI stimulation of C2 spike-evoked Ca2+. A micropipette containing serotonin (1 mM) was positioned near the ventral surface of the pedal ganglion (Fig. 7A). Pressure application of 5-HT (10 psi, 500 ms) mimicked the effect of DSI stimulation by increasing the amplitude of C2 spike-evoked Ca2+ signals without directly causing a Ca2+ signal (Fig. 7Bi). Similar results were observed in four preparations (Fig. 7Bii).
FIG. 7.
Pressure ejection of serotonin mimicked the neuromodulatory effects of DSI but also had additional actions. A: experimental setup: imaging from the distal neurites of C2 with an electrode in the soma of C2 and a puff electrode filled with 10−3 M serotonin positioned just above the ventral surface of the pedal ganglion (illustration of a serotonin puff shown in red). Bi: a puff of serotonin (10 psi, 500 ms, red bar below the C2 voltage traces) given 15 s before the C2 spikes (5 spikes at 10 Hz) mimicked the modulatory effects of DSI on C2 spike-evoked Ca2+ signaling (Bi; black trace, C2 alone; magenta trace, C2 stimulation following the serotonin puff; corresponding C2 voltage traces are shown below the C2 Ca2+ signals; C2's spikes were truncated to highlight the depolarization caused by the serotonin). Bii: averaged data for serotonin puffs given 15 s prior to the C2 spikes in which the serotonin puff did not change resting Ca2+ levels in C2. In these preparations, serotonin significantly enhanced the C2 spike-evoked Ca2+ signals [P < 0.05, paired t-test (mean before serotonin puff = 125.27 ± 23.02, mean after serotonin puff = 161.25 ± 22.96), n = 4], thereby mimicking the actions of synaptically released serotonin. C: in a different preparation from Bi, doubling the pressure and duration of the serotonin puff (20 psi, 1 s) caused a depolarization in the C2 membrane potential recorded in this soma and an increase in basal Ca2+ in C2's distal neurites and increased the size of spike-evoked Ca2+ signals (black trace, C2 alone; magenta trace, C2 stimulation following the serotonin puff; corresponding C2 voltage traces are shown below the C2 Ca2+ signals; C2's spikes were truncated to highlight the depolarization of C2 caused by the serotonin puff). Note that the depolarization of C2 caused by the serotonin puff preceded the increase in C2 basal Ca2+ by ∼5 s (dashed lines at the onset of the C2 membrane potential depolarization and at the onset of the increase in C2 basal Ca2+).
Increasing the amount of 5-HT released from the pipette produced additional effects on Ca2+ signals in the C2 distal neurites that did not mimic the effect of DSI stimulation. Longer-duration 5-HT puffs (20 psi, 1 s) directly caused a large membrane potential depolarization (recorded in the C2 soma) and a slowly rising baseline Ca2+ signal (Fig. 7C). Similar results were observed in six preparations. The C2 membrane potential depolarization preceded the onset of the Ca2+ signal by ∼5 s, suggesting either that the depolarization itself did not cause the Ca2+ signal or that there was a voltage threshold for evoking Ca2+ signals. Thus although a serotonin puff applied to the ventral surface of the pedal ganglion could mimic the effects of synaptically released serotonin, we found that increasing the amount of serotonin applied could also result in actions that were never observed with DSI stimulation. Similarly, bath-applied serotonin produced variable results on spike-evoked Ca2+ signals in C2, as well as changes in baseline Ca2+ (data not shown).
DISCUSSION
In Tritonia, stimulation of a serotonergic swim interneuron (DSI) at physiological spike rates heterosynaptically enhances the synaptic output from C2, another swim interneuron (Katz and Frost 1995a,b; Katz et al. 1994). Here we found that DSI stimulation also enhanced spike-evoked Ca2+ signals in C2. Although modulation of presynaptic Ca2+ is a likely mechanism for mediating heterosynaptic modulation of transmitter release, it has been demonstrated only in cultured neurons, where stimulation of a peptidergic interneuron reduces spike-evoked Ca2+ transients (Richmond et al. 1991). To our knowledge, the present results are the first demonstration that stimulation of an interneuron can alter spike-evoked Ca2+ signals in another interneuron in situ.
DSI enhancement of C2 spike-evoked Ca2+ signals may underlie the DSI potentiation of C2 synaptic strength
Several lines of evidence suggest that the DSI enhancement of spike-evoked Ca2+ signals in C2 may underlie the DSI potentiation of C2 to pedal follower cell synapses. 1) The restriction of the enhancement of spike-evoked Ca2+ to C2's distal neurites corresponds with the location of the synapses being modulated. 2) The time course of neuromodulatory action of DSI on C2 spike-evoked Ca2+ signaling corresponded well with the time course of the enhancement of C2 synaptic strength; both effects took more than two seconds to reach their maxima and both decayed within 10 s after the end of the DSI spike train. 3) Both actions were independent of the DSI-evoked depolarization of C2's distal neurites. 4) Both actions were blocked by the serotonin receptor antagonist methysergide. Taken together, these data support the model that release of serotonin from DSI enhances spike-evoked Ca2+ signals in C2, contributing to heterosynaptic facilitation of transmitter release.
There was some disparity between the effect of DSI on presynaptic Ca2+ signals and synaptic strength. For example, DSI increased C2 synaptic output proportionally more than it did C2 spike-evoked Ca2+ signals. This could be accounted for by the observation that the relationship between the magnitude of Ca2+ signals and neurotransmitter release is nonlinear (Sabatini and Regehr 1995; Sabria et al. 1995), such that even modest changes in presynaptic Ca2+ can lead to large changes in neurotransmitter release (Sabatini and Regehr 1997). Therefore the relatively small enhancement of C2 spike-evoked Ca2+ signals could underlie the larger potentiation of C2 synaptic strength by DSI. Another explanation for the disparity could be that our imaging methodology did not detect small or highly localized changes in spike-evoked Ca2+ signals in C2. For instance, it is conceivable that DSI stimulation at 5 Hz did enhance C2 Ca2+ signals in highly restricted microdomains, which would have led to the increase in synaptic output from C2. This issue could be addressed by imaging with a higher powered objective, however, in this study, we found it necessary to use a lower power (×5) objective to simultaneously impale both C2 and DSI with intracellular microelectrodes.
Although the enhancement of C2 spike-evoked Ca2+ signal and the enhancement of C2 synaptic strength were both dependent on the rate DSI stimulation, we found that the thresholds eliciting a statistically significant change differed. We found that a 5-Hz DSI spike train caused a significant increase in EPSP amplitude but not in spike-evoked Ca2+ signals. Once again, this discrepancy could be attributed to either methodological detection limits or the nonlinearity of the relationship between Ca2+ and synaptic release.
The discrepancies between the increase Ca2+ signal and the increase in EPSP magnitude do not disprove the hypothesis that the increase in Ca2+ causes the increase in synaptic output. However, the observations that support the hypothesis show correlation rather than causation. It is difficult to test a causal relationship because of the necessity of Ca2+ in both C2 basal release and in release of 5-HT from DSI.
DSI stimulation did not directly increase Ca2+ in C2
DSI stimulation did not directly produce a detectable Ca2+ signal in C2 but rather only affected Ca2+ signals produced by C2 action potentials. Thus the effect of DSI is conditional on the production of an action potential in C2. Because the effect of DSI on C2 is transient, the modulatory action is only expressed when C2 fires an action potential within 10 s of the end of the DSI spike train.
Serotonin puffs could mimic this conditional effect of DSI stimulation; they could produce an increase in C2 spike-evoked Ca2+ signals without affecting basal Ca2+. However, larger puffs of serotonin, in addition to enhancing spike-evoked Ca2+, also directly caused a Ca2+ signal in the C2 distal neurites, something never observed with DSI stimulation. Presumably, this discrepancy is due to the difference in the amount of serotonin reaching C2 and possibly the extra-synaptic location of the serotonin. We interpret the effect of 5-HT puffs on basal Ca2+ as nonphysiological because they don't correspond to the actions of the DSIs. Without prior knowledge of the modulatory effects of synaptically released serotonin on C2 Ca2+ signaling, we would not have known which of the results represented the physiological actions of 5-HT on C2 Ca2+ signaling.
In Helix, stimulation of a serotonergic neuron increases basal intracellular Ca2+ levels in another interneuron (Balaban et al. 2004). In other systems serotonin and other neurotransmitters have been shown to cause elevation of basal Ca2+ levels, such as leech Retzius neurons (Beck et al. 2002). Activation of nicotinic acetylcholine receptors directly causes a Ca2+ influx and indirectly leads to Ca2+ influx through voltage-gated Ca2+ channels (Girod et al. 2000). Ca2+ from both of these sources can be sufficient to induce presynaptic facilitation. It would be of interest to know in these other systems if the effect of the exogenous neurotransmitter on basal Ca2+ is reflective of the action of the neurons that would release them or if any of these actions represent nonphysiological effects.
Possible mechanisms by which DSI enhances C2 spike-evoked Ca2+ signaling
Subthreshold depolarization can lead to Ca2+ influx and an enhancement of neurotransmitter release (Shapiro et al. 1980; Turecek and Trussell 2001). However, our data, and those of Katz and Frost (1995b) suggest that this is not the mechanism by which DSI enhances C2 distal neurite spike-evoked Ca2+ signals or transmitter release. Methysergide blocked the neuromodulatory effects of DSI on C2 spike-evoked Ca2+ signaling but did not reduce the depolarization of C2 membrane potential.
Spike-broadening has been implicated in mediating increases in synaptic strength. In Manduca, serotonin inhibits K+ channels, leading to spike-broadening and presumably greater influx of Ca2+ per spike (Mercer et al. 1996). In Aplysia, serotonin-induced increases in spike width correlate well with increased somatic Ca2+ influx, suggesting that longer-duration action potentials in synaptic terminals could contribute to greater Ca2+ influx and hence to potentiation of synaptic strength (Eliot et al. 1993). We did not observe spike-broadening in our recordings of C2 in response to DSI stimulation, consistent with the lack of a change in spike-evoked Ca2+ signals in the proximal neurites. We were unable to reliably record from C2's distal neurites to observe whether the increase in spike-evoked Ca2+ correlated with spike-broadening in those parts of the neuron that are electronically distant from the soma. Thus spike broadening in the distal neurites of C2 remains as a possible mechanism for the modulatory effects of DSI on C2 Ca2+ signaling.
Alternatively, serotonin might enhance spike-evoked Ca2+ signaling in C2 distal neurites by enhancing Ca2+ entry through voltage-gated Ca2+ channels. In spinal motoneurons, serotonin alters the voltage-sensitivity of L-type Ca2+ channels (Perrier et al. 2002). In Aplysia, serotonin increases sensory neuron voltage-dependent Ca2+ currents (Braha et al. 1993) as well as Ca2+ currents during action potentials (Yu et al. 2001). In Helix, serotonin inhibits Ca2+ currents in some neurons while potentiating it in others (Kostyuk et al. 1992).
Serotonin might also affect Ca2+-induced-Ca2+-release (CICR) from C2 internal stores. At Schaffer collateral-CA1 synapses, CICR from presynaptic Ca2+ stores contributes to nicotine-induced increases in evoked transmitter release (Le Magueresse and Cherubini 2007). Similarly, at basket cell to Purkinje cell synapses, Ca2+ released from presynaptic Ca2+ stores enhances the amplitude of evoked synaptic currents (Galante and Marty 2003). Serotonergic potentiation of neurotransmitter release at the crayfish neuromuscular junction is also modulated by Ca2+ released from internal stores (Dropic et al. 2005). Recent evidence at another Tritonia synapse has shown that Ca2+ release from internal stores is necessary for serotonergic potentiation of synaptic strength (Sakurai et al. 2007). Thus increased release of Ca2+ from C2 internal stores could lead to the enhanced spike-evoked Ca2+ signals in the distal neurites of C2 that we observed following DSI stimulation.
Functional significance for the Tritonia swim CPG
The function of the DSI enhancement of C2 Ca2+ signaling and on C2 synaptic output are likely intimately involved with the generation of the rhythmic motor output of the Tritonia swim CPG. C2 and DSI are both members of the escape swim CPG and are both rhythmically active during the swim motor pattern, with a period of 7–10 s. Furthermore, previous results suggest that such modulatory actions are necessary for switching the network into a rhythmic state (Calin-Jageman et al. 2007). The results in this study suggest that DSI activity during the swim motor pattern would enhance spike-evoked Ca2+ signaling in the distal neurites of C2 on a cycle by cycle basis.
The lack of a modulatory effect of DSI in the proximal neurites suggests that there is a functional segregation in C2. We predict that if C2 has output synapses in the cerebral ganglion, they would not be modulated by DSI. Furthermore, the results suggest that the synaptic integration zone for the swim CPG is not located in the cerebral ganglia but rather in the pedal ganglia.
Our results demonstrate that a serotonergic interneuron dynamically and locally enhances spike-evoked Ca2+ signaling in another interneuron. The characteristics of this neuromodulatory action implicate it as a potential mechanism mediating heterosynaptic facilitation of transmitter release, which is likely to be of short-term functional relevance to operation of the neural circuit.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke grant NS-035371 to P. S. Katz.
Supplementary Material
Acknowledgments
We thank B. Neuhaus for technical assistance with the confocal microscope and J. Lillvis for important comments on this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Balaban et al. 2004.Balaban PM, Korshunova TA, Bravarenko NI. Postsynaptic calcium contributes to reinforcement in a three-neuron network exhibiting associative plasticity. Eur J Neurosci 19: 227–233, 2004. [DOI] [PubMed] [Google Scholar]
- Bayliss et al. 1997.Bayliss DA, Li Y-W, Talley EM. Effects of serotonin on caudal raphe neurons: inhibition of N- and P/Q-type calcium channels and the afterhyperpolarization. J Neurophysiol 77: 1362–1374, 1997. [DOI] [PubMed] [Google Scholar]
- Beck et al. 2002.Beck A, Lohr C, Berthold H, Deitmer JW. Calcium influx into dendrites of the leech Retzius neuron evoked by 5-hydroxytryptamine. Cell Calcium 31: 137–149, 2002. [DOI] [PubMed] [Google Scholar]
- Blumenfeld et al. 1990.Blumenfeld H, Spira ME, Kandel ER, Siegelbaum SA. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in Aplysia sensory neurons. Neuron 5: 487–499, 1990. [DOI] [PubMed] [Google Scholar]
- Braha et al. 1993.Braha O, Edmonds B, Sacktor T, Kandel ER, Klein M. The contributions of protein kinase A and protein kinase C to the actions of 5-HT on the L-type Ca2+ current of the sensory neurons in Aplysia. J Neurosci 13: 1839–1851, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin-Jageman et al. 2007.Calin-Jageman RJ, Tunstall MJ, Mensh BD, Katz PS, Frost WN. Parameter space analysis suggests multi-site plasticity contributes to motor pattern initiation in Tritonia. J Neurophysiol 98: 2382–2398, 2007. [DOI] [PubMed] [Google Scholar]
- Clemens and Katz 2001.Clemens S, Katz PS. Identified serotonergic neurons in the Tritonia swim CPG activate both ionotropic and metabotropic receptors. J Neurophysiol 85: 476–479, 2001. [DOI] [PubMed] [Google Scholar]
- Clemens and Katz 2003.Clemens S, Katz PS. G protein signaling in a neuronal network is necessary for rhythmic motor pattern production. J Neurophysiol 89: 762–772, 2003. [DOI] [PubMed] [Google Scholar]
- Diaz-Rios et al. 2007.Diaz-Rios M, Dombeck DA, Webb WW, Harris-Warrick RM. Serotonin modulates dendritic calcium influx in commissural interneurons in the mouse spinal locomotor network. J Neurophysiol 98: 2157–2167, 2007. [DOI] [PubMed] [Google Scholar]
- Dropic et al. 2005.Dropic AJ, Brailoiu E, Cooper RL. Presynaptic mechanism of action induced by 5-HT in nerve terminals: possible involvement of ryanodine and IP3 sensitive Ca2+ stores. Comp Biochem Physiol A 142: 355–361, 2005. [DOI] [PubMed] [Google Scholar]
- Eliot et al. 1993.Eliot LS, Kandel ER, Siegelbaum SA, Blumenfeld H. Imaging terminals of Aplysia sensory neurons demonstrates role of enhanced Ca2+ influx in presynaptic facilitation. Nature 361: 634–637, 1993. [DOI] [PubMed] [Google Scholar]
- El Manira and Wallen 2000.El Manira A, Wallen P. Mechanisms of modulation of a neural network. NIPS 15: 186–191, 2000. [DOI] [PubMed] [Google Scholar]
- Foehring 1996.Foehring RC Serotonin modulates N- and P-type calcium currents in neocortical pyramidal neurons via a membrane-delimited pathway. J Neurophysiol 75: 648–659, 1996. [DOI] [PubMed] [Google Scholar]
- Galante and Marty 2003.Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci 23: 11229–11234, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting 1981.Getting PA Mechanisms of pattern generation underlying swimming in Tritonia. I. Neuronal network formed by monosynaptic connections. J Neurophysiol 46: 65–79, 1981. [DOI] [PubMed] [Google Scholar]
- Getting 1983.Getting PA Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol 49: 1036–1050, 1983. [DOI] [PubMed] [Google Scholar]
- Getting et al. 1980.Getting PA, Lennard PR, Hume RI. Central pattern generator mediating swimming in Tritonia. I. Identification and synaptic interactions. J Neurophysiol 44: 151–164, 1980. [DOI] [PubMed] [Google Scholar]
- Girod et al. 2000.Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology 39: 2715–2725, 2000. [DOI] [PubMed] [Google Scholar]
- Hill and Katz 2006.Hill ES, Katz PS. Heterosynaptic enhancement of spike-evoked Ca2+ signals by a serotonergic interneuron. Soc Neurosci Abst 32: 350.3, 2006. [Google Scholar]
- Hill and Katz 2007.Hill ES, Katz PS. Role of membrane potential in calcium signaling during rhythmic bursting in Tritonia swim interneurons. J Neurophysiol 97: 2204–2214, 2007. [DOI] [PubMed] [Google Scholar]
- Katz 2007.Katz PS Tritonia. Scholarpedia 2: 3504, 2007. [Google Scholar]
- Katz and Frost 1995a.Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol 74: 2281–2294, 1995a. [DOI] [PubMed] [Google Scholar]
- Katz and Frost 1995b.Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: The serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci 15: 6035–6045, 1995b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz et al. 1994.Katz PS, Getting PA, Frost WN. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature 367: 729–731, 1994. [DOI] [PubMed] [Google Scholar]
- Kostyuk et al. 1992.Kostyuk PG, Lukyanetz EA, Doroshenko PA. Effects of serotonin and cAMP on calcium currents in different neurons of Helix pomatia. Pfluegers Arch 420: 9–15, 1992. [DOI] [PubMed] [Google Scholar]
- Ladewig et al. 2004.Ladewig T, Lalley PM, Keller BU. Serotonergic modulation of intracellular calcium dynamics in neonatal hypoglossal motoneurons from mouse. Brain Res 1001: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- Le Magueresse and Cherubini 2007.Le Magueresse C, Cherubini E. Presynaptic calcium stores contribute to nicotine-elicited potentiation of evoked synaptic transmission at CA3-CA1 connections in the neonatal rat hippocampus. Hippocampus 17: 316–325, 2007. [DOI] [PubMed] [Google Scholar]
- Leonard et al. 2000.Leonard CS, Rao SR, Inoue T. Serotonergic inhibition of action potential evoked calcium transients in NOS-containing mesopontine cholinergic neurons. J Neurophysiol 84: 1558–1572, 2000. [DOI] [PubMed] [Google Scholar]
- Mercer et al. 1996.Mercer AR, Kloppenburg P, Hildebrand JG. Serotonin-induced changes in the excitability of cultured antennal-lobe neurons of the sphinx moth Manduca sexta. J Comp Physiol [A] 178: 21–31, 1996. [DOI] [PubMed] [Google Scholar]
- Perrier et al. 2002.Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev 40: 223–229, 2002. [DOI] [PubMed] [Google Scholar]
- Rhee et al. 1996.Rhee JS, Ishibashi H, Akaike N. Serotonin modulates high-voltage-activated Ca2+ channels in rat ventromedial hypothalamic neurons. Neuropharmacology 35: 1093–1100, 1996. [DOI] [PubMed] [Google Scholar]
- Richmond et al. 1991.Richmond JE, Funte LR, Smith WL, Price DA, Haydon PG. Activation of a peptidergic synapse locally modulates postsynaptic calcium influx. J Exp Biol 161: 257–271, 1991. [DOI] [PubMed] [Google Scholar]
- Sabatini and Regehr 1995.Sabatini BL, Regehr WG. Detecting changes in calcium influx which contribute to synaptic modulation in mammalian brain slice. Neuropharmacology 34: 1453–1467, 1995. [DOI] [PubMed] [Google Scholar]
- Sabatini and Regehr 1997.Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci 17: 3425–3435, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabria et al. 1995.Sabria J, Pastor C, Clos MV, Garcia A, Badia A. Involvement of different types of voltage-sensitive calcium channels in the presynaptic regulation of noradrenaline release in rat brain cortex and hippocampus. J Neurochem 64: 2567–71, 1995. [DOI] [PubMed] [Google Scholar]
- Sakurai et al. 2007.Sakurai A, Calin-Jageman RJ, Katz PS. The potentiation phase of spike timing-dependent neuromodulation by a serotonergic interneuron involves an increase in the fraction of transmitter release. J Neurophysiol 98: 1975–1987, 2007. [DOI] [PubMed] [Google Scholar]
- Sakurai and Katz 2003.Sakurai A, Katz PS. Spike timing-dependent serotonergic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. J Neurosci 23: 10745–10755, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro et al. 1980.Shapiro E, Castellucci VF, Kandel ER. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci USA 77: 629–633, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghert and Willows 1978.Taghert PH, Willows AOD. Control of a fixed action pattern by single, central neurons in the marine mollusk, Tritonia diomedea. J Comp Physiol 123: 253–259, 1978. [Google Scholar]
- Turecek and Trussell 2001.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature 411: 587–590, 2001. [DOI] [PubMed] [Google Scholar]
- Wu and Saggau 1997.Wu L-G, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci 20: 204–212, 1997. [DOI] [PubMed] [Google Scholar]
- Yu et al. 2001.Yu B, Gamkrelidze GN, Laurienti PJ, Blankenship JE. Serotonin directly increases a calcium current in swim motoneurons of Aplysia brasiliana. Am Zool 41: 1009–1025, 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.