Abstract
The purpose of the study was to examine the influence of practice on time to failure of a submaximal contraction with the elbow flexor muscles and on reflex inhibition from brachioradialis afferents onto biceps brachii motor neurons. Fifteen subjects practiced sustaining an isometric contraction (20% of maximum) with the elbow flexors until failure. Spike-triggered stimulation was used to assess the influence of radial nerve stimulation on the discharge of single motor units in biceps brachii before and after three practice sessions. Time to failure increased from 760 ± 333 s in session 1 to 1,103 ± 415 s in session 3 (P < 0.03) and was accompanied by a slower rate of increase in electromyographic (EMG) activity of the short head of biceps brachii (P < 0.05). Stimulation of the radial nerve prolonged the interspike interval before practice (n = 56; 7.2 ± 6.8 ms; P < 0.001), and this effect was reduced after practice (n = 62; 2.3 ± 3.6 ms; P < 0.01). The reduction was greater for motor units in the short head of biceps brachii than for those in the long head (P < 0.05) and was associated with a slower rate of increase in EMG (r = 0.57, P = 0.03). The decrease in reflex inhibition was the main predictor of the increase in time to failure (r2 = 0.60, P = 0.001). These results demonstrate that practice reduced an antagonistic inhibition and improved the ability of the muscles to perform a synergistic action of elbow flexion.
INTRODUCTION
The plasticity of the nervous system in response to increases and decreases in physical activity has been well documented in healthy and diseased individuals (Aagaard 2003; Semmler et al. 2000; Wolpaw and Tennissen 2001). Practice-related adaptations in the nervous system can include changes in corticospinal excitability (Jensen et al. 2005; Perez et al. 2007), somatosensory feedback to the motor neurons (Geertsen et al. 2007; Zehr 2006), and the output of the motor neuron pool to muscle (Del Balso and Cafarelli 2007; Duchateau et al. 2006). The sites of plasticity in the nervous system are specific to the task performed and can differ, for instance, between motor skill training and strength training (Jensen et al. 2005; Semmler and Nordstrom 1998). Furthermore, practice of a task evokes both short- and long-term adaptations in the nervous system that can differ among individuals (Duchateau and Enoka 2002; Zehr 2006).
A previous study on practice of a submaximal isometric contraction sustained until failure with the elbow flexors demonstrated that some individuals were able to increase time to failure (responders) across three sessions, whereas others experienced no change in the time to failure (Hunter and Enoka 2003). The 60% increase in time to failure achieved by the responders was accompanied by adaptations in the motor output to the muscles. For example, reductions in the rates of increase in the coefficient of variation for force and the amplitude of the surface EMG and less bursting in the surface EMG indicated a delayed recruitment of motor units after practice (Hunter et al. 2002; Riley et al. 2008b). The more gradual change in motor output during the fatiguing contractions suggested that three practice sessions were sufficient to change the excitatory and inhibitory inputs received by the involved motor neuron pools.
One pathway that might contribute to the prolongation of time to failure is an inhibitory reflex from the branch of the radial nerve that innervates brachioradialis onto biceps brachii motor neurons (Naito et al. 1996; Barry et al. 2008). Activation of this inhibitory pathway decreases the discharge rate of biceps brachii motor units during isometric contractions with the elbow flexor muscles. The strength of the inhibitory reflex onto biceps brachii motor neurons depends on the position of the forearm with greater inhibition when the forearm is neutral compared with supinated (Barry et al. 2008), which might explain why the time to failure for a sustained elbow flexor contraction is longer when the forearm is in a supinated position than when it is in a neutral position (Rudroff et al. 2005, 2007). A suppression of this inhibitory pathway with practice might attenuate the decline in the discharge rate of motor units, delay the recruitment of new motor units, and prolong the time to task failure. The purpose of the present study was to determine the influence of practice on the time to failure of a submaximal contraction with the elbow flexor muscles and on reflex inhibition from brachioradialis afferents onto biceps brachii motor neurons.
METHODS
Fifteen healthy adults (7 men, 8 women; 21.9 ± 3.5 yr; range, 18–28 yr) participated in the study. All subjects reported being free of neurological disorders. None of the subjects had performed a strength-training program with the upper body in the six months preceding the study, and only 2 of the 15 subjects had reported any previous strength-training experience. The Human Research Committee at the University of Colorado in Boulder approved the procedures and the experiments were performed in accordance with the declaration of Helsinki. All subjects gave written informed consent prior to participating in the study.
Each participant completed five experimental sessions, and each session was separated by ≥72 h. The first and last sessions involved reflex testing, and the middle three sessions were used to practice the fatiguing contraction. Single motor units were recorded from either head of biceps brachii during the reflex testing sessions as the subject performed low-force contractions to match a target force that was displayed on a monitor. The subject received visual and auditory feedback of motor-unit discharge, and two to five motor units were recorded in each session. The practice sessions involved the subject performing a single fatiguing contraction to failure at 20% of maximal voluntary contraction (MVC) force.
Experimental setup
Subjects were seated upright in a chair with the upper arm vertical and slightly abducted from the trunk and the elbow resting on a support. The elbow was flexed to 1.57 rad with the forearm horizontal and in a neutral position (midway between pronation and supination). The hand and forearm were secured with a modified wrist-hand orthosis (Orthomerica; Newport Beach, CA) and the force exerted by the elbow flexor muscles was measured with a force-moment sensor (JR-3, 900-N range, 89.4 N/V; JR-3, Woodland, CA). The transducer was attached to the orthosis at the level of the wrist. Subjects were instructed to exert a force with the elbow flexor muscles and to minimize the involvement of other muscles. The force was displayed on a 17-in monitor that was located at eye level ∼1.2 m in front of the subject.
Single motor-unit recordings
Single motor-unit potentials were recorded from the long and short heads of biceps brachii during the reflex testing sessions using stainless-steel wires (50 μm diam, California Fine Wire, GroverBeach, CA) that were insulated with Formvar and glued together at the recording tips. The insulation was only absent from the recording tip of each wire, and two or three wires were included in each electrode. The wires were inserted into the muscle belly using a 27- or 30-gauge hypodermic needle that was removed after the wires were in place. Two electrodes were inserted at the beginning of each reflex testing session, and an additional electrode was inserted if one of the electrodes failed to record a distinct motor-unit potential. The electrode was repositioned occasionally to improve the quality of the signal. A reference electrode was placed on the skin over the lateral epicondyle. The single motor-unit recordings were amplified (1,000–5,000 times) and band-pass filtered between 0.3 and 8 kHz (Coulbourn Instruments, Allentown, PA). The motor-unit signal was sampled at 20 kHz with a Power1401 (CED), stored on a computer, and the single motor-unit potentials were later identified off-line using Spike2 software (v.5.16, CED, Cambridge, UK).
Surface EMG recordings
Surface EMG activity during the reflex testing sessions was recorded from the long head of biceps brachii, short head of biceps brachii, and lateral head of the triceps brachii muscles with pairs of bipolar electrodes (8-mm diam; silver-silver chloride) and from brachioradialis and extensor carpi radialis muscles with smaller electrodes (4-mm diam; silver-silver chloride). The activity of these muscles was also recorded during the fatiguing contractions performed in the practice sessions except that for extensor carpi radialis. Reference electrodes were placed over the acromion or lateral epicondyle of the same limb. The surface EMG signals were amplified (1,000 times) and band-pass filtered (13–1,000 Hz; Coulbourn Instruments, Allenstown, PA) prior to data acquisition.
Stimulation paradigm
The influence of radial nerve stimulation on the tonic discharge of motor units in biceps brachii during a voluntary contraction was assessed with spike-triggered stimulation during the reflex testing sessions (Nafati et al. 2005). A Grass S48 stimulator with constant-current and isolation units (CCU1 and SIU-5) was used to stimulate the radial nerve (0.5-ms rectangular pulse) through adhesive electrodes attached to the skin (Conmed, Utica, NY). The site of stimulation (∼4 cm proximal to the lateral epicondyle between the borders of biceps and triceps brachii) was determined by adjusting a probe electrode until a clear brachioradialis M wave was present in the surface electromyograph (EMG), and there was no response in extensor carpi radialis at intensities of 1.3 times motor threshold (MT) for brachioradialis. Eight control stimulus responses were recorded at 0.9, 1.0, and 1.3 times brachioradialis MT.
The task involved isolating a single motor unit that could be discriminated on-line (Time-Amplitude Window Discriminator, Bak Electronics, Mount Airy, MD) and used to trigger a stimulus pulse or control pulse randomly within an interval of 1.5–2 s. The stimulation was delivered 30 ms after a selected motor-unit discharge (Barry et al. 2008) at an intensity of 0.9 times brachioradialis MT, and an average of 77 ± 26 triggers were recorded for each motor-unit trial. Control pulses (no stimulation) were recorded to demonstrate that there was no effect due to differences in discharge rate.
Protocol
The practice sessions comprised three tasks: assessment of MVC force for the elbow flexors and extensors, a sustained contraction at 20% MVC force until the force declined <16% MVC force for >2 s, and an immediate measurement of MVC force for the elbow flexors. Verbal encouragement was provided throughout each practice session. Subjects were not informed of the times to failure until after the third session.
The reflex protocol was performed in sessions before and after the three practice sessions and comprised four tasks: identification of the stimulus site and measurement of control M waves, identification of a single motor unit that discharged at ∼10–11 pps during a low-force contraction, measurement of control M waves, and assessment of MVC force for the elbow flexors and extensors. The second task was usually repeated several times after moving the electrode or switching to another electrode to record the discharge of a different motor unit. Single motor units were identified by having the subject slowly increase and decrease force within a window of ∼10% of MVC force until one unit could be easily discriminated. The subject was then instructed to match a target force that ensured the discharge rate was within the prescribed range. Subjects rested for ∼5 min after each trial to minimize fatigue, and then the procedures were repeated with another motor unit.
At least two isometric MVCs in the flexion direction and one in the extension direction were performed in each of the five sessions. The subjects performed the MVCs by increasing force from zero to maximum over 3 s and then holding it for 3 s. Subjects rested for 90–120 s between trials. If the MVC forces for the two elbow flexor trials were within 5% of each other, the two values were averaged, recorded as the maximum, and used as a reference for the sustained contraction. Otherwise, additional MVC trials were performed until the 5% criterion was achieved.
Data analysis
Surface EMG signals were rectified and normalized to a 0.5-s epoch from the peak EMG during the MVC before the sustained contraction for each muscle. The average EMG (aEMG) values were determined for each 1% of time to failure. The coefficient of variation for force was also measured in 1% intervals of time to failure. The rates of change in aEMG and CV for force were quantified by the coefficient (ea) of an exponential fit to the data.
The rate of bursting activity in each of the surface EMG signals was measured during the practice sessions for each one-third of time to failure. This involved using an algorithm developed by Hunter et al. (2002) and validated by Riley et al. (2008b) to detect the transient recruitment of motor units from the surface EMG. The steps included: rectification of the signals, low-pass filtering at 2 Hz, differentiation, and normalization to the average EMG amplitude of the entire signal. The threshold for the beginning and end of each burst of activity was defined as the point in time when the smoothed and differentiated surface EMG signal exceeded 2.5 SD above the mean that had been calculated from 25 samples (12.5 ms) at the beginning of the contraction. The minimal time between two consecutive bursts was constrained to 2 s, and the minimal burst duration was 0.5 s.
Single motor-unit potentials for each reflex-testing trial were discriminated off-line using the template-matching feature of Spike2. Mean discharge rate and the CV for interspike interval (ISI) were calculated from the epoch of activity during which the stimuli were delivered. Mean force was also calculated from the same region. The three ISIs prior to stimulation were averaged (ISI-pre) and the ISI that included the stimulation (ISI-0) was measured. The difference between the two values was used to indicate the strength of the reflex inhibition. In addition, the four ISIs immediately after the ISI that included the stimulation (ISI +1 to ISI +4) were recorded to assess the time course of the afferent volley. The ISIs were averaged across the total number of stimuli within a single motor-unit trial. The same procedure was applied to the control pulses.
The influence of stimulus intensity on muscle activation was measured by averaging the EMG from the brachioradialis and extensor carpi radialis muscles after stimulation. The peak-to-peak amplitude of the brachioradialis M wave in response to the 1.3 times motor threshold intensity was recorded before and at the end of each reflex-testing session to ensure that stimulation conditions did not change. The waveform was also averaged during the motor-unit trials to ensure there was no motor response to the stimulation during the low-force contractions.
Statistics
One-way ANOVAs (session) were used to compare the initial MVC force, time to failure, final MVC force, and the rate of increase in CV for force across the three practice sessions. The rate of increase in aEMG for each muscle was compared across practice sessions with a two-factor, repeated-measures ANOVA (session × muscle), and the surface EMG burst rates (bursts/min) for each muscle were examined with a three-factor, repeated-measures ANOVA (session × time × muscle). Burst rate from the practice sessions was analyzed at absolute times for each session and relative to the duration of the shortest session for each individual. The first session had the shortest duration for three subjects, and the second session was the shortest for the other subjects.
The differences between the ISI that included the stimulation (ISI-0) and the average of the 3 ISIs before stimulation (ISI-pre) were compared for each stimulus condition and before and after practice with a paired-samples t-test. The ISI difference was also used in a three-factor ANOVA (stimulation × muscle × session), with repeated measures, to assess stimulation and control conditions, the differences between long and short heads of biceps brachii, and the influence of practice. A three-factor, repeated-measures ANOVA (stimulation × time × session) was also used to examine the time course of changes in ISI (ISI-pre –ISI +4) for stimulation and control conditions.
A Pearson correlation was used to examine the associations between time to failure (% change), the change in the rate of increase in aEMG, and the change in reflex inhibition (ms) for each biceps brachii muscle. A multiple-stepwise, linear regression model was used to identify predictors (CV for force, aEMG, burst rate, reflex inhibition) of the change in time to failure.
Tukey post hoc tests were used to identify differences when appropriate. A statistical level of P < 0.05 was used to identify significant differences. All statistical analyses were performed in SPSS v.15 and v.16 (Chicago, IL). Data are presented in the text as means ± SD and in figures as means ± SE.
RESULTS
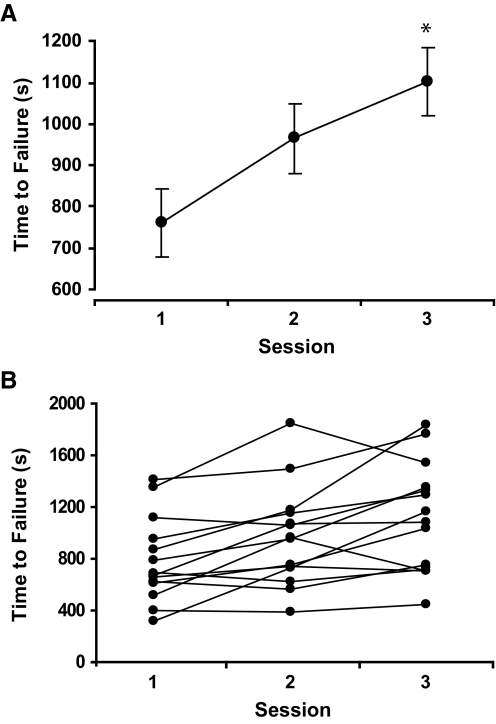
Time to failure for the practice sessions increased from 760 ± 333 s in session 1 to 1,103 ± 415 s in session 3 (P < 0.03, Fig. 1A). All subjects increased time to failure, ranging from 3.3 to 264.2% (mean: 57.5 ± 67.5%). MVC force for all subjects was similar at the beginning of each practice session (P = 0.9, Table 1). In addition, the decline in MVC force was similar across all three sessions (P = 0.9) and was significantly reduced by 29.1 ± 13.7% MVC force at the end of the fatiguing contractions (P < 0.01).
FIG. 1.
Increase in time to failure for all subjects (A) and each subject (B) from session 1 to session 3 (P < 0.03). Data are presented as the means ± SE.
TABLE 1.
Force and EMG measurements during the practice sessions
| Session 1 | Session 2 | Session 3 | |
|---|---|---|---|
| Time to failure, s | 759 ± 333 | 964 ± 384 | 1102 ± 415 |
| MVC force, N | |||
| Before | 201 ± 54 | 203 ± 38 | 204 ± 41 |
| After | 144 ± 37 | 141 ± 32 | 146 ± 33 |
| Burst Rate (bursts/min) | |||
| Brachioradialis | |||
| Last third | 3.5 ± 3.1 | 3.5 ± 4.1 | 3.7 ± 3.1 |
| Relative to shortest session | 3.6 ± 3.2 | 3.1 ± 2.4 | 3.0 ± 1.8 |
| Biceps Brachii Long Head | |||
| Last third | 3.8 ± 2.4 | 4.2 ± 2.8 | 4.4 ± 3.0 |
| Relative to shortest session | 3.7 ± 2.3 | 3.7 ± 2.5 | 3.0 ± 2.4 |
| Biceps Brachii Short Head | |||
| Last third | 2.8 ± 2.4 | 3.5 ± 2.1 | 3.3 ± 2.8 |
| Relative to shortest session | 2.9 ± 2.7 | 2.8 ± 2.2 | 2.3 ± 1.9 |
| Triceps Brachii | |||
| Last third | 2.8 ± 2.3 | 2.8 ± 2.2 | 3.0 ± 3.2 |
| Relative to shortest session | 2.8 ± 2.4 | 2.3 ± 1.5 | 2.4 ± 1.9 |
Maximal voluntary contraction (MVC) forces are before and after the sustained contraction. Burst rate is reported for the last third of time to failure for each session and for last third of the time to failure relative to the duration of the shortest session.
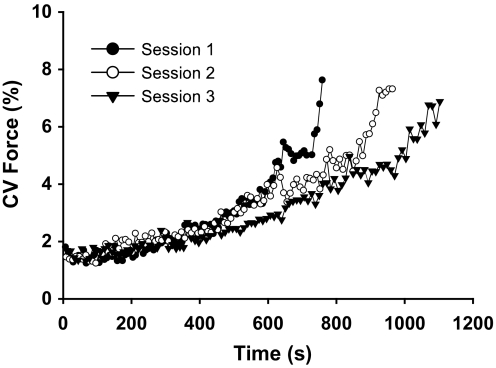
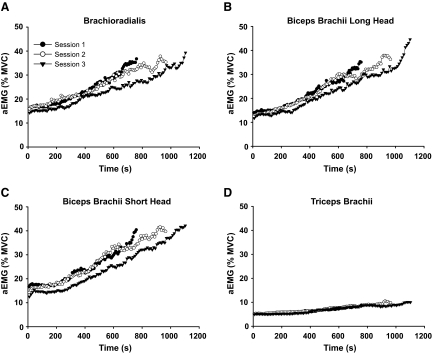
CV for force reached similar values at failure for each practice session (P = 0.9). However, the rate of increase in CV for force (ea) was less in session 3 compared with session 1 (1.0035 ± 0.0015 and 1.0018 ± 0.0008, respectively; P = 0.007; Fig. 2). The aEMG for all muscles increased significantly with time in each session (P = 0.01, Fig. 3, A–D). There was a main effect for muscle due to a lower rate of increase in aEMG for triceps brachii compared with the elbow flexor muscles (P < 0.001). The rate of increase in aEMG for triceps brachii was similar in all three practice sessions (Fig. 3D). There was a reduced rate of increase in aEMG for the short head of the biceps brachii in session 3 compared with session 1 (P < 0.05, Fig. 3C). The rate of increase in aEMG activity for the other muscles did not change with practice (P > 0.15). The rate of bursting activity in the EMG increased with contraction time for all of the muscles (P < 0.05), although there were no differences between individual muscles (P = 0.8, Table 1) or changes across practice sessions (P > 0.7).
FIG. 2.
Coefficient of variation for force at 1% intervals of time to failure for the duration of each practice session. The r2 values for the exponential fits were >0.87 for each session. The rate of increase in CV for force was lower in session 3 compared with session 1 (P < 0.007).
FIG. 3.
Average electromyograph (EMG) at 1% intervals of time to failure for the duration of each practice session for the brachioradialis, long head of biceps brachii, short head of biceps brachii, and triceps brachii (A–D). The r2 values across sessions and muscles for the exponential fits were all >0.87. The rate of increase in biceps brachii short head EMG (C) was significantly lower for session 3 compared with session 1 (P < 0.05).
Reflex testing sessions
Fifty-six motor units were recorded from biceps brachii (31 long head, 25 short head) during the reflex testing session before practice, and 62 motor units (36 long head, 26 short head) were recorded after practice. The responses to an average of 77 ± 27 and 76 ± 21 stimuli before and after practice, respectively, were recorded for each motor unit during the reflex testing sessions. Average force, discharge rate, and the coefficient of variation for ISI were similar before and after practice (Table 2; P > 0.2).
TABLE 2.
Motor-unit characteristics during spike-triggered stimulation before and after practice
| Before | After | |
|---|---|---|
| Number of motor units | 56 | 62 |
| Trial duration, s | 159 ± 58 | 154 ± 48 |
| Number of stimuli | 77 ± 27 | 76 ± 21 |
| Mean force, % MVC | 3.9 ± 3.8 | 3.6 ± 4.3 |
| Mean discharge rate, pps | 10.5 ± 1 | 10.5 ± 0.9 |
| Coefficient of variation for ISI (%) | 19.4 ± 7.7 | 17.7 ± 6.4 |
ISI, interspike interval.
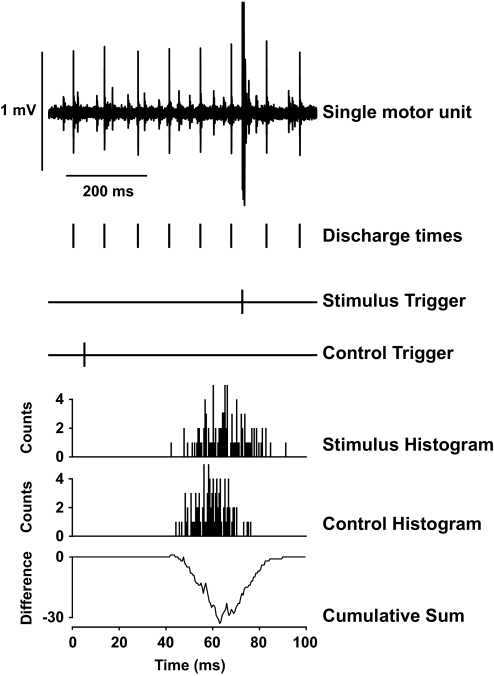
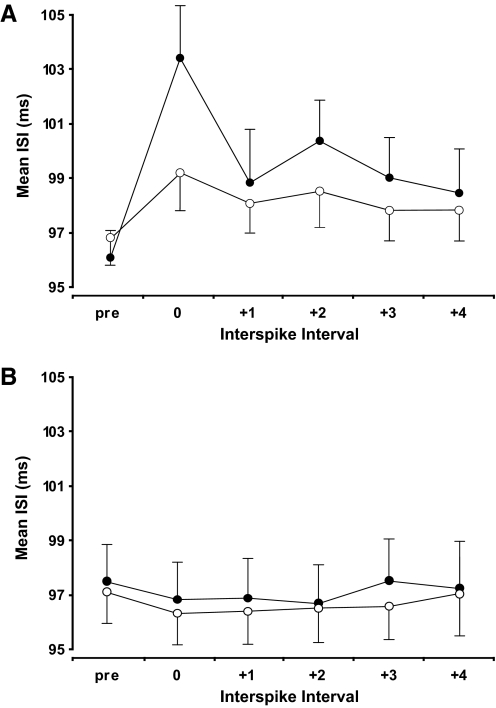
Fifty-four of the 56 motor units (96%) before practice demonstrated inhibition in response to radial nerve stimulation. Figure 4 illustrates the influence of stimulating the nerve to brachioradialis on the discharge of the isolated motor unit in biceps brachii. The ISI during stimulation (ISI-0) was significantly longer than the average of the three preceding ISIs (103.4 ± 14.1 and 96.2 ± 10.2 ms, respectively; P < 0.01). The difference between these two ISIs (7.2 ± 6.8 ms) was significantly greater than the control measurements when stimulation was not given (–0.6 ± 1.7 ms; P < 0.001; Fig. 5A). When the response to the stimulus was averaged across all motor units for each subject, all subjects demonstrated inhibition (range: 0.8 –23.1 ms). The three subsequent ISIs after the interval that included the stimulation (ISI +1 to ISI +3) were also significantly longer than the matched control ISIs (P < 0.05; Fig. 6A). There was no difference in the amplitude of the M wave before (2.7 ± 2.1 V) and after (2.8 ± 2.1 V) the trials, which indicated that the stimulation conditions did not change within each session (P = 0.8).
FIG. 4.
The influence of spike-triggered stimulation applied to the nerve innervating the brachioradialis muscle on the discharge of a motor unit in biceps brachii. The single motor unit discharged action potentials at 10.6 pps, and the stimulation was delivered at 30 ms after a randomly selected discharge time. The interspike intervals during stimulation and control conditions (n = 100 each) were used to construct the poststimulus time histograms. The cumulative sum procedure was applied to the difference between the stimulation and control histograms; the negative deflection denotes an inhibitory effect of the stimulation that prolonged the time to the next discharge of an action potential by the motor unit.
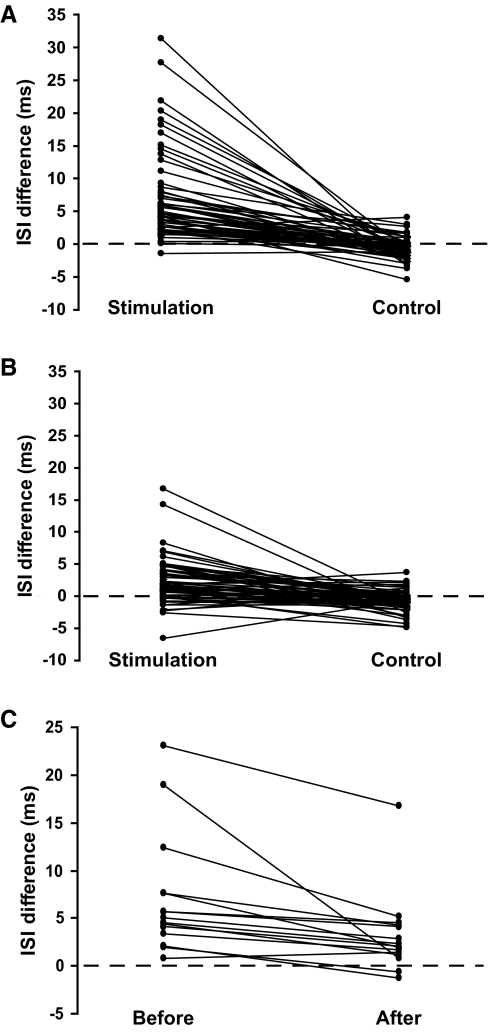
FIG. 5.
The difference between the poststimulus interspike interval (ISI-0) and the 3 ISIs preceding the stimulation (ISI-pre) for stimulus and control conditions in each of the 56 motor units before practice (A) and the 62 motor units after practice (B). The difference between the ISI-0 and ISI-pre averaged for all motor units within a subject (n = 15) before and after practice (C).
FIG. 6.
The durations of the ISIs around the time of the brachioradialis nerve stimulation as measured before (○) and after (•) practice (A). The baseline duration is indicated by the average of the 3 ISIs (pre) that preceded the stimulation. The subsequent intervals represent the ISI during stimulation (ISI-0) and then the next 4 intervals (ISI +1 to ISI +4). The control measurements are indicated in a similar format (B) and demonstrate the consistent discharge rate before and after practice. Data are presented as the means ± SE.
After three practice sessions of the fatiguing contraction, the discharge of 52 of the 62 motor units (84%) was inhibited by the stimulation and 14 of the 15 individuals displayed inhibition. The ISI that included the stimulation (99.2 ± 9.9 ms) was significantly longer than the preceding ISIs (96.9 ± 8.6 ms; P < 0.01). In addition, the difference between the two ISI values (2.3 ± 3.6 ms) was greater than for the control measurements (–0.8 ± 1.6 ms; P < 0.01; Fig. 5B). The additional discharges following stimulation up to ISI +3 were significantly prolonged with stimulation when compared with the control measurements after practice (P < 0.03; Fig. 6B). Stimulus intensity remained the same before (2.4 ± 1.5 V) and after (2.4 ± 1.6 V) each session (P = 0.8).
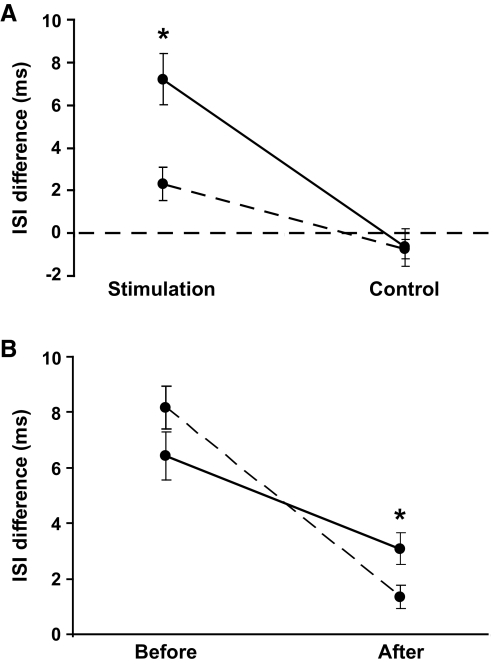
Only one of the subjects did not experience a reduction in the amount of reflex inhibition after the three practice sessions (Fig. 5C). This individual had the smallest response to stimulation before practice. A significant main effect for practice indicated that stimulation increased the ISI duration less after practice (7.2 ± 6.8 to 2.3 ± 3.6 ms, P < 0.01; Fig. 7A). There were no changes after practice in the control condition (P = 0.8). There was also a muscle effect due to less reflex inhibition in the short head of biceps brachii after practice than in the long head (1.2 ± 2.8 and 3.1 ± 3.9 ms; P < 0.05; Fig. 7B).
FIG. 7.
The difference between the ISI that included the stimulation (ISI-0) and the 3 ISIs preceding the stimulation (ISI-pre) for stimulus and control conditions averaged for all motor units recorded before (—) and after (- - -) practice (A). The difference in ISIs for the stimulation condition declined after practice (P < 0.001). The change in the difference between ISI-0 and ISI-pre before and after practice (B) was greater for the short head of biceps brachii (- - -) compared with the long head (—). The inhibition was less in the short head than the long head after practice (P < 0.05). Data are presented as the means ± SE.
There was no correlation between the increase in time to failure with practice and the reduction in the reflex inhibition of biceps brachii motor units (short and long head combined) for the 15 subjects (r = –0.39, P = 0.15). However, there was a significant negative correlation between the increase in time to failure and the reduction in the reflex inhibition for the short head of biceps brachii (r = –0.77, P = 0.001). The reduction in reflex inhibition of the short head of biceps brachii was also correlated with the decline in the rate of increase in aEMG activity in the short head (r = 0.57, P = 0.03), and the change in the rate of increase in aEMG for the short head of biceps brachii was correlated with the increase in the time to failure (r = –0.68, P = 0.005). The multiple-stepwise linear regression analysis indicated that the decrease in reflex inhibition of the short head of biceps brachii was the main predictor of the increase in the time to failure (r2 = 0.60, P = 0.001).
DISCUSSION
The main findings of the current study were that practice of a sustained submaximal contraction with the elbow flexor muscles increased time to failure, reduced the rate of increase in EMG activity for the short head of biceps brachii, and decreased the reflex inhibition from brachioradialis radial nerve afferents onto motor units in the short and long heads of biceps brachii. There was an association between the change in aEMG for the short head of biceps brachii and the reflex inhibition of its motor units, and the increase in time to failure was predicted by the reduction in reflex inhibition for the short head of biceps brachii. The results demonstrate that practice attenuated the reflex inhibition between two of the elbow flexor muscles and prolonged the duration that the submaximal contraction could be sustained.
Change in time to failure
The average time to failure increased by ∼60% from the first to the third session in the current study, which is similar to the mean increase observed for the responders by Hunter and Enoka (2003). One difference between the two studies, however, was that subjects in the Hunter and Enoka (2003) study were classified as responders (9 subjects) and nonresponders (5 subjects) based on clear cluster centers with the breakpoint at a 15% increase in time to failure. All participants in the present study experienced an increase in the time to failure with practice (range: 3–264%), and with no clear divisions in the data all subjects were placed in a single group. In both of these studies, the MVC forces before and immediately after the fatiguing contraction were similar across all three sessions, which indicated that the target force and the depression of MVC force remained the same. Although the subjects in the current study were a little weaker (MVC force: 201 ± 54 vs. 289 ± 117 N), the time to failure for the contraction sustained at 20% MVC force was longer in the previous study (1,265 ± 753 vs. 760 ± 333 s). Nonetheless the performances within each study were consistent across the three practice sessions and the average increases in time to failure were comparable.
Prolongation of the time to failure by the third session was accompanied by adaptations in the motor output to the elbow flexor muscles in both studies. The changes were manifested as reductions in the rates of increase in force fluctuations and EMG amplitude. Although the task was to maintain a constant force, the force exerted by a muscle or a group of muscles is never constant during a voluntary contraction but fluctuates about an average value due to the discharge characteristics of the activated motor units (Barry et al. 2007; Moritz et al. 2005). Furthermore the amplitude of the normalized (coefficient of variation) force fluctuations increases during a fatiguing contraction due to changes in motor-unit activity (Maluf et al. 2005; Mottram et al. 2005; Rudroff et al. 2007). In the current study, the coefficient of variation for force increased from 1.6 ± 0.5% during the first 60 s of the fatiguing contraction performed in session 1 to 7.6 ± 2.8% during the last 60 s. Although a similar value was reached during the last 60 s of session 3 (7.1 ± 2.1%), the longer time to failure for the third session meant a slower rate of increase in the coefficient of variation for force. Hunter and Enoka (2003) reported similar reductions in the rate of increase in force fluctuations by the third session. Taken together, these results indicate that motor-unit activity changed more gradually by the third performance of the fatiguing contraction despite the requirement to exert the same net force.
The lower rate of increase in the force fluctuations was accompanied by corresponding changes in EMG activity. Contractions sustained at a submaximal intensity are characterized by a progressive increase in the amplitude of the EMG activity and the appearance of bursts of activity due to the transient recruitment of higher-threshold motor units (Carpentier et al. 2001; Hunter et al. 2002; Kouzaki and Shinohara 2006; Riley et al. 2008a). The adaptations achieved by the subjects differed in the two practice studies, presumably due to clustering of groups based on time to failure for the participants in the study by Hunter and Enoka (2003). In this previous work, Hunter and Enoka (2003) reported lower rates of increase in EMG amplitude and burst frequency for biceps brachii and brachioradialis for the responders. In contrast, the changes in EMG activity across the three sessions were more limited in the current study even though all subjects increased the time to failure; there were no statistical differences across sessions in the rates of increase in either the frequency of the EMG bursts or, with one exception, EMG amplitude for any of the recorded muscle activity. The exception was the rate of increase in EMG amplitude for the short head of biceps brachii, which declined with practice and was associated with the increase in the time to failure (r = –0.68). When the criterion used by Hunter and Enoka (2003) to distinguish responders and nonresponders was applied to the current study, three subjects were removed from the group analysis and the rate of increase in biceps brachii long head EMG activity was significantly reduced with practice (P < 0.05), and there was a trend for a reduction in EMG activity for brachioradialis (P = 0.09). In both studies, coactivation of the antagonist muscle (triceps brachii) reached final values of <10% of the MVC value during the last 60 s of the fatiguing contraction and changed minimally across practice sessions, and could not account for the differences between studies.
The absence of a change in surface EMG across sessions does not necessarily indicate that there were no adaptations in motor-unit activity. Due to limitations in the interference signal (Farina et al. 2004; Keenan et al. 2005), modest changes in motor-unit activity can be masked in measurements of EMG amplitude (Mottram et al. 2005). In the current study, for example, practice reduced the reflex inhibition of motor units in both the short and long heads of biceps brachii, but only the short head experienced a lower rate of increase in EMG amplitude in the third performance of the fatiguing contraction.
Change in inhibition
The strength of the inhibitory reflex to the long and short heads of biceps brachii decreased significantly after practice. A number of studies have demonstrated that the amplitude of reflex responses can change with practice (Chen et al. 2006; Geertsen et al. 2007; Perez et al. 2007; Voigt et al. 1998). For example, both healthy and spinal-cord injured subjects were able to decrease the amplitude of the stretch reflex in biceps brachii after 24 training sessions in which subjects were provided with feedback of the response and a reward for producing the desired adaptation (Segal and Wolf 1994; Wolf and Segal 1996). In the present study, however, the adaptation was likely an indirect consequence of the practice (Zehr 2006), as reported for the H reflex in the lower limb (Schneider and Capaday 2003). Presumably changes in reflex responses that parallel improvements in performance indicate a causal association between the transmission of information over the reflex pathway and the ensuing adaptation in motor output.
Although the current study focused on a single reflex pathway, there were surely other changes in the modulation of sensory feedback that influenced biceps brachii muscle activity and contributed to the improvement in performance. For example, the capacity to change the stretch reflex in biceps brachii with operant conditioning suggests that there are modifications in the excitatory reflex pathway from afferents that connect monosynaptically onto the motor neurons that innervate biceps brachii (Evatt et al. 1989; Wolf and Segal 1996). Given that the intervention comprised only three sessions, the adaptations were likely limited to the modulation of input to the motor neurons rather than involving the plastic changes that have been observed in spinal and cortical neurons and circuits with more prolonged training (Carroll et al. 2002; Jensen et al. 2005; Thompson et al. 2006). In addition to the attenuation of reflex inhibition from brachioradialis to biceps brachii, the increase in time to failure may have involved a reduction in recurrent inhibition, which declines during a sustained contraction (Löscher et al. 1996), and an increase in excitatory input to biceps brachii motor neurons from afferents arising from forearm muscles (Cavallari and Katz 1989). In contrast, the adaptation likely did not involve a change in disynaptic reciprocal inhibition because the low level of EMG activity in triceps brachii did not change across the practice sessions (Perez et al. 2007). These pathways are difficult to test in biceps brachii, however, due to the challenges associated with measuring the H reflex in this muscle (Klass et al. 2008; Miller et al. 1995a,b).
As observed in a previous study (Barry et al. 2008), the strength of the inhibitory inputs from brachioradialis afferents was similar for the two heads of the biceps brachii, at least before the three practice sessions. However, there was a greater reduction in the strength of reflex inhibition to the short head of biceps brachii after practice. Because the two heads of the biceps brachii have a similar morphology (Murray et al. 2000) and a common tendon insertion at the elbow and are both innervated by the musculocutaneous nerve, the two muscles are often considered to act as a single entity (Buchanan et al. 1989; Cavallari and Katz 1989; van Zuylen et al. 1988). When tasks involve the production of elbow flexion and supination torques, however, some motor units in the long head are active during the production of flexion and supination torques whereas others are only active when producing a flexion torque (ter Haar Romeny et al. 1984; van Zuylen et al. 1988). In contrast, motor units in the short head of biceps brachii are activated when producing a combination of flexion and supination torques. The sensory pathway investigated in the present study involved afferents from the brachioradialis muscle that produces flexion as well as pronation and supination torques depending on forearm position (Buchanan et al. 1989; Jamison and Caldwell 1993; Zhang et al. 1998). This can correspond to the synergistic flexion action and antagonistic supination action of motor units in biceps brachii, especially for those of the short head. An attenuation of the antagonistic interaction between brachioradialis and biceps brachii should enhance the flexion action to which both muscles contribute.
Indeed practice reduced the reflex inhibition of motor units in the short head of biceps brachii, which lowered the rate of increase in EMG for this muscle and predicted the changes in time to failure after practice. The decrease in the strength of reflex inhibition presumably slowed the decline in discharge rate and delayed the recruitment of additional motor units (Carpentier et al. 2001; Mottram et al. 2005). Consistent with this interpretation, the time to failure for a sustained submaximal contraction is longer when the forearm is supinated and the strength of inhibition from afferents in the radial nerve innervating brachioradialis is lower (Barry et al. 2008; Rudroff et al. 2005). The spinal mechanisms responsible for the decrease in motor-unit activity in biceps brachii most likely involves an oligosynaptic pathway that produces an inhibitory postsynaptic potential in the motor neurons (Naito et al. 1996). The inhibition could also include presynaptic inhibition of excitatory inputs onto biceps motor units because this source of inhibition can last several hundred milliseconds (Hultborn et al. 1987) as suggested by the increase in the duration of the additional ISIs after stimulation in the current study. The influence of the stimulation on the additional ISIs was reduced after practice (Fig. 6A); suggesting that the mechanisms mediating short- and long-lasting inhibition were both changed with practice.
This study demonstrates plasticity of a reflex between muscles that are activated as synergists to support elbow flexion torques and antagonists in some forearm positions to produce supination-pronation torques. Although it is acknowledged that this specific reflex pathway is only one of many that converge on the biceps brachii motor neuron pool, the association between the change in the reflex and time to failure suggests that the reflex is functionally important in controlling the output of the motor-unit pool. The observed depression of reflex inhibition underscores the specificity of the adaptations, including the importance of limb position, in the activation of upper limb muscles.
GRANTS
The work was supported byNational Institute of Neurological Disorders and Stroke Grant NS-043275 to R. M. Enoka.
Acknowledgments
We thank K. E. Taylor for assistance with data collection and Prof. Jacques Duchateau for comments on a draft of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aagaard 2003.Aagaard P Training-induced changes in neural function. Exerc Sport Sci Rev 31: 61–67, 2003. [DOI] [PubMed] [Google Scholar]
- Barry et al. 2007.Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007. [DOI] [PubMed] [Google Scholar]
- Barry et al. 2008.Barry BK, Riley ZA, Pascoe MA, Enoka RM. A spinal pathway between synergists can modulate activity in human elbow flexor muscle. Exp Brain Res 190: 347–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan et al. 1989.Buchanan TS, Rovai GP, Rymer WZ. Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol 62: 1201–1212, 1989. [DOI] [PubMed] [Google Scholar]
- Carpentier et al. 2001.Carpentier A, Duchateau J, Hainaut K. Motor unit behavior and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534: 903–912, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll et al. 2002.Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol 544: 641–652, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari and Katz 1989.Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurons supplying biceps and triceps muscles in man. Exp Brain Res 78: 465–478, 1989. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2006.Chen XY, Chen L, Chen Y, Wolpaw JR. Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol 96: 2144–2150, 2006. [DOI] [PubMed] [Google Scholar]
- Del Balso and Cafarelli 2007.Del Balso C, Cafarelli E. Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol 103: 402–411, 2007. [DOI] [PubMed] [Google Scholar]
- Duchateau and Enoka 2002.Duchateau J, Enoka RM. Neural adaptations with chronic activity patterns in able-bodied humans. Am J Phys Med Rehabil 81: S17–27, 2002. [DOI] [PubMed] [Google Scholar]
- Duchateau et al. 2006.Duchateau J, Semmler JG, Enoka RM. Training adaptations in the behavior of human motor units. J Appl Physiol 101: 1766–1775, 2006. [DOI] [PubMed] [Google Scholar]
- Evatt et al. 1989.Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: preliminary studies. Neurosci Lett 105: 350–355, 1989. [DOI] [PubMed] [Google Scholar]
- Farina et al. 2004.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol 96: 1486–1495, 2004. [DOI] [PubMed] [Google Scholar]
- Geertsen et al. 2008.Geertsen SS, Lundbye-Jensen J, Nielsen JB. Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol (June 28, 2008). [DOI] [PubMed]
- Hultborn et al. 1987.Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibers: a study in man and the cat. J Physiol 389: 729–756, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter and Enoka 2003.Hunter SK, Enoka RM. Changes in muscle activation can prolong the endurance time of a submaximal isometric contraction in humans. J Appl Physiol 94: 108–118, 2003. [DOI] [PubMed] [Google Scholar]
- Hunter et al. 2002.Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol 88: 3087–3096, 2002. [DOI] [PubMed] [Google Scholar]
- Jamison and Caldwell 1993.Jamison JC, Caldwell GE. Muscle synergies and isometric torque production: influence of supination and pronation level on elbow flexion. J Neurophysiol 70: 947–960, 1993. [DOI] [PubMed] [Google Scholar]
- Jensen et al. 2005.Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol 99: 1558–1568, 2005. [DOI] [PubMed] [Google Scholar]
- Keenan et al. 2005.Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 98: 120–131, 2005. [DOI] [PubMed] [Google Scholar]
- Klass et al. 2008.Klass M, Lévénez M, Enoka RM, Duchateau J. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99: 1096–1104, 2008. [DOI] [PubMed] [Google Scholar]
- Kouzaki and Shinohara 2006.Kouzaki M, Shinohara M. The frequency of alternate muscle activity is associated with the attenuation in muscle fatigue. J Appl Physiol 101: 715–720, 2006. [DOI] [PubMed] [Google Scholar]
- Loscher et al. 1996.Loscher WN, Cresswell AG, Thorstensson A. Recurrent inhibition of soleus alpha-motoneurons during a sustained submaximal plantar flexion. Electroencephalogr Clin Neurophysiol 101: 334–338, 1996. [DOI] [PubMed] [Google Scholar]
- Maluf et al. 2005.Maluf KS, Shinohara M, Stephenson JL, Enoka RM. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res 167: 165–177, 2005. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1995a.Miller TA, Mogyoros I, Burke D. Homonymous and heteronymous monosynaptic reflexes in biceps brachii. Muscle Nerve 18: 585–592, 1995a. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1995b.Miller TA, Mogyoros I, Kiernan M, Burke D. Reproducibility of a heteronymous monosynaptic reflex in biceps brachii. Electroencephalogr Clin Neurophysiol 97: 318–325, 1995b. [DOI] [PubMed] [Google Scholar]
- Moritz et al. 2005.Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. [DOI] [PubMed] [Google Scholar]
- Mottram et al. 2005.Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392, 2005. [DOI] [PubMed] [Google Scholar]
- Murray et al. 2000.Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. J Biomech 33: 943–952, 2000. [DOI] [PubMed] [Google Scholar]
- Nafati et al. 2005.Nafati G, Schmied A, Rossi-Durand C. Changes in the inhibitory control exerted by the antagonist Ia afferents on human wrist extensor motor units during an attention-demanding motor task. J Neurophysiol 93: 2350–2353, 2005. [DOI] [PubMed] [Google Scholar]
- Naito et al. 1996.Naito A, Shindo M, Miyasaka T, Sun YJ, Morita H. Inhibitory projection from brachioradialis to biceps brachii motoneurons in human. Exp Brain Res 111: 483–486, 1996. [DOI] [PubMed] [Google Scholar]
- Perez et al. 2007.Perez MA, Lundbye-Jensen J, Nielsen JB. Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol 98: 3677–3687, 2007. [DOI] [PubMed] [Google Scholar]
- Riley et al. 2008a.Riley ZA, Maerz AH, Litsey JC, Enoka RM. Motor unit recruitment in human biceps brachii during sustained voluntary contraction. J Physiol 586: 2183–2193, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley et al. 2008b.Riley ZA, Terry ME, Mendez-Villanueva A, Litsey JC, Enoka RM. Motor unit recruitment and burst of activity in the surface electromyogram during a sustained contraction. Muscle Nerve 37: 745–753, 2008b. [DOI] [PubMed] [Google Scholar]
- Rudroff et al. 2007.Rudroff T, Barry BK, Stone AL, Barry CJ, Enoka RM. Accessory muscle activity contributes to the variation in time to task failure for different arm postures and loads. J Appl Physiol 102: 1000–1006, 2007. [DOI] [PubMed] [Google Scholar]
- Rudroff et al. 2005.Rudroff T, Poston B, Shin IS, Bojsen-Moller J, Enoka RM. Net excitation of the motor unit pool varies with load type during fatiguing contractions. Muscle Nerve 31: 78–87, 2005. [DOI] [PubMed] [Google Scholar]
- Schneider and Capaday 2003.Schneider C, Capaday C. Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol 89: 648–656, 2003. [DOI] [PubMed] [Google Scholar]
- Segal and Wolf 1994.Segal RL, Wolf SL. Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol 130: 202–213, 1994. [DOI] [PubMed] [Google Scholar]
- Semmler et al. 2000.Semmler JG, Kutzscher DV, Enoka RM. Limb immobilization alters muscle activation patterns during a fatiguing isometric contraction. Muscle Nerve 23: 1381–1392, 2000. [DOI] [PubMed] [Google Scholar]
- Semmler and Nordstrom 1998.Semmler JG, Nordstrom MA. Motor unit discharge and force tremor in skill- and strength-trained individuals. Exp Brain Res 119: 27–38, 1998. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny et al. 1984.ter Haar Romeny BM, van der Gon JJ, Gielen CC. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Exp Neurol 85: 631–650, 1984. [DOI] [PubMed] [Google Scholar]
- Thompson et al. 2006.Thompson AK, Stein RB, Chen XY, Wolpaw JR. Modulation in spinal circuit and corticospinal connections following nerve stimulation and operant conditioning. Conf Proc IEEE Eng Med Biol Soc 1: 2138–2141, 2006. [DOI] [PubMed] [Google Scholar]
- van Zuylen et al. 1988.van Zuylen EJ, Gielen CC, Denier van der Gon JJ. Coordination and inhomogeneous activation of human arm muscles during isometric torques. J Neurophysiol 60: 1523–1548, 1988. [DOI] [PubMed] [Google Scholar]
- Voigt et al. 1998.Voigt M, Chelli F, Frigo C. Changes in the excitability of soleus muscle short latency stretch reflexes during human hopping after 4 weeks of hopping training. Eur J Appl Physiol 78: 522–532, 1998. [DOI] [PubMed] [Google Scholar]
- Wolf and Segal 1996.Wolf SL, Segal RL. Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol 75: 1637–1646, 1996. [DOI] [PubMed] [Google Scholar]
- Wolpaw and Tennissen 2001.Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 24: 807–843, 2001. [DOI] [PubMed] [Google Scholar]
- Zehr 2006.Zehr EP Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol 101: 1783–1794, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 1998.Zhang L, Butler J, Nishida T, Nuber G, Huang H, Rymer WZ. In vivo determination of the direction of rotation and moment-angle relationship of individual elbow muscles. J Biomech Eng 120: 625–633, 1998. [DOI] [PubMed] [Google Scholar]