Abstract
Previous studies using muscimol inactivations in the frontal eye fields (FEFs) have shown that saccades generated by recall from working memory are eliminated by these lesions, whereas visually guided saccades are relatively spared. In these experiments, we made reversible inactivations in FEFs in alert macaque monkeys and examined the effect on saccades in a choice response task. Our task required monkeys to learn arbitrary pairings between colored stimuli and saccade direction. Following inactivations, the percentage of choice errors increased as a function of the number of alternative (NA) pairings. In contrast, the percentage of dysmetric saccades (saccades that landed in the correct quadrant but were inaccurate) did not vary with NA. Saccade latency increased postlesion but did not increase with NA. We also made simultaneous inactivations in both FEFs. The results following bilateral lesions showed approximately twice as many choice errors. We conclude that the FEFs are involved in the generation of saccades in choice response tasks. The dramatic effect of NA on choice errors, but the lack of an effect of NA on motor errors or response latency, suggests that two types of processing are interrupted by FEF lesions. The first involves the formation of a saccadic intention vector from associate memory inputs, and the second, the execution of the saccade from the intention vector. An alternative interpretation of the first result is that a role of the FEFs may be to suppress incorrect responses. The doubling of choice errors following bilateral FEF lesions suggests that the effect of unilateral lesions is not caused by a general inhibition of the lesioned side by the intact side.
INTRODUCTION
The frontal eye fields (FEFs) have been shown to be associated with the planning and execution of saccadic eye movements (see Goldberg and Segraves 1989 for a review). The results observed following the placement of lesions in the FEF have established a causal role for the FEF in such planning and generation. Unilateral surgical ablation of the FEF leads to a transient neglect of the contralateral hemifield, increases in saccade latencies, and targeting errors (Schiller et al. 1980). More permanent effects on saccades are found when target selection for the saccade is biased by the asynchronous onset of paired or multiple targets (Schiller and Chou 1998). More pronounced and longer lasting effects of surgical ablations of the FEFs are produced for working memory-guided saccades (Deng et al. 1986). Reversible inactivations made with muscimol injections in the FEFs have produced more definitive effects on saccade generation (Dias and Segraves 1999; Sommer and Tehovnik 1997). FEF inactivations produced a major disruption of memory-guided saccades, but much smaller effects on visually guided saccades. This finding has led to the belief that visually guided saccade execution is relatively unaffected by FEF lesions. For memory-guided saccades, lesioned animals made fewer saccades to targets situated at the retinotopic location coded by the lesioned site in the FEF than to other locations in the visual field. Saccades that were made to the lesioned locus were inaccurate and had slower velocities and longer latencies. The lesioned animals also showed a fixation disorder. They made frequent premature saccades to targets appearing in the ipsilateral field in the memory-guided task.
Target selection deficits following muscimol-induced lesions in FEFs were shown in a recent study (Schiller and Tehovnik 2003). In this study, monkeys were tested in a task that required target selection between two peripheral targets that appeared with asynchronous onset. The target located at the retinotopic location of the lesioned site in FEF was compromised in the selection process in comparison to the other target. Similar target selection deficits were shown in the same study in an oddity detection, visual search task. McPeek (2004) also showed that muscimol inactivations in FEF degraded monkeys' performance (increased targeting to a distractor in the visual array of stimuli) in a similar visual search task, and the deficit became more pronounced when the perceptual difference between the oddball target and the distractors was decreased. This is consistent with a target selection deficit rather than a purely motor impairment.
In this study, we investigate the effect of muscimol inactivations in FEFs on saccade target selection in a task that requires the arbitrary pairing of each member of a set of nonspatially coded colored visual targets with a particular saccade vector (Lee et al. 2005). This task differs significantly from both the traditional visuospatial working memory-guided saccade task (Dias and Segraves 1999) and the visual search task (McPeek 2004; Schiller and Tehovnik 2003) previously used in reversible lesion studies in FEF. The working memory-guided saccade task requires the retention in visuospatial memory of the location of a single flashed visual target. Following a delay period after target extinction, a saccade is required to the same location held in memory. However, the location of the remembered target and the required saccade vector to this memory are in spatial register requiring only a simple memory to motor transformation. The visual search task requires the saliency-driven detection of an odd target among distractors. In contrast, the task used in these experiments requires the learned association of a particular color cue that appears centrally with a particular saccade vector. This saccade selection task requires access to associative memory, not working memory, for successful performance. This task also differs from other target selection tasks in that the difficulty of the task is augmented by increasing the number of arbitrary associations (NAs) that must be made, not by increasing the perceptual difficulty. In our task the perceptual difficulty of defining the correct target color remains constant across all NA conditions. A recent functional MRI (fMRI) study has indicated that the FEF is involved in the execution of this task (Lee et al. 2006). Therefore in this study, we concentrate on determining the effect of NA on saccades guided by recall from associative memory following muscimol inactivations of FEF.
METHODS
Subjects and surgical procedures
Two adult male rhesus monkeys (Macaca mulatta) weighing between 6 and 8 kg were used. Experimental procedures were approved by the Smith-Kettlewell Animal Care and Use Committee and were in compliance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals.
A head-restraint post and titanium recording cylinders were implanted under isoflurane anesthesia and sterile surgical conditions. The recording cylinders (15 mm ID) were positioned over craniotomies centered on the left and right arcuate sulci in both animals. These sulci were visible through the intact dura at the time of surgical implantation.
Behavioral tasks
COLOR-CUED ASSOCIATIVE MEMORY-GUIDED SACCADE TASK.
At the beginning of each trial, a gray fixation spot appeared at the center of the visual field (Fig. 1). While the monkey was holding fixation on the spot, an array of colored potential targets separated uniformly in direction from each other appeared in the peripheral visual field. The colored array was illuminated for 400 ms, and all target alternatives turned gray so that they were no longer distinguishable by color. After a delay randomly chosen between 400 and 800 ms, the gray fixation spot changed into one of the former target array colors. This color change at the fixation point was a cue to make a saccade and indicated which target to choose. To receive a liquid reward, the animal was required to saccade to the gray target at the array location that previously had the same color as the cue. The arbitrary association between the nonspatial color cue and the required saccade vector was held constant throughout the training and experiments. The number of alternative targets (NA) was constant at one, two, or four within a block of trials. The color presented at the fixation point was randomly sequenced among four colors, each associated with a different saccade vector. The animal was rewarded only if a saccade occurred within 1,000 ms from the cue onset and the endpoint was within 2 and 15° from the correct target in amplitude and direction, respectively. We were able to monitor saccade accuracy on-line within each block of trials. In the postlesion condition, saccades that went to the correct target quadrant became more inaccurate. We increased the size of the reward window to increase the percent of rewarded trials that were inaccurate but still went to the correct target quadrant. This procedure kept the animals working on the task.
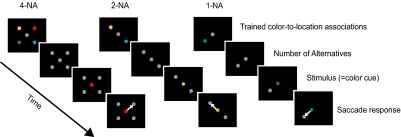
FIG. 1.
Task-related events in the associative memory paradigm are shown as a function of time. The number of alternatives (NAs) for the full set (NA = 4) of learned associations of color and movement direction is shown on the left. The 2-NA condition and the 1-NA condition are shown in the middle and on the right, respectively. The learned associations were shown as a reminder of the correct pairings at the beginning of each trial. The array of alternative targets also indicated how many alternatives were available in this block of trials. The colors of the alternative targets turned to gray, rendering them indiscriminate at the time of the cue onset. After a further delay, the cue shown at the center was 1 of the alternative colors presented in the array of potential targets shown at the beginning of the trial. The monkeys were required to make a saccade to the target location that was associated with the cue color. In the 2-NA condition, 2-alternative color/location associations were shown, either the red/green pair (data not shown) or the yellow/blue pair. In the 1-NA condition, the alternative target array contained only 1 target, but all 4 color cues were randomly shown. Blocks of trials with fixed 1-NA, 2-NA, or 4-NA were randomly interleaved.
Figure 1 shows the task for the 4-NA, 2-NA, and 1-NA condition, from left to right, respectively. The fixation spot and the targets were disks 1° in diameter. When the locations of the potential targets were >10° from the fovea, larger disks of 1.5° in diameter were used as targets to enhance the visibility. The eccentricity of the potential targets was the same as the amplitude of saccades evoked by electrical microstimulation at the injection sites. The colors used for the targets and the fixation spot were equi-luminant and chosen based on the CIE 1976 (L*a*b*) space, which is approximately uniform in perception of color difference (Wyszecki and Stiles 1982), such that the colors were at the same distance in color space from the two neighboring ones and the gray. A chromometer (CS-100, Minolta Photo Imaging USA, Mahwah, NJ) was used for measuring luminance and chromaticity of the colors. The luminance of all the visual stimuli was 1.24 cd/m2 presented against a background luminance of 0.12 cd/m2.
Our aim in using the 1-NA condition was to see whether the deficit in performance following FEF inactivations was dependent on the number of alternatives in such a way that it was present only in multiple-choice situations. Although there is only one choice present in the 1-NA condition, this task may be processed differently from the typical one-target visually guided or working memory-guided saccade task, both tasks widely used in defining the neural basis of saccade generation. In the latter tasks, only a single target appears, and the animal must suppress a saccade to its location (still present or held in memory) until the fixation light goes off. In the 1-NA condition of the choice task, only a single target is present, but the animal has to wait until the fixation light changes color. We believe that the animals treat trials of this type that appear in a block of trials intermixed with blocks of the 2-NA or 4-NA condition as a choice response. This hypothesis is supported by our previous work (Lee and Keller 2008) in which we showed that saccade latency was significantly longer in the 1-NA condition than it was for the one target, delayed-saccade task even though a single target was present in both cases.
Note that all potential targets were gray when the cue was given, so that the target selection had to be made by recalling which of the peripheral locations was associated with the color now presented in the cue and not by a visual matching between cue and targets. Because the association of color and target locations was pretrained and fixed through all blocks of trials, the monkeys performed the task relying on a long-term symbolic association between color and target location. Therefore even though the location of the target colors were shown at the beginning of each trial (Fig. 1), a visual working memory of target configuration was not crucial for performing the task.
The two monkeys were trained on the associative-memory saccade task for a period of ∼6 wk. During the training period and the subsequent experimental sessions, blocks of trials with a fixed NA (randomly selected from 1-, 2-, or 4-NA) were used, and the red cue was associated with the gray target located in a direction 45° into the top right visual quadrant with the yellow cue associated with the target in the top left, the green cue with the target in the bottom left, and the blue cue with the target in the bottom right quadrants, respectively. In each block, ∼10 trials were recorded for each of the four potential target positions (40 trials/block).
VISUALLY GUIDED DELAYED SACCADE TASK.
While the animal fixated at a central, gray fixation point, a single gray target appeared at selected peripheral locations. The monkey was required to hold fixation until the fixation point was turned off after a random interval of 300–1,000 ms. The offset of the fixation point signaled the animal to make a saccade to the peripheral target to receive a liquid reward. The peripheral target was presented at the same set of locations used in the associative memory task.
GAP-SACCADE TASK.
The monkey fixated at a central gray fixation point that was turned off after a delay. After an interval of 200 ms following fixation off, a single gray target appeared at selected peripheral locations. The animal was required to maintain fixation at the location of the extinguished fixation point until the peripheral target appeared and to make a saccade to the latter target.
Neural recording, microstimulation, and location of injecting sites
Testing was performed in a dimly illuminated room. A custom real-time program controlled experimental events and data collection, running on a personal computer with dual Xeon processors and Windows XP equipped with two National Instrument multi-channel IO boards. Visual targets were displayed on a screen at a distance of 107 cm from the animal's eyes. Eye position and velocity were recorded with a video-based eye tracker (Eyelink2, SR Research, Mississauga, Ontario, Canada) and sampled at 500 Hz. The activity of neurons was recorded using tungsten microelectrodes lowered through the recording cylinder within a sharpened guide tube which abraded, but did not penetrate the dura. Before any lesions were made, we conducted a series of microelectrode recording/stimulating studies to establish the topological map of the FEFs with respect to the center of each cylinder. We noted the depth on each track where the microelectrode penetrated the dura. The depths over which neural responses were recorded were noted, and the response fields of single units or multiunit activity were roughly determined by on-line analysis.
Electrical microstimulation was used to determine the depth on each penetration at which saccades were evoked at minimal current in the gap-saccade task. Pulse-train stimulation consisting of biphasic stimuli (0.25 ms each phase at 250 Hz) was delivered 100 ms after the offset of the fixation point at microelectrode advancement intervals of ∼1 mm. We determined the mediolateral and dorsoventral boundries of the FEFs in the anterior bank of the arcuate sulcus where the threshold for evoking saccades was 50 μA or less. These topographically organized spatial maps of the FEFs resembled those reported by Sommer and Wurtz (2000).
We occasionally evoked smooth pursuit eye movements at some very lateral and ventral sites in the FEFs. None of the muscimol injections were made in regions where pursuit eye movements were evoked.
Muscimol inactivations
After completing the mapping of the bilateral FEF for the low-threshold region for evoking saccades, we initiated a series of experiments using muscimol injections to determine the effect of inactivation of FEF on saccades controlled by recall from associative memory. In each of the two monkeys, we attempted to spread the location of the injection sites from lateral to medial throughout the anterior bank where the amplitude of the evoked saccades varied from 3 to 15°. For each injection site, we first lowered a microelectrode inside a guide tube. After the electrode had penetrated the dura and eye movement–related activity began to be recorded, we applied electrical stimulation at sites separated by 1 mm of electrode advancement. We located the site associated with the lowest current threshold for evoking a saccade on each penetration. The depth of this site was carefully measured on the microdrive, and the electrode was withdrawn leaving the guide tube in place. A 33-gauge hypodermic cannula was inserted into the guide tube and was lowered until its tip was located at the same depth previously occupied by the tip of the stimulating electrode. Injections of muscimol were made through the cannula using pressure injection from a Harvard minipump. The concentration of the muscimol solution was kept constant at 5 μg/μl. The normal volume of solution injected was 1 μl over a period of ∼2 min. The amount of solution injected was monitored by watching the movement of a small bubble against fiducial marks located on the Teflon tubing connecting the pump to the injection cannula. Following the injection, the cannula was left in place for ∼10 min and was withdrawn. Data collection began 0.5 h after the onset of the injection, but at this time, the effect of the inactivations was small and variable so most of the data were collected starting at 1 h. At a few sites we continued to collect data on the effect of inactivations on eye movements made in the associative memory task for times up to several hours.
In both monkeys, we also made simultaneous bilateral injections of muscimol, one in each hemifield, in addition to the unilateral injections. Because of the length of time involved in the setup for these bilateral injections, we did not use microelectrode penetrations to find the low threshold site before lowering the injecting cannula into the anterior bank. Instead, we located the cannula penetrations and their depths based on previous microelectrode results where we had good information about the size and direction of the evoked saccade. Sites were located in each cylinder where saccades of similar amplitude had been previously evoked at currents <50 μA. We advanced the two cannulas directly to these known depths before the injections were made. Pressure injections were made simultaneously through both cannulas. The concentration of the muscimol solution remained at 5 μg/μl, and the amount injected at each site was ∼1 μl. Following the completion of the postlesion data collection, the animal was returned to its home cage. Control data were collected the following day, and full recovery was always noted.
In each monkey, we made at least one injection of 1 μl of sterile saline in each hemifield and recorded a complete set of data after a hiatus of 1 h to control for the possibility that any negative effects produced by muscimol inactivation might have been caused by local pressure on cortical neurons or local dilution of the extracellular fluid at the injection sites. The animal's general behavior was monitored throughout the experiment by an infrared video camera.
Data analysis
Saccade behavior was measured off-line using programs written in MATLAB (The Mathworks). Markers were available for all experimental events used in each task. The onset and offset of saccades were determined by velocity criteria (30°/s radial velocity for onset and 10°/s for offset). Correct placement of these marks was checked manually and adjusted where necessary.
RESULTS
We made 12 unilateral and 4 bilateral muscimol injections in each of the two monkeys, referenced as monkey S and monkey N.
Effects on choice performance
Figures 2 and 3 (top plots) show the control performance of the two monkeys for the 4-NA condition before placement of the lesions. The data were pooled from one or two blocks of trials for each NA condition taken immediately before making the inactivations. The figures are organized with data for monkey S in Fig. 2 and that for monkey N in Fig. 3. Each plot shows the topological arrangement of the cue (colored disk at the center) and the four candidate target locations from the animals' viewpoint at the time that the cue color has been presented. The yellow cue signals the candidate target at the top left position is the correctly associated target. The red cue signals the target at the top right, the green cue the target at the bottom left, and the blue cue the target at the bottom right. The black symbols show each animal's saccadic endpoints on individual trials for data sorted into responses made to each of the colored cues.
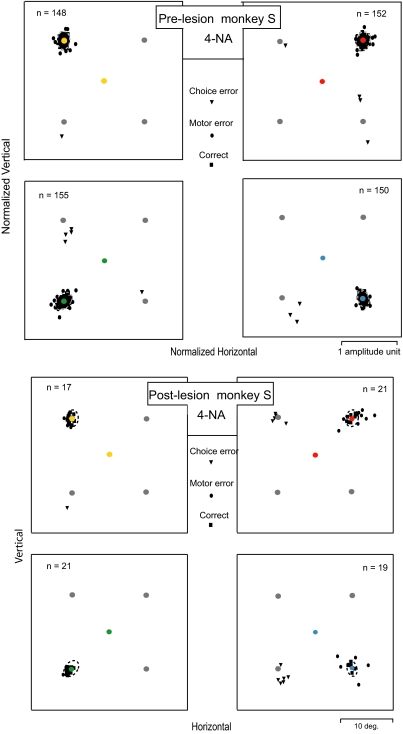
FIG. 2.
The top 4 plots show prelesion performance on the 4-NA task for monkey S pooled over all experimental sessions. The 4 plots represent data sorted by the color of the cue presented at the center (fixation point). The eccentric disks show the location of the candidate targets. The candidate target that is correctly associated with the cue color on that trial is shown in the same color for ease of reader understanding, but the monkeys saw only 4 gray disks as candidate targets at the time of cue appearance. The type of saccade produced on each trial is shown by the black symbols. In each plot, the dashed outlines show the bivariate 95% confidence ellipses computed from saccade endpoints in correct trials for each target location for the control data. Square symbols indicate saccades whose endpoints were located inside the ellipses. Circular symbols indicate saccades whose endpoints were located outside the ellipses, but in the correct quadrant (motor errors). Triangle symbols indicate saccades that were directed to incorrectly associated targets (saccades landing in wrong quadrants), which are called choice errors in the text. Saccade amplitudes are normalized so that data from all inactivation sessions with different target amplitudes could be combined. The normalized amplitude calibration applies to all 4 plots. The bottom 4 plots show postlesion results obtained from 1 example unilateral muscimol injection in the left frontal eye field (FEF) in monkey S. The format is the same as in the top 4 prelesion plots. The error ellipses are repeated from the control data obtained for monkey S shown in the top 4 plots. The rate of choice errors increased for trials in which the cue (red or blue) indicated that the correct target was located in the contralateral (right) hemifield. There was also an increase in the rate of inaccurate saccades (motor errors) for saccades directed into the contralateral hemifield.
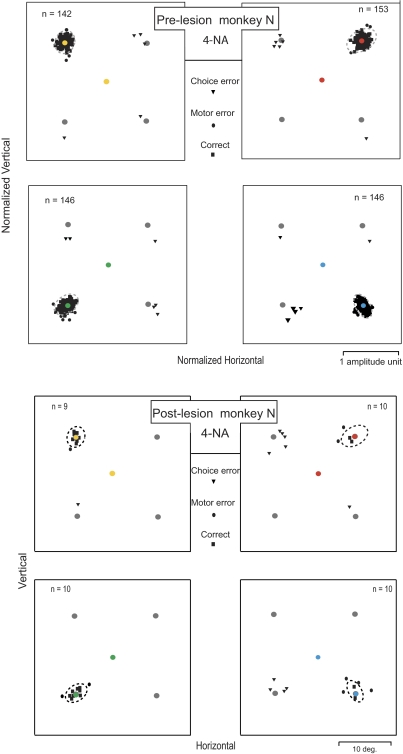
FIG. 3.
Same format as Fig. 2 except all data shown for the 4-NA condition in monkey N. The top 4 plots show prelesion controls. The bottom 4 plots show postlesion results obtained from 1 example unilateral muscimol injection in the left FEF in monkey N.
The eccentricity of the array of potential targets was adjusted to match the radial amplitude of the saccade evoked by microstimulation at the injection site. The prelesion control data for each animal was combined across all experimental sessions by normalizing the radial eye position reached at the end of the saccade on each trial by the radial amplitude of the potential target locations. We computed the 95% confidence, bivariate contour ellipses (Heinen and Skavenski 1992) for the saccadic endpoints for correct saccades (dashed ellipses for the prelesion results shown for each animal in the top 4 plots in Figs. 2 and 3). Correct saccades were defined as movements that ended in the correct target quadrant and inside that quadrant's 95% confidence ellipse. Correct saccades are indicated by filled squares in Figs. 2 and 3. We defined “choice errors” as movements that landed in target quadrants incorrectly associated with the color shown at the center on that trial. Choice errors are indicated by filled triangles. For example, in the top right plot in Fig. 2, for monkey S, the three triangles in the bottom right quadrant mark choice error responses in which the red cue indicted that the correctly associated target was in the top right quadrant, but the animal instead made these three saccades toward the target in the bottom right quadrant (a target that should have been associated with blue). In the control data for the 4-NA condition taken before muscimol injections, the performance was almost perfect, with a total of 13 choice errors in 605 trials for monkey S and 23 choice errors in 587 trials in monkey N (an error rate of ∼2 and 4%, respectively). In the control data, the choice errors appeared to be randomly distributed into the three wrong quadrants, but the small number of choice errors prevented statistical verification of this claim. The filled circles in Figs. 2 and 3 show movement endpoints that landed outside the 95% confidence ellipses but were directed into the correct quadrant associated with the cue color presented at the fixation point. We will refer to errors of this type as “motor errors.” By definition, each animal had ∼5% motor errors in its prelesion data. Trials with motor errors might or might not be rewarded. The criteria for reward are given in methods.
The bottom four subplots in Fig. 2 show typical results following a muscimol injection for the 4-NA condition obtained 1 h after a unilateral lesion was placed in the left FEF in monkey S. The bottom four subplots in Fig. 3 show example results for monkey N for the 4-NA condition following the placement of a lesion in the left FEF. In both animals, the saccade evoked by prelesion electrical stimulation was 10° in amplitude and was directed into the right hemifield. Therefore the array of potential targets in both experiments was located with an eccentricity of 10°. In these typical results, the animals make a larger percentage of choice errors after the inactivation in comparison to this animal's prelesion control values. In the example data for monkey S (Fig. 2), the percentage of total choice errors increased to 15% (12 choice errors in 78 trials) compared with the prelesion control value for this animal of 2% error. The majority of these choice errors occurred for trials in which the correct target was located in the contralateral hemifield with respect to the side of the lesion (2 right plots in the bottom 4 panels in Fig. 2). There were 11 contralateral and 1 ipsilateral choice errors (with respect to the side of the correct target). The responses for the 4-NA condition during this FEF inactivation in monkey S also showed much greater saccadic endpoint scatter (•) for saccades directed into the correct quadrant (motor errors), particularly when the correct target was located in the hemifield contralateral to the lesion. The percentage of total motor errors for this particular inactivation was ∼25% (17 motor errors in 66 trials), a value well above the 5% motor error rate for prelesion controls. Again, the majority of motor errors occurred when the correct target was located in the contralateral field with respect to the side of the lesion (13 for contralateral and 4 for ipsilateral field). The number of trials with choice errors are subtracted from the total number of trials before the percentage of motor errors is computed.
In the example data for monkey N (Fig. 3, bottom 4 plots), the percentage of total choice errors increased to 28% (11 choice errors in 39 trials) compared with the prelesion control value for this animal of 4% error. Again the majority of these choice errors occurred for trials in which the correct target was located in the contralateral hemifield (2 right plots in the bottom panels in Fig. 3). There were 10 contralateral and only 1 ipsilateral choice errors. The responses for the 4-NA condition during this FEF inactivation in monkey N also showed much greater saccadic endpoint scatter (•) for saccades directed into the correct quadrant (motor errors). The percentage of total motor errors for this particular inactivation was ∼32% (9 motor errors in 28 trials), a value well above the 5% motor error rate for prelesion controls. Again, the majority of motor errors occurred when the correct target was located in the contralateral field (6 for contralateral and 3 for the ipsilateral field targets).
Figure 4 shows data for the 1-NA condition in both animals for the same two example inactivation sites shown in the bottom four plots in Figs. 2 and 3. In contrast to the 4-NA condition, there are no choice errors in either example for this condition. However, the percentage of motor errors was 35% (14 errors in 40 trials) in monkey S and 26% (10 motor errors in 38 trials) in monkey N, predominantly for movements directed to the contralateral side with respect to the lesion.
FIG. 4.
Example results from the same 2 experimental lesion sessions shown in Figs. 2 and 3 except that the data are from the 1-NA condition. The top 4 plots are for monkey S and the bottom 4 plots are for monkey N. There were no choice errors in either animal, but there was an increased rate of motor errors in comparison to the rate for the prelesion controls. The amplitude calibration shown below the bottom 4 plots also applies to the top 4 plots.
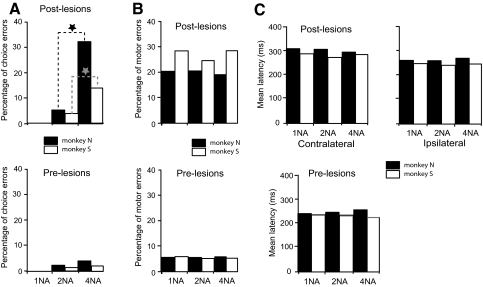
Figure 5A presents summary performance (percent choice errors) for each animal as a function of NA pooled across all unilateral FEF inactivations. The bottom bar graph shows prelesion control values and the top graph shows the summary postlesion results. There were no choice errors for the 1-NA condition pre- or postlesion in either monkey. The number of choice errors prelesion for the 2-NA condition increased to 2 and 1% for monkeys N and S, respectively. The postlesion errors for the 2-NA condition were 5% for monkey N and 4% for monkey S. The percentage of choice errors in the 4-NA condition postlesion increased dramatically and well beyond proportionality in comparison to the 2-NA condition. This increase in choice errors for the 4-NA condition compared with the 2-NA condition was significant (Wilcoxon rank sum test, P < 0.001 in both animals). Figure 5B shows, in contrast, that the motor error rate does not increase significantly as a function of NA (ANOVA, P > 0.25 in both animals) for either the prelesion (bottom bar graph) or the postlesion (top bar graph). We did not see a decrease in the number of saccades attempted when the correct target was located in the contralateral hemifield, only an increase in the number of saccades made in error when the correct target was located in the contralateral hemifield with respect to the lesion.
FIG. 5.
Summary data for all unilateral inactivations in both monkeys. A: bottom: the prelesion percentage of total choice errors (both contralateral and ipsilateral) averaged across all unilateral inactivation experiments as a function of NA condition. In the prelesion tests, there were no choice errors in either monkey for the 1-NA condition and only a few for the 2-NA condition. There was a small increase in choice errors for the 4-NA condition. Results for monkey S shown by open bars and for monkey N by filled bars. Top: the postlesion percentage of choice errors averaged across all inactivations as a function of NA condition. There were no choice errors in either monkey for the 1-NA condition and only a few for the 2-NA condition, but the percentage of errors increased dramatically in the postlesion data for both animals for the 4-NA condition. Stars indicate that the differences in error rate between the 2-NA and 4-NA conditions in the postlesion data were significant for both animals. B: in contrast, the rate of total motor errors (combined contralateral and ipsilateral) did not vary as a function of NA condition as shown in the top plot. The bottom plot shows the prelesion data for motor errors. C: top: left set of bars show the effect of unilateral muscimol injections on saccade latency as a function of NA for contralaterally directed, correct movements (trials without choice errors). Similar data for ipsilaterally directed, correct movements are shown by the right set of bars. Latency did not increase systematically as a function of NA for movements directed into either hemifield. Bottom: the mean prelesion saccadic latencies as a function of NA condition. For these prelesion values, left and right saccades were combined.
The bottom bar graph in Fig. 5C shows that mean saccadic latencies were not significantly different across NA condition in the control data taken before FEF inactivations [monkey S: 232 ± 15 (SD) ms for 1-NA, 233 ± 39 ms for 2-NA, 227 ± 25 ms for 4-NA; ANOVA, P = 0.62; monkey N: 240 ± 34 ms for 1-NA, 246 ± 27 ms for 2-NA, 249 ± 21 ms for 4-NA; ANOVA, P = 0.54). Postlesion saccade latencies (top bar graph) also did not increase systematically as a function of NA for either the contralateral or ipsilateral direction (where direction is with respect to the side of the injection). However, statistically there was a small, but significant effect of NA as a factor on saccade latency for both directions of saccades (ANOVA, P < 0.05 for both animals). Saccades with motor errors were included in the analysis of saccade latency, but choice errors were not. A postlesion increase in latency in comparison to controls (compare top bar graph to the bottom graph) occurred for each NA condition for saccades directed toward the contralateral hemifield, and these increases were significant (Wilcoxon rank sum test, P < 0.01 in each animal). Mean latencies for contralateral saccades in the 4-NA condition increased to 291 ms for monkey N and to 284 ms in monkey S after the FEF inactivations. There was a much smaller but significant increase in latency for ipsilateral movements compared with prelesion controls for each NA condition (Wilcoxon rank sum test, P < 0.05 in each animal).
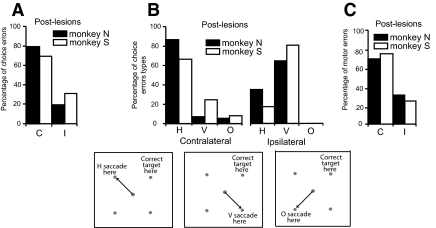
The spatial pattern of choice errors and motor errors for the 4-NA condition is examined in Fig. 6. This figure shows pooled data for each monkey in which behavior for the postlesion 4-NA condition is combined across all unilateral injection sites. Figure 6A separates the choice error rate into contralateral (C) and ipsilateral (I) hemifield errors for each animal, where the terms contralateral and ipsilateral errors refer to errors that occurred when the correct target was in the field contralateral or ipsilateral to the lesion, respectively. Contralateral errors occur more frequently than errors made when the correct target is located in the ipsilateral field (4 and 2.3 times more frequently in monkey N and monkey S, respectively). Figure 6B breaks the choice errors in the 4-NA condition down further according to direction relative to the correct target. The inset schematics in this figure clarify the definition of the directional choice errors. Each schematic is constructed for lesions placed on the left side (correct target appears on the right side). Horizontal (H) errors land near the incorrect visual stimulus that is located at the mirror image location in the opposite visual hemifield, vertical (V) errors land near the incorrect target located in the same hemifield, and oblique errors (O) land near the target diagonally opposite from the correct target. In both animals, the errors when the correct target is located contralateral to the lesion are predominantly type H. Type V and O errors are relatively rare. When the correct target is located ipsilateral to the lesion site, the predominant type of choice error among the much smaller percentage of total choice errors is type V with no oblique errors. Figure 6C shows the pooled data for motor errors. This graph shows the percentage of motor errors made when the target appeared in the contralateral (C) and ipsilateral hemifields (I) with respect to the side of the lesion separately for each animal. Motor errors like choice errors were predominantly for targets cued to the contralateral side.
FIG. 6.
The effect of laterality of target location and relative direction on error rate in postlesion data. Data pooled across all unilateral inactivation experiments for the 4-NA condition. A: percentage of postlesion choice errors as a function of target location in the contralateral (C) or ipsilateral (I) hemifield. Contralateral refers to the correct target being located in the visual field contralateral to the lesioned FEF and ipsilateral to the field ipsilateral to the lesion. B: percentage of choice errors further differentiated by relative direction of the error saccades with respect to the correct target location. Errors that occurred when the correct target was in the contralateral field (left set of bars) and in the ipsilateral field (right set of bars) are differentiated into horizontal errors (H), vertical errors (V), and directly opposite errors (O) errors. The definition of these types of error is clarified by the insets located below the plots in B. C: percentage of motor errors as a function of target location in the contralateral (C) or ipsilateral (I) hemifield.
Finally, there was an additional type of error not shown in the data presented thus far. This deficit occurred when the animal broke fixation before the fixation point was turned off in the delayed-saccade paradigm or before the cue was presented in the 4-NA task. This type of error is called “broke fixation” in the remainder of the text. Trials with broke fixation errors were not counted in the total number of trials used in the calculations of percentage of choice errors and motor errors. We used data from both the delayed-saccade task and the 4-NA condition in the associative memory task to analyze broke fixation deficits. Deficits of this type of were rare in both animals for the delayed-saccade task before the placement of the muscimol injections (Fig. 7 A, bottom bar graph) in the FEF (2% in monkey S and 2.5% in monkey N). Here contralateral (C) and ipsilateral (I) refer to the side of the visual field where the correct target appeared with respect to the side of the lesion. The percentages of both ipsilateral and contralateral broke fixation errors in the postlesion data for the delayed-saccade task are shown in the top graph in Fig. 7A. The rate of broke fixations increased significantly (Wilcoxon rank sum test, P < 0.01 for both animals and for both the contralateral and ipsilateral directions). The increase in the percentage of broke fixations in the delayed-saccade task was greater when the target appeared in the ipsilateral hemifield (Fig. 7A).
FIG. 7.
Comparison of saccadic behavior for the delayed and 4-NA tasks. A: bottom: the percentage of trials in which the animals were unable to maintain fixation at the center until the go signal was given in the prelesion control data for the delayed-saccade task. Data pooled over all experimental sessions for the delayed, visually guided task in which the disappearance of the fixation point was the go signal. Data are divided into trials in which the correct target appeared on the contralateral (C) or ipsilateral (I) side. Direction of the saccade carrying the eyes away from fixation was not material. Top: the percentage of trials in which the animals were unable to maintain fixation at the center until the go signal was given in the postlesion data. Data pooled over all inactivations for the delayed, visually guided task. Data are divided into trials in which the target appeared on the contralateral (C) or ipsilateral (I) side. Direction of the saccade carrying the eyes away from fixation was not material. Stars indicate the rate of postlesion broke fixations was significantly different from control prelesion values for both fields and for both animals. B: comparison of the percentage of broke fixations for the delayed-saccade task (D) and the 4-NA task (4NA). Data for both contralateral and ipsilateral targets are combined. C: percentage of motor errors compared for the delayed-saccade and the 4-NA task.
In the 4-NA task, the animal cannot determine where the correct target is located until the color cue at the center appears, and this cue also serves as the go signal for a saccade to the location of the correctly associated target. Therefore we expected that there would be a lower rate of broke fixations following inactivations for this task in comparison to the rate for the delayed-saccade task, but surprisingly, in the postlesion data for one animal, there was a higher rate of total broke fixations in the 4-NA condition than for the delayed saccade task (Fig. 7B, monkey N). However, this difference was not significant (Wilcoxon rank sum test, P = 0.23). In the other animal, the broke fixation rate for the 4-NA condition was less than that for the delayed-saccade task as expected (Fig. 7B, monkey S), but again the difference was not significant (Wilcoxon rank sum test, P = 0.18). The data plotted in Fig. 7B are combined for both directions of saccades.
In Fig. 7C, we compare the percentage of motor errors for the delayed-saccade task and the 4-NA task. The percentage of motor errors was not significantly different in either animal between these two tasks (Wilcoxon rank sum test, P = 0.321).
We examined the behavior 1 h after injection for the four saline injections (1 injection in each hemifield in each monkey). There were no differences in the results obtained with the four injections so we combined the data for the four injections. The percentage of choice errors in the 4-NA condition was 3% for monkey S and 3% for monkey N. The percentage of motor errors was 6% for monkey S and 4% for monkey N. None of these performance measures was significantly different from the combined prelesion controls (Wilcoxon rank sum test, P > 0.05).
Effect of bilateral lesions in the FEF
Previous studies of the FEF using muscimol have only made unilateral inactivations (Dias and Segraves 1999; McPeek 2004; Schiller and Tehovnik 2003; Sommer and Tehovnik1997). Clear unilateral deficits involving working memory-guided saccades and target selection in popout visual search tasks have been shown in these previous studies. Nevertheless, it is possible that these deficits following unilateral lesions were caused by an unbalance in the operation of the bilateral FEFs and not a specific deficit in the neural mechanisms underlying these two types of visuospatial processing. The bilateral FEFs are known to inhibit each other (Schlag et al. 1998) under selective conditions. Thus a lesion weakening one FEF may lead to the tonic inhibition of that FEF by the other that could prevent the cognitive processing required for memory-guided or target selection behavior to operate, while sparing the more reflexively driven visually guided saccades.
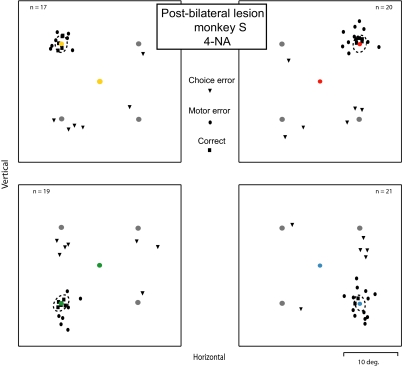
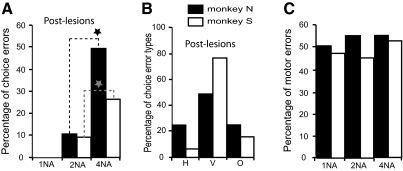
We made bilateral, reversible lesions in the FEFs in both animals (4 bilateral sites in monkey S and 4 bilateral sites in monkey N) to directly test the hypothesis that the dysfunction in associate memory behavior that we found following unilateral lesions was caused by specific impairments in FEF processing crucial in this behavior and not by bilateral FEF unbalance and tonic inhibition of one side of the cortex by the intact FEF. Figure 8 shows example results for the 4-NA condition from one bilateral injection experiment. The percentage of choice errors in this example was 32%, and these errors were about equally divided between those for the correct target on the right or on the left. This postlesion error rate was significantly larger than the postlesion population choice error rate for unilateral lesion sites in animal S (cf. with Fig. 5A, top graph; Wilcoxon rank sum test, P < 0.01). Figure 9A shows postlesion summary results for the percentage of choice errors as a function of NA for all the bilateral injection sites separately for each animal. Similarly to the results of unilateral lesions, the choice error rate increased significantly as a function of NA in both animals (Wilcoxon rank sum test, P < 0.001 in both animals). Comparison of the results for the 4-NA condition shown in Figs. 9A (bilateral lesions) and 5A (unilateral lesions) indicates that the error rate increased in both animals for the bilateral lesions in comparison to the rate for unilateral lesions (from 14 to 26% in monkey S and from 31.5 to 49.5% in monkey N). These increases in the rate of choice errors for bilateral lesions are 1.9 and 1.6 times larger in comparison to the rate for unilateral lesions. The directional pattern of the choice errors also changed with the bilateral lesions. Whereas the direction of the errors for unilateral lesions was predominantly to the visual stimulus horizontally opposite in the other hemifield, at least for contralateral errors (Fig. 6B, bars labeled H). In the bilateral inactivation case (Fig. 9B), the predominant type of choice error was made to the visual stimulus located in the vertical direction in the same hemifield (V type errors). Figure 9C shows the motor error rate for the bilateral lesions combined for both hemifields as a function of NA. The motor error rate was not significantly different as a function of NA in either animal (ANOVA, P > 0.05). The total motor error rate for the 4-NA condition for both hemifields combined was 52 and 55% for monkeys S and N, respectively. When comparable total motor error rates for unilateral inactivations are computed from the contralateral and ipsilateral data shown in Fig. 6C, the total rates were 58 and 48% for monkeys S and N, respectively. The small decrease in the rate of motor errors in monkey S for the bilateral case in comparison to the unilateral case was not significant (Wilcoxon rank sum test, P = 0.27), whereas the small increase in rate for monkey N was significant (Wilcoxon rank sum test, P < 0.05).
FIG. 8.
The effect of bilateral lesions in the FEF. Example results from 1 bilateral inactivation experiment in monkey S. The symbols and layout are the same as in Fig. 2. The candidate target that is correctly associated with the cue color on that trial is shown in the same color for ease of reader understanding, but the monkeys saw only 4 gray disks as candidate targets at the time of cue appearance. The percentage of both choice errors and motor errors increased in this experiment in comparison to data obtained for unilateral injections.
FIG. 9.
Pooled data for all bilateral inactivation experiments. A: bar graph shows the postlesion percentage of choice errors for each animal as a function of NA. B: this bar graph shows the altered pattern for the directionality of the choice errors for bilateral lesions in the 4-NA condition. The meaning of the directional errors is the same as shown in the inset in Fig. 5. Type V errors are now predominant. C: percentage of total motor errors following bilateral lesions in the 4-NA condition as a function of NA condition.
DISCUSSION
The inactivation results presented here establish an essential role for the FEFs in the generation of saccadic eye movements guided by recall from associative memory. This outcome supports a previous fMRI study in human subjects that showed FEF activity correlated with saccadic choice in the same associative memory task used here (Lee et al. 2006). We find in monkeys in the associative memory task that the percentage of choice errors increases dramatically with the NA choices present. This dependence of behavior on NA occurs even though the perceptual difficulty and motor behavior remain unchanged over all NA conditions.
Previous inactivation studies of FEFs
Previous studies using chemical inactivation of the FEFs have established that this cortical structure is necessary for saccades generated by recall from visuospatial working memory (Dias and Segraves 1999; Sommer and Tehovnik 1997). These studies have described several distinct types of saccadic behavioral deficits following FEF inactivations. There was a tendency for no saccades to be produced when the retinotopic location of the memory coincided with the location represented at the injection site. Those movements that were directed toward the affected region or targets adjacent to this region showed less accurate endpoint control. There was degradation in the monkeys' ability to hold fixation during the delay period, especially when the target was presented in the ipsilateral field. Finally, there was an increase in saccadic latency for movements directed into the affected field. All these deficits following inactivations were greatly reduced when visually guided saccades to single targets were studied. These observations suggest that motor execution to visible targets was relatively less affected by FEF lesions than that produced by recall from memory.
Similar effects were noted following FEF inactivations in a popout visual search task (McPeek 2004; Schiller and Tehovnik 2003). In addition, because there were multiple potential visual stimuli present on each trial (the odd target and distractors), these experiments produced an increased number of targeting deficits (saccades to distractor locations) when the target was located in the retinotopic region coded by the injection site. Saccadic endpoint control was more inaccurate when the movement was directed toward a target at or adjacent to the affected spatial location (McPeek 2004). Again these deficits were milder when visually guided saccades to single targets were performed.
Comparison of the deficits produced by FEF inactivations in the traditional memory-guided task and our associative memory task
The most significant difference we report using our associative memory task is the strong dependence of the percent of choice errors produced on NA. Choice errors when multiple alternatives were present were not possible to study using the traditional memory-guided task, because only a single target location was stored in memory on each trial. In addition, we found that inaccuracy in saccadic endpoint (motor errors) did not vary with NA. Taken together, these differences in the dependence of deficits on NA suggest that two FEF mechanisms are involved in producing the results we observed. The first mechanism involves the formation of a saccadic intension vector from memory inputs and the second is the accurate execution of the saccade vector from the information present in the intension vector.
Only the first mechanism is heavily dependent on the number of choices present in associative memory. A second major difference in the two tasks is that in the traditional working memory paradigm the difficulty of the task is increased by lengthening the duration of the delay period over which the memory trace must be maintained. In the associative memory task there is no delay period—the monkey can make a saccade as soon as the color cue is revealed. Difficulty, at least for choice behavior, in this task is increased by increasing NA. Execution errors (motor errors) do not increase with NA.
Although we focus our explanation of the effects of the inactivations on role of the FEFs in the conversion from memory to intention, our results do not rule out an alternative role for the FEFs. The role of the FEFs in our task may instead be to aid in the suppression of incorrect, alternative-choice processes. Our previous neural recording study in FEF (Lee and Keller 2008) provides some support for this alternative interpretation of these results. In the previous study, we showed that the postcue buildup of activity in visuomotor cells in FEFs was actually more rapid in the 4-NA condition than in the 2- or 1-NA condition. To account for this unexpected finding, we suggested that fixation activity in other cells in the FEFs was raised in the behaviorally more difficult 4-NA condition and that this increased level of fixation activity raised the effective neural threshold for generating a saccade.
Associative memory involves the learned association of a set of nonspatial sensory stimuli with spatially directed saccades, whereas the visuospatial working memory task involves a single visual stimulus and a saccadic eye movement, both of which have the same spatial coordinates. In both types of experiments, it seems unlikely that the object memory coding of color is located in the FEFs (Bichot et al. 1996; Ferrera et al. 1999). For the working memory task, at least major portions of the memory are located in the dorsolateral prefrontal cortex rostral to the FEFs (Sawaguchi and Iba 2001). Associative memory is located in portions of inferior temporal cortex and the prefrontal cortex (see Wise and Murray 2000 for a review). Both these regions project to the FEFs (Leichnetz and Goldberg 1988), possibly providing a pathway over which the nonspatial object information and/or association information is directed to the FEFs where the saccadic intention vector is formed. It seems unlikely that we affected the memory itself with our FEF inactivations. Rather, the effect is more likely to be produced at the level of memory projections to topographically arranged neural circuits in the FEFs where a saccadic intention vector could be formed from the nonspatially coded memory inputs. In this hypothesis, saccades to single visual targets would be executed by the remaining distributed nodes of the saccadic system even in the face of FEF lesions, but with less accuracy than that observed in controls. The lateral intraparietal area may be one of these nodes because inactivation lesions there do not produce deficits in target selection or traditional memory-guided saccades (Wardak et al. 2002).
The postlesion data presented in Fig. 7C show that visually guided saccades to single targets and choice saccades in the 4-NA condition have equal motor error rates that are much larger than those observed for prelesion controls support this hypothesis. To arrive at this conclusion, it is important to remember that we first eliminated trials in which choice errors occurred before the rate of motor errors was computed. Saccadic execution, if directed to the correct quadrant, shows the same degree of saccadic dysmetria for the 4-NA task as for visually guided saccades to single targets.
Comparison of visual search tasks and the associative memory task
Popout visual search paradigms following FEF inactivations show similar problems in target selection and execution to those present in our FEF inactivations, but the results for visual search have not been quantified, so it is difficult to make comparisons across tasks (McPeek 2004; Schiller and Tehovnik 2003). The spatial extent (usually a whole hemifield) of both types of deficits in our inactivations was broader than that reported for visual-search tasks and memory-guided saccade tasks. We found no consistent difference in the percentage of choice errors between trials in which the target was located at either position in the affected hemifield, so we pooled data for the trials in which the target was in the hemifield contralateral or ipsilateral to the lesion site.
The lack of a differential effect of target direction within the contralateral field on the rate of choice errors may be caused by the diffusion of muscimol, although we used similar amounts of the drug and measured effects at similar postlesion times to those used in the study by Dias and Segraves (1999). The latter authors found more pronounced effects within a quadrant of the visual field but did see smaller effects within a whole hemifield. Alternatively, our more spatially distributed effects may be caused by the rapid change of directional preference as a function of penetration depth through the FEFs as shown in Bruce et al. (1985). These investigators reported directional differences as great as 90° in electrically evoked saccades with electrode advances in FEFs of only a couple of millimeters. A third explanation may be that the translation process from sensory discrimination to saccade intention is spatially much more widely distributed within FEF in the multiple choice task so that lesions of the same size produced a wider directional deficit for our choice task than they did for the working memory task in previous studies using muscimol lesions in the FEFs.
Saccade latency
We found no significant difference in saccade latency across NA conditions in the control data collected before the inactivations (Fig. 5C, bottom graph). This lack of effect of NA on saccadic reaction time in the control data in the two highly trained monkeys used in this experiment stands in contrast to our previous report of an increase in latency with NA in less well-trained monkeys (Thiem et al. 2008). Saccade latency did increase following inactivations, particularly for contralateral movements, but this increase did not consistently depend on NA.
The lack of an increase in saccade latency as a function of NA found in our experimental animals is an apparent violation of Hick's Law (Hick 1952). However, in Hick's work in human subjects, it is important to note that error rate was held constant across NA condition. With human subjects, it is relatively easy to achieve this with the proper instructions to the subjects, but it is more difficult to do in monkey subjects. In our two animals, the choice error rate increased as function of NA in the prelesion controls (see Fig. 5A). Thus we do not believe that our results refute Hick's Law.
Directional effects
We found an interesting directional pattern for the choice errors in the 4-NA condition following inactivations. The large majority of choice errors occurred when the central color cue was associated with a correct target located in the contralateral hemifield. Among the preponderance of total contralateral errors, we found that most were directed toward the visual stimulus located horizontally in the ipsilateral hemifield (H type errors) with respect to the correct target. Only a few were made to the visual stimulus located vertically in the same hemifield (V errors) or to the stimulus located diagonally opposite the target (O errors). We speculate that unilateral FEF inactivation weakens the networks in the FEFs receiving associative memory inputs for potential targets located in the contralateral hemifield that accounts for the contralateral preponderance of choice errors. The cause of the horizontal bias in contralateral choice errors is not so clear but may result from the fact that the potential target in the H position is closer in both physical space and color space to the correct target than the only other target in the ipsilateral field, the potential target at the O position.
Deficits following bilateral lesions
The deficits produced by unilateral inactivations in the FEFs in this study and in previous studies (Dias and Segraves 1999; McPeek 2004; Sawaguchi and Iba 2001; Schiller and Tehovnik 2003; Sommer and Tehovnik 1997) may have been caused by an imbalance in the operation of the bilateral FEF and not by a specific deficit in the neural mechanisms underlying memory or target selection. The bilateral FEF are known to inhibit each other under selective conditions (Schlag et al. 1998). Thus a lesion weakening one FEF may lead to the tonic inhibition of that FEF by the other that could prevent the cognitive processing required for memory-guided or target selection behavior to operate while sparing the more reflexively driven visually guided saccades.
To address this possibility, we also made, for the first time, simultaneous inactivations in both FEF. The results following bilateral lesions showed that approximately twice as many choice errors appeared in comparison to the results obtained for unilateral inactivations. We conclude that the FEF are involved in the generation of saccades from both working and associative memory. The approximate doubling of choice errors following bilateral FEF lesions in comparison to the unilateral lesions suggests that the effect of unilateral lesions is not to cause a general inhibition of the lesioned side, but instead involves specific FEF mechanisms concerned with the translation of memory into spatially directed action.
Comparison of Figs. 5B and 8B shows that the spatial pattern of choice errors shifts from a predominance of the H pattern for unilateral lesions to a predominance of the V type for bilateral lesions. When bilateral lesions are made, targets in neither hemifield are relatively disadvantaged as they are for unilateral lesions. This condition allows the closest target in physical space (V type errors) to predominate.
GRANTS
This research was partial supported by National Institutes of Health Grant R01-EY-006860 to E. L. Keller, Brain Research Center of The 21st Century Frontier Research Program, Republic of Korea, to K.-M. Lee, and the Smith Kettlewell Eye Research Institute.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bichot et al. 1996.Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature 381: 697–699, 1996. [DOI] [PubMed] [Google Scholar]
- Bruce et al. 1985.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734, 1985. [DOI] [PubMed] [Google Scholar]
- Deng et al. 1986.Deng S-Y, Goldberg ME, Segraves MA, Ungerleider LG, Mishkin M. The effect of unilateral ablation of the frontal eye fields on saccadic performance in the monkey. In: Adaptive Processes in the Visual and Oculomotor Systems, edited by Keller EL, Zee DS. Oxford, UK: Pergamon Press, 1986, p. 201–208.
- Dias and Segraves 1999.Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol 81: 2191–2214, 1999. [DOI] [PubMed] [Google Scholar]
- Ferrera et al. 1999.Ferrera VP, Cohen JK, Lee BB. Activity of prefrontal neurons during location and color delayed matching tasks. Neuroreport 10: 1315–1322, 1999. [DOI] [PubMed] [Google Scholar]
- Goldberg and Segraves 1989.Goldberg ME, Segraves MA. The visual and frontal cortices. In: The Neurobiology of Saccadic Eye Movements, edited by Wurtz RH, Goldberg ME. New York: Elsevier, 1989, p. 283–313. [PubMed]
- Heinen and Skavenski 1992.Heinen SJ, Skavenski AA. Adaptation of saccades and fixation to bilateral foveal lesions in the adult monkey. Vision Res 32: 365–373, 1992. [DOI] [PubMed] [Google Scholar]
- Hick 1952.Hick WE On the rate of gain of information. Q J Exp Psychol 4: 11–26, 1952. [Google Scholar]
- Lee and Keller 2008.Lee KM, Keller EL. Neural activity in the frontal eye fields modulated by the number of alternatives in target choice. J Neurosci 28: 2242–2251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. 2005.Lee KM, Keller EL, Heinen SJ. Properties of saccades generated as a choice response. Exp Brain Res 162: 278–286, 2005. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2006.Lee KM, Wade AR, Lee BT. Differential correlation of frontal and parietal activity with the number of alternatives for cued choice saccades. Neuroimage 33: 307–315, 2006. [DOI] [PubMed] [Google Scholar]
- Leichnetz and Goldberg 1988.Leichnetz GR, Goldberg ME. Higher centers concerned with eye movement and visual attention: cerebral cortex and thalamus. In: Neuroanatomy of the Oculomotor System, edited by Buettner-Ennever JA. Amsterdam: Elsevier, 1988, p 365–429. [PubMed]
- McPeek 2004.McPeek RM Comparing the effects of frontal eye field and superior colliculus inactivation on saccade target selection. Soc Neurosci Abstr 186.13: 2004.
- Sawaguchi and Iba 2001.Sawaguchi T, Iba M. Prefrontal cortical representation of visuospatial working memory in monkeys examined by local inactivation with muscimol. J Neurophysiol 86: 2041–2053, 2001. [DOI] [PubMed] [Google Scholar]
- Schiller and Chou 1998.Schiller PH, Chou I. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci 1: 248–253, 1998. [DOI] [PubMed] [Google Scholar]
- Schiller and Tehovnik 2003.Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci 18: 3127–3133, 2003. [DOI] [PubMed] [Google Scholar]
- Schiller et al. 1980.Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye field and superior colliculus ablations. J Neurophysiol 44: 1175–1189, 1980. [DOI] [PubMed] [Google Scholar]
- Schlag et al. 1998.Schlag J, Dassonville P, Schlag-Rey M. Interaction of the two frontal eye fields prior to saccade onset. J Neurophysiol 79: 64–72, 1998. [DOI] [PubMed] [Google Scholar]
- Sommer and Tehovnik 1997.Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res 116: 229–249, 1997. [DOI] [PubMed] [Google Scholar]
- Sommer and Wurtz 2000.Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83: 1979–2001, 2000. [DOI] [PubMed] [Google Scholar]
- Thiem et al. 2008.Thiem PD, Hill JA, Lee K-M, Keller EL. Behavioral properties of saccades generated as a choice response. Exp Brain Res 186: 355–364, 2008. [DOI] [PubMed] [Google Scholar]
- Wardak et al. 2002.Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci 22: 9877–9884, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise and Murray 2000.Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci 23: 271–276, 2000. [DOI] [PubMed] [Google Scholar]
- Wyszecki and Stiles 1982.Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. New York: John Wiley, 1982.