Abstract
Within a neuron, spike propagation can occur in a complex manner, with spikes propagating into some processes but not others. We study this phenomenon in an experimentally advantageous mechanoafferent in Aplysia, neuron B21. B21 has two processes within the CNS. One is ipsilateral to the soma and is referred to as the lateral process. The second travels into the contralateral hemiganglion and is referred to as the contralateral process. Previously we characterized spike propagation to the lateral process, which is an output region that contacts follower motor neurons. Spikes fail to actively propagate to the lateral process when B21 is peripherally activated at its resting potential. This propagation failure can be relieved if the medial regions of B21 are centrally depolarized during peripheral activation. This study examines spike propagation to the contralateral process. We show that, unlike the lateral process, active spike propagation in the contralateral process occurs when B21 is peripherally activated at its resting membrane potential. Thus spike propagation occurs selectively, favoring the contralateral process. Interestingly, the contralateral process of one B21 is immediately adjacent to the medial region of the bilaterally symmetrical cell. The B21 neurons are electrically coupled, suggesting that spikes propagating in the contralateral process of one cell could modify propagation in the sister neuron. Consistent with this idea, we show that lateral process propagation failures observed when a single B21 is peripherally activated can be relieved by central coactivation of the contralateral cell. These results imply that stimuli that coactivate the B21 neurons bilaterally are more apt to generate afferent activity that is transmitted to followers than stimuli that activate one cell.
INTRODUCTION
Many neurons have complex patterns of axonal arborizations. In some neurons where this occurs, spikes reliably propagate throughout the axonal tree (Cox et al. 2000; Raastad and Shepherd 2003). In other cases, axonal spike propagation is more variable and can occur selectively (i.e., spikes actively propagate into some parts of the axonal tree and not others) (Debanne 2004). This phenomenon has generated considerable interest, both from the point of view of questions concerning its physiological significance and questions concerning underlying mechanisms. Significant progress has been made in studies investigating both issues in a variety of preparations (for review, see Debanne 2004) including experimentally advantageous invertebrate preparations such as the subject of this study, Aplysia californica (Tauc 1962; Tauc and Hughes 1963; Weiss et al. 1986).
This report studies one such system, the Aplysia mechanoafferent B21 (Rosen et al. 2000). B21 has two processes within the CNS (Fig. 1A). One process travels laterally in the buccal hemiganglion containing the B21 soma (the lateral process). This process is an output region of B21, being the primary point of contact with follower neurons, the B8 radula closer motor neurons (Borovikov et al. 2000). The second is a branch of the medial process that passes into the hemiganglion contralateral to the soma (the contralateral process). Previous work characterized spike propagation to the lateral process (Evans et al. 2003a,b, 2005). This study characterizes spike propagation in the contralateral process (Fig. 1A).
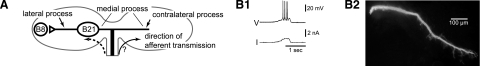
FIG. 1.
A: schematic representation of central regions of B21. Previous experiments in B21 studied spike propagation to the lateral process (left), showing that, although the lateral process is excitable, it is separated from incoming afferent activity by the inexcitable soma. Consequently, when B21 is peripherally activated at its resting potential, spikes actively propagating in the radula nerve fail to actively propagate to the lateral process (indicated by dashed arrow). This study focuses on the contralateral process (right). B: the contralateral process is excitable. The contralateral process was mechanically isolated from the rest of the cell and impaled with an intracellular electrode. When depolarizing current was injected (trace labeled I), spikes were evoked (trace labeled V) (B1). At the conclusion of physiological experiments, electrode placement was verified via the injection of carboxyfluorescein (B2).
In previous studies, we showed that, although the lateral process is excitable (Evans et al. 2003b), it is separated from the source of incoming afferent activity (the radula nerve) by a relatively inexcitable region (the B21 soma; Fig. 1A) (Evans et al. 2007). Consequently, spikes fail to actively propagate to the lateral process when B21 is peripherally activated at its resting membrane potential (Evans et al. 2003b). Here we show that the contralateral process, like the lateral process, is excitable. The contralateral and lateral processes differ, however, in that incoming afferent activity does not pass through the inexcitable soma to reach the contralateral process. When B21 is peripherally activated at its resting membrane potential, spikes do actively propagate to the contralateral process. Thus spike propagation is selective with spikes propagating to the contralateral process but failing to propagate to the lateral process. We studied the possible physiological significance of this phenomenon and show that it can impact afferent transmission when the two bilaterally symmetrical B21 neurons are coactivated by a peripherally applied stimulus.
METHODS
Experiments were conducted in 200- to 300-g Aplysia californica (Marinus, CA) maintained in holding tanks at 14–16°C. Animals were anesthetized by injection of isotonic MgCl2. Experiments were conducted at ∼16°C in artificial seawater (ASW; in mM: 460 NaCl, 10 KCl, 11 CaCl2, 55 MgCl2, and 10 HEPES, pH 7.6).
Electrophysiological equipment included Getting Model 5A amplifiers modified for 100-nA current injection (Getting Instruments, Iowa City, IA), Tektronix AM 502 amplifiers (Tektronix, Wilsonville, OR), and a storage oscilloscope. Data were digitized using a Digidata (Axon Instruments, Union City, CA) and were acquired and analyzed using pClamp version 9 software (Axon Instruments).
To record from the somata of neurons, electrodes were filled with 3 M potassium acetate and 30 mM potassium chloride and were beveled so that their impedances were generally <10 MΩ. To record from processes, electrodes were ∼50 MΩ, and the tip was filled with 3% 5(6)-carboxyfluorescein dye in 0.1 M potassium citrate. Carboxyfluorescein was injected after physiological experiments to verify recording sites. During physiological experiments, the impalement of processes was facilitated by injecting Fast Green dye into the B21 soma and allowing it to diffuse (Evans et al. 2003b). Processes were severed using a glass micropipette. To evoke afferent activity, the subradula tissue was peripherally stimulated using a mini-speaker controlled by a stimulator (Grass Instruments) (Cropper et al. 1996). Time differences between spikes were determined by measuring the peak-to-peak difference.
Five neurons were iontophoretically injected with 200 mM Alexa Fluor 488 or Alexa Fluor 568 (Molecular Devices, Sunnyvale, CA) for confocal microscopy. Microscopy was performed using a Leica TCS-SP inverted system (Leica Microsystems, Wetzlar, Germany) with a ×10 0.3 NA PL Fluotar lens. Multiple stacks of images were taken serially every 2.1 μm and averaged over four scans to reduce noise. Images were combined using Amira 3.1 3D (Mercury Computer Systems, Chelmsford, MA).
Statistical tests were performed with Kaleidagraph (Synergy Software, Essex Junction, VT). Unless otherwise noted, two group comparisons used a paired t-test, and n indicates the number of preparations in which data were obtained. Data are reported as means ± SE. The minimum level of significance for all tests was 0.05.
RESULTS
Selective spike propagation in the contralateral process
To determine whether the contralateral process is excitable, we mechanically isolated it from the rest of the neuron and injected depolarizing current intracellularly. Spikes were triggered (Fig. 1B1; n = 2). These results indicate that the contralateral process is excitable.
To contrast contralateral and lateral spike propagation, we performed experiments under the two sets of conditions where spike propagation failures to the lateral process have been shown (Evans et al. 2003b). First, we performed experiments in which we used a mechanical stimulus to peripherally activate B21 at its resting membrane potential. Lateral process propagation failures under this condition are shown in Fig. 2B (left, top trace). Initial experiments that monitored signal transmission to the contralateral process used the electrode placement shown in Fig. 2A, electrode 4 (referred to as the near contralateral recording site). Recording here is technically less difficult than recording further into the contralateral hemiganglion. When B21 was peripherally activated, what appeared to be regenerative spikes were recorded (mean peak amplitude, 49.7 ± 2.9 mV; n = 12; Fig. 2B, left, bottom trace).
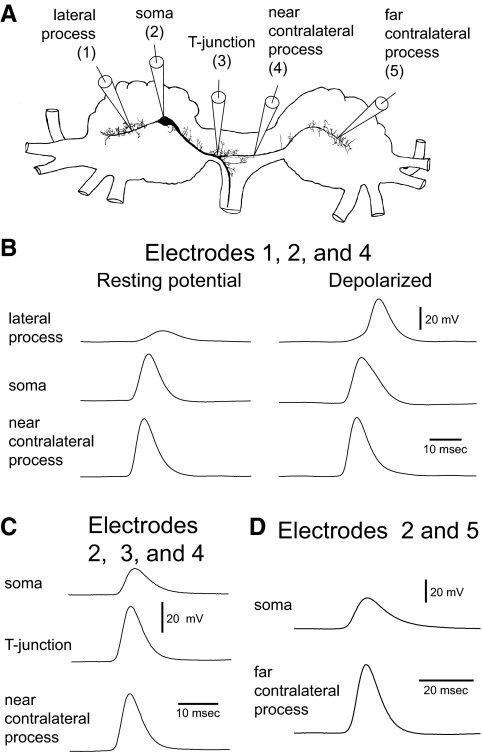
FIG. 2.
Selective spike propagation to the contralateral process with peripheral activation of B21 at its resting membrane potential. A: Camera Lucida drawing of a B21 neuron showing the electrode placement used in B–D. B: left: response to a single mechanical stimulus applied to the subradula tissue with B21 at resting membrane potential. Three intracellular recordings were simultaneously obtained with electrodes at positions labeled 1, 2, and 4 in A. Note that spikes failed to actively propagate to the lateral process (top trace) but that spikes appear regenerative at the near contralateral recording site (bottom trace). Right: reapplication of the mechanical stimulus when B21 was centrally depolarized before (and during) activation. Note that spikes are now recorded in both the lateral and contralateral processes (top and bottom traces). C: experiment as in B with electrodes at positions 2, 3, and 4 in A. Note that spikes at the T-junction and near contralateral recording sites are of similar amplitude (middle vs. bottom traces) but that the somatic impulse (top trace) is smaller. D: experiment as in B with electrodes in B21 at positions 2 and 5 in A. Note that spikes appear regenerative at the far contralateral recording site (bottom trace).
Several further observations are consistent with the idea that these depolarizing impulses are active spikes. Their peak amplitude was not significantly different from the peak amplitude of regenerative spikes recorded in the part of the medial process where bifurcation occurs. We refer to this region as the T-junction to reflect the fact that it resembles the letter of the alphabet (Fig. 2A). In preparations in which we simultaneously recorded from the soma, T-junction, and contralateral process (electrodes 2, 3, and 4 in Fig. 2A), impulses were 42.8 ± 3.2 mV in the contralateral process and 45.8 ± 3.9 mV in the T-junction (Fig. 2C, middle vs. bottom trace; t = 1.4; df = 3; P = 0.3; n = 4). The peak amplitude of contralateral spikes was therefore not significantly different from the peak amplitude of spikes that we have previously shown are actively generated (Evans et al. 2007).
We did, however, observe significant amplitude differences when we compared contralateral spikes to impulses previously identified as electrotonic, i.e., those in the lateral process and soma (Evans et al. 2003b, 2007). In experiments with simultaneous recordings from the lateral process, soma, and contralateral process (electrodes 1, 2, and 4 in Fig. 2A), spikes in the contralateral process had a mean amplitude of 50.9 ± 1.1 mV, whereas impulses in the lateral process had a mean amplitude of 18.4 ± 6.4 mV (t = 5.9; df = 2; P = 0.03; n = 3; Fig. 2B, left, top vs. bottom trace). In experiments with simultaneous recordings from the soma and contralateral process (electrodes 2 and 4 in Fig. 2A), spikes in the contralateral process had a mean amplitude of 49.7 ± 2.9 mV, whereas impulses in the soma had a mean amplitude of 37.6 ± 3.9 mV (t = 5.3; df = 11; P = 0.0002; n = 12; Fig. 2B, left, middle vs. bottom trace; Fig. 2C, top vs. bottom trace).
Finally, depolarizing changes in the holding potential significantly alter the peak amplitude of lateral process recordings because they enable active spiking (Evans et al. 2003b) (also see Fig. 2B, left, top vs. right top trace). In contrast, changes in holding potential had no significant effect on the amplitude of contralateral impulses (the mean amplitude just before somatic depolarization was 51.7 ± 1.3 mV and the mean amplitude after central depolarization was 52.6 ± 1.0 mV; t = 1.6; df = 2; P = 0.2; n = 3; Fig. 2B, left, bottom trace vs. right bottom trace). Our data therefore indicate that when B21 is peripherally activated at its resting membrane potential, spikes actively propagate to regions of the contralateral process that are relatively close to the bifurcation point of the medial process. As might be expected, this activity is progressively transmitted from the T-junction region to the contralateral process. Spikes are initially recorded in the T-junction region and only subsequently recorded in the contralateral process (the mean time difference being 0.45 ± 0.17 ms when recordings were made at the near contralateral site; n = 4).
As the contralateral process passes into the contralateral hemiganglion, a propagation failure could occur. To determine whether this is the case, we simultaneously recorded from the soma and distal parts of the contralateral process (electrode 5 in Fig. 2A, referred to as the far recording site). When B21 was peripherally activated at its resting potential, spikes still appeared to be regenerative at this recording site (mean amplitude, 56.1 ± 4.3 mV; n = 4; Fig. 2D, bottom trace).
The above data indicate that spikes selectively propagate into the contralateral process under one set of conditions, i.e., when B21 is peripherally activated at its resting membrane potential. Our next set of experiments studied spike propagation under the second set of conditions where spike propagation failures to the lateral process have been shown (Evans et al. 2003a), i.e., under conditions where B21 is centrally depolarized and additionally receives inhibitory synaptic input from the B4/5 neurons. Under physiological conditions, this occurs during the radula retraction phase of motor programs (Evans et al. 2003b). Initially, we used an ipsilateral B4/5 (Fig. 3A), because ipsilateral input has been shown to inhibit lateral process propagation (Evans et al. 2003a) (also see Fig. 3B). B21 was centrally depolarized so that spikes would propagate to the lateral process, and B21 was repeatedly peripherally stimulated (at ∼1 Hz; Fig. 3B). A B4/5 neuron was activated to generate firing frequencies approximately twice those observed during motor programs (i.e., 20–30 Hz). Firing frequencies were relatively high so that inhibitory postsynaptic potentials (IPSPs) in B21 would summate, and the relative timing of peripheral activation with respect to IPSP induction would not be a factor. Additionally, high frequencies were used to compensate for the fact that we were only able to stimulate one of the two B4/5 neurons at a time. Although B4/5 stimulation inhibited lateral process spike propagation, it did not significantly alter spike propagation in the contralateral process, i.e., the peak amplitude of the contralateral spike before B4/5 activation was 43.1 ± 3.5 mV and the peak amplitude after B4/5 stimulation was 42.5 ± 3.1 mV (t = 1.2; df = 3; P = 0.3; n = 4; Fig. 3, B and C, right).
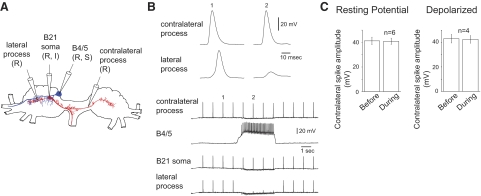
FIG. 3.
Selective spike propagation in the contralateral process when B21 is centrally depolarized and receives inhibitory synaptic input from ipsilateral B4/5 neurons. A: Camera Lucida drawing of a B21 (red) and the ipsilateral B4/5 (blue) showing the electrode placement for the experiment in B. B: B21 was centrally depolarized and peripherally activated at ∼1 Hz when a probe contacted the subradula tissue. The bottom traces are low-speed intracellular recordings from all 4 intracellular electrodes. The top traces are high-speed recordings of the contralateral and lateral responses (1) before B4/5 stimulation and (2) during B4/5 stimulation. Note that B4/5 stimulation selectively inhibits spike propagation to the lateral process. C: right: group data for experiments as shown in B (depolarized). Left: group data for experiments in which effects of B4/5 stimulation were determined when B21 was peripherally activated at its resting membrane potential.
To determine whether the depolarized holding potential in B21 was a factor in determining whether contralateral process spike propagation was altered, we also performed experiments with peripheral activation of B21 at its resting membrane potential. Again, contralateral spike propagation was not altered by B4/5 activation. The mean peak amplitude before B4/5 stimulation was 41.3 ± 3.0 mV, and the mean peak amplitude during B4/5 stimulation was 40.9 ± 2.6 mV (t = 0.6; df = 5; P = 0.6; n = 6; Fig. 3C, left).
Although ipsilateral B4/5 activation was not effective, contralateral activation could possibly produce different results. Note that the contralateral B4/5 is in the same hemiganglion as B21's contralateral process (Fig. 4A). We found, however, that this was not the case. The mean peak amplitude of the B21 contralateral spike before B4/5 stimulation was 53.2 ± 4.9 mV, and the mean peak amplitude during B4/5 stimulation was 52.3 ± 4.8 mV (t = 1.6; df = 5; P = 0.2; n = 6; Fig. 4, B and C). Our data therefore indicate that there are at least two conditions in which selective spike propagation can occur in B21's central processes. This can occur when B21 is peripherally activated at its resting membrane potential and when B21 is centrally depolarized and receives inhibitory input from B4/5.
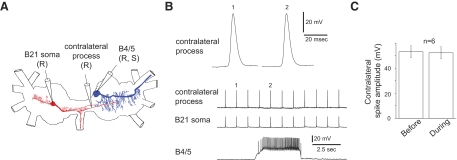
FIG. 4.
Contralateral B4/5 input does not inhibit spike propagation in the contralateral process. A: Camera Lucida drawings of a B21 (red) and a contralateral B4/5 (blue) showing the electrode placement for the experiment shown in B. B: B21 was peripherally activated at ∼1 Hz when a probe contacted the subradula tissue. The bottom traces are low-speed intracellular recordings from all 3 intracellular electrodes. The top trace is a high-speed recording of contralateral responses (1) before B4/5 stimulation and (2) during B4/5 stimulation. Note that B4/5 stimulation does not inhibit contralateral spike propagation. C: group data for experiments as shown in B.
What are the consequences of the selective spike propagation?
We have shown that the lateral process of B21 is an output region, being the primary point of contact with the B8 radula closer motor neurons (Fig. 1A) (Borovikov et al. 2000). Mechanisms that regulate spike propagation to the lateral process therefore regulate ipsilateral mechanoafferent transmission to B8. We showed that the regulation of lateral process spike propagation is, however, not necessarily accompanied by regulation of contralateral spike propagation. Consequently, an important question becomes: does the contralateral process contact the contralateral B8? If so, contralateral B21 mechanoafferent transmission to B8 cells could occur under conditions where ipsilateral transmission fails.
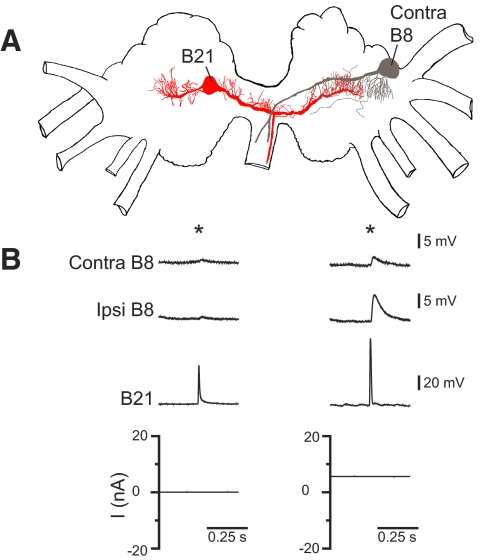
When B21 and contralateral B8 neurons were visualized, possible points of contact became apparent, but regions where processes appeared to overlap were not extensive (Fig. 5A; n = 3). When we peripherally activated B21 at its resting membrane potential and recorded from the contralateral B8, PSPs were virtually nonexistent (Fig. 5B, left top trace; n = 5). Small PSPs only became apparent when B21 was centrally depolarized before peripheral activation (Fig. 5B, right top trace). Contralateral PSPs were significantly smaller than those recorded ipsilaterally. For example, with peripheral activation at depolarized potentials, contralateral PSPs had mean amplitudes of 1.3 ± 0.3 mV, whereas ipsilateral PSPs had mean amplitudes of 3.9 ± 1.0 mV (t = 2.9; df = 4; P = 0.04; n = 5). These data indicate that there is a connection between the contralateral process of B21 and the contralateral B8 neuron. This is, however, a weak connection that only becomes apparent when B21 is centrally depolarized before and during spike initiation. Importantly, mechanoafferent information is not transmitted contralaterally to B8 when transmission to the lateral process fails.
FIG. 5.
The connection between the contralateral process of B21 and the contralateral B8. A: Camera Lucida drawing of a B21 (red) and a contralateral B8 (gray). B: left: B21 was peripherally activated at its resting membrane potential (at the point indicated by the *). Postsynaptic potentials (PSPs) were not observed in the contralateral B8 (despite the fact that spikes actively propagate to the contralateral process under these conditions). Right: when B21 was centrally depolarized before peripheral activation (*), PSPs did become apparent in the contralateral B8, but these PSPs were significantly smaller than the PSPs simultaneously induced in the ipsilateral B8.
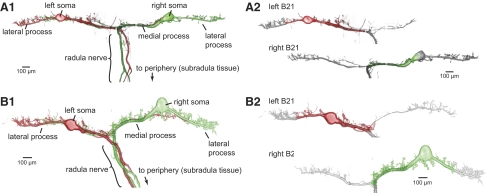
When the two B21 are simultaneously imaged with confocal microscopy, it becomes apparent that the contralateral process of each cell is immediately adjacent to parts of the bilaterally symmetrical neuron (Fig. 6). More specifically, contact most obviously appears to occur throughout the medial and somatic regions of the two cells (colored regions in Fig. 6, A2 and B2), although contact in other regions of the cell would be possible (n = 5 preparations). The medial and somatic regions are of interest because depolarization here before (and during) peripheral activation can modify lateral process spike propagation (Evans et al. 2003b).
FIG. 6.
Confocal image of the 2 bilaterally symmetrical B21 neurons visualized with Alexa Fluor 488 and Alexa Fluor 568. Note that contralateral processes of each neuron contacts medial and somatic regions of the sister cell. This is most apparent in B, which shows the same 2 neurons pseudocolored gray, except for regions where proximity to a contralateral process is observed.
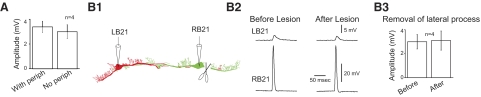
The two B21 neurons are electrically coupled (Rosen et al. 2000). To determine whether this coupling occurs in the somatic and medial regions of close proximity identified in morphological experiments we performed experiments in the isolated buccal ganglion (no periphery present) and induced coupling potentials in one cell by evoking a spike in the other neuron. These coupling potentials were not significantly smaller than those observed in preparations in which the periphery was present (Fig. 7A; 3.4 ± 0.5 vs. 3.0 ± 0.6 mV; t = 0.5; df = 6; P = 0.6; n = 4). We lesioned the stimulated cell by removing its lateral process (Fig. 7B1). This did not significantly decrease coupling potential peak amplitude (Fig. 7, B2 and B3). The mean peak amplitude before the lesion was 3.0 ± 0.6 mV. After the lesion, it was 3.1 ± 0.8 mV (t = 0.4; df = 3; P = 0.7; n = 4). Thus there is a connection between the two B21 neurons in regions where spikes are either actively propagating (i.e., medial and contralateral processes) or where transmission is electrotonic but little decay has occurred (the soma). Potentially, therefore, afferent activation of one cell could impact spike propagation in the other.
FIG. 7.
The 2 B21s are electrically coupled in the medial and somatic region. A: the peak amplitude of coupling potentials is not significantly altered by removal of the periphery. B1: Camera Lucida drawing of the 2 B21 neurons showing electrode placement and the lesion made in the experiment shown in B2. B2: coupling potentials before and after removal of the lateral process. Note that the peak amplitude is virtually unchanged. B3 group data for experiments as shown in B2.
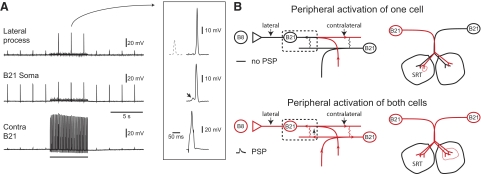
In previous work, B21 was peripherally activated with a weak mechanical stimulus that most commonly selectively activates one of the two bilaterally symmetrical neurons. To determine whether spike propagation in one cell can be altered by coactivation of its homologue, we used this stimulus (Fig. 8A). One cell was peripherally activated, and spike propagation was monitored with and without central activation of the contralateral neuron. To activate contralateral cells, spikes were induced via the injection of brief current pulses. This evoked coupling potentials in the contralateral cell, which were larger in somatic recordings (Fig. 8A, see arrow in inset). Experiments were conducted under two conditions. Initially, the two B21 neurons were coactivated at resting membrane potential. This did not induce lateral process spike propagation (data not shown). Second, one or both cells were centrally depolarized to simulate conditions observed during the retraction phase of motor programs (i.e., DC current was injected to depolarize cells by ∼6 mV). An additional benefit of this manipulation was that spikes could be evoked with the injection of relatively small current pulses. Consequently, most (i.e., ∼70%) of the depolarization in the contralateral neuron was caused by the coupling potential (and not current injection). Under these conditions, B21 coactivation did induce lateral process spike propagation (Fig. 8A). Electrotonic potentials recorded in the lateral process before the activation of the contralateral were on average 13.0 ± 1.0 mV, whereas the full-size spikes observed with contralateral activation were on average 51.4 ± 2.2 mV (t = 23.8; df = 3; P = 0.0001633; n = 4). These results suggest that stimulus detection will be impacted by whether or not B21 coactivation occurs when other radula mechanoafferent input is present (e.g., during radula retraction).
FIG. 8.
A: spiking in 1 B21 can modify spike propagation in the contralateral cell. One B21 was repeatedly peripherally activated at a membrane potential that was above resting potential but was not sufficient to induce spike propagation to the lateral process. Action potentials were triggered in the contralateral cell via the injection of brief current pulses during the period of time indicated by the bar under the bottom trace. This induced coupling potentials, which are primarily observed in somatic recordings (see arrow in the middle trace in the inset). These potentials modified spike propagation. In the inset, compare the electrotonic potential shown with the dashed line (which was recorded before the activation of the contralateral neuron) to the full-size spike recorded during the period of stimulation (recording shown with the solid line). B: left: proposed model of spike propagation when only 1 B21 neuron is activated (top) and when both cells are activated (bottom). For simplicity, our discussion is focused on the region shown in the dashed box. When only 1 cell is activated (top), spikes actively propagate in the regions shown in red. Active propagation fails in the somatic region of the neuron, in part because of the fact that this region is inexcitable. When both cells are peripherally activated (bottom), the somatic region of each neuron receives additional depolarizing input in the form of coupling potentials generated by the spiking in the contralateral process of the sister neuron. This will tend to promote lateral process spike propagation and afferent transmission to B8. Right: stimulus properties will determine the pattern of B21 activation. Top: the B21 neurons have bilateral, partially overlapping receptive fields. If a stimulus is applied to a region where receptive fields do not overlap, the B21 neurons will be selectively activated (i.e., 1 cell is shown in red). Bottom: if a stimulus is applied to a region where the receptive fields overlap, both cells will be peripherally activated (i.e., both are shown in red).
DISCUSSION
Our experiments studied spike propagation in B21, a radula mechanoafferent used during feeding in the mollusk Aplysia. We showed that peripherally triggered action potentials can selectively propagate within B21's central processes. Although selective spike propagation has been shown in a number of other cells, it is not always clear how it is mediated. Modeling and theoretical studies have shown that preferential propagation cannot necessarily be explained simply by relative differences in branch diameter (Goldstein and Rall 1974; Segev and Schneidman 1999). Additional asymmetries must be present. In the neuron B21, propagation is presumably influenced by the fact that impulses must be transmitted through the relatively inexcitable somatic region of B21 to reach the lateral process. Somatic impulse transmission has been shown to induce propagation failures and reduce excitability in other neurons (Antic et al. 2000; Graubard and Hartline 1991; Luscher et al. 1994), in part as a result of the loading effect of the soma. Additionally, in previous work, we have shown that outward currents are generated by depolarization in the somatic region of B21 (Evans et al. 2007), which can impact excitability (Graubard and Hartline 1991). Somatic transmission does not, however, inhibit contralateral spike propagation. This may be because of the fact that the bifurcation point in the medial process (the T-junction) is not directly adjacent to the soma, i.e., a length of medial process separates the T-junction and soma. In dorsal root ganglion (DRG) neurons, an apparently similar “stem” region serves an important function, i.e., it promotes and speeds conduction by masking the electrical load of the soma (Luscher et al. 1994). Thus spikes presumably propagate into the contralateral process of B21 because the loading effect of the soma is masked. In contrast, when spikes propagate into the lateral process, effects of somatic transmission are manifested.
Previous physiological experiments that studied spike propagation in B21 focused on the lateral process (Evans et al. 2003a,b, 2005). The lateral process contacts ipsilateral follower neurons (the B8 cells; Fig. 1A). Our results indicate that inhibition of ipsilateral spike propagation is not necessarily accompanied by inhibition of contralateral spike propagation. An important question therefore becomes: does the contralateral process make contact with the contralateral B8 neurons? If so, previously characterized processes that inhibit lateral process spike propagation might only be inhibiting B21–B8 mechanoafferent transmission ipsilaterally. We showed that, although there is a connection between the contralateral process of B21 and the contralateral B8 neurons, the connection is weak. Importantly PSPs are not observed in the contralateral B8 under conditions where spike propagation to the lateral process fails. Thus selective propagation does not seem to induce contralateral B8 activation under conditions where ipsilateral activation fails.
A further question is why PSPs were not observed in the contralateral B8 when B21 was activated at its resting membrane potential. We showed that spikes actively propagate to the contralateral process, and the data shown in Fig. 5B indicate that there is a connection between the contralateral process and the contralateral B8. A likely possibility is that synaptic transmission is determined by holding potential, as has been shown for other Aplysia neurons (Shapiro et al. 1980). Thus at resting potential, spikes propagate, but there is relatively little transmitter release. With further depolarization, synaptic transmission is potentiated, e.g., by a change in the steady-state calcium level.
We showed that the contralateral process of one B21 neuron is in close proximity to the medial process (and somatic region) of its homolog. The two B21 neurons are electrically coupled to each other (Rosen et al. 2000), and we showed that the coupling occurs in medial regions of both cells. This suggests that spiking activity in one B21 neuron could modify action potential propagation in the contralateral cell (as shown schematically in Fig. 8B, left). Thus when one cell is peripherally activated, spike propagation fails as activity is transmitted through the inexcitable somatic region of the neuron. When both cells are activated, the somatic region will be depolarized as a result of the spikes actively propagating in the contralateral process of its electrically coupled homolog. Our data showed that this depolarizing input can modify spike propagation. An important consequence of this arrangement is that stimuli that bilaterally activate the two B21 neurons are more apt to induce radula mechanoafferent activity that is transmitted to follower motor neurons than stimuli that selectively activate one cell.
The B21 neurons have bilateral receptive fields that are partially overlapping (Fig. 8B, right) (Rosen et al. 2000). Consequently, application of a mechanical stimulus to the subradula tissue can produce either selective activation of a single cell or coactivation of both neurons. Selective activation will occur if a stimulus is applied to a region where receptive fields do not overlap (Fig. 8B, top right). Coactivation will occur if the stimulus is applied to an overlapping region (Fig. 8B, bottom right). Under experimental conditions, selective activation of the B21 neurons has only been observed when a relatively small diameter probe has been used to deliver a highly localized mechanical stimulus (e.g., Fig. 8A). With more widespread mechanical stimulation, both cells are activated (Miller et al. 1994). This suggests that, under physiological conditions, stimulus properties will determine the pattern of mechanoafferent activation. For example, selective activation of the B21 neurons may occur with the ingestion of a small piece of food (e.g., algae). In contrast, coactivation of the B21 neurons is most apt to occur with the ingestion of a larger piece of food (e.g., seaweed).
This arrangement is likely to be of physiological significance because it will tend to promote radula mechanoafferent transmission to follower motor neurons under conditions where it will be particularly beneficial. Thus follower motor neurons contacted by the B21 cells are the B8 cells, which are radula closer motor neurons (Morton and Chiel 1993). Although the B8 neurons are phasically driven during the radula retraction phase of ingestive motor programs in the absence of mechanoafferent activation, their firing frequency is significantly increased by additional excitatory input from the B21 cells (Shetreat-Klein and Cropper 2004). Increases in the B8 firing frequency produce stronger closing movements (Orekhova et al. 2001). Stronger closing movements are likely to be particularly important when the piece of ingested food is large. Enhanced closing will tend to prevent food from slipping between the radula halves as it is pulled through the buccal cavity. We suggest therefore that modification of spike propagation induced by radula mechanoafferent coactivation will tend to couple afferent transmission to stimulus properties.
A further question that arises is how this type of coupling will impact the phasic control of mechanoafferent transmission that has been characterized in previous work. Thus we have shown that B21 receives phasic depolarizing input during feeding motor programs via its electrical coupling with pattern generating interneurons (Evans et al. 2003b). This depolarization promotes lateral process spike propagation and afferent transmission to B8 and occurs during the radula retraction phase of ingestive motor programs (Evans et al. 2003b). We have argued that this is a type of central control that insures that responses to peripheral activation occur at the behaviorally appropriate time. Obviously, however, the phasic nature of the afferent regulation would be altered if radula mechanoafferent coactivation were sufficient to induce spike propagation in the absence of the central input (i.e., during the protraction phase of motor programs). Interestingly, however, we found that we were not able to induce spike propagation in one B21 via the activation of its homolog if both cells were at their resting membrane potential. We show therefore that bilateral activation of the B21 neurons on its own is not sufficient to induce afferent transmission. Central depolarization is also necessary. This suggests that the type of regulation that we characterize in this study is most apt to be of physiological significance when B21 is centrally depolarized but the depolarization is not sufficient to induce lateral spike propagation. This occurs during the early part of radula retraction (Evans et al. 2000). Thus we do not expect that the mechanism that we characterize in this study alters the phasic control of afferent transmission in that it induces protraction phase excitation of B8. Instead we expect that it modifies the extent to which radula mechanoafferent transmission occurs during radula retraction.
To summarize, taken together, results of this study and our previous work showed that active spike propagation in the radula mechanoafferent B21 is in part determined by input from pattern generating interneurons during feeding motor programs. This central control determines the phase of the motor program in which afferent transmission occurs. In this report, we show that spike propagation can also be promoted by radula mechanoafferent coactivation. We suggest that the latter is likely to be a mechanism that couples afferent transmission to stimulus properties.
GRANTS
This work was supported by National Institute of Mental Health Grant MH-51393. Some of the Aplysia used in this study were provided by the National Resource for Aplysia of the University of Miami under Division of Research Resources Grant RR-10294. Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from National Institutes of Health-NCI Shared Resources Grant 5R24 CA-095823-04, National Science Foundation Major Research Instrumentation Grant DBI-9724504, and National Institutes of Health Shared Instrumentation Grant 1 S10 RR-0 9145-01.
Acknowledgments
We thank K. R. Weiss and B. Ludwar for valuable comments on an earlier version of this manuscript and A. H. Gillard for expert assistance with the preparation of the figures of the Camera Lucida drawings of B4/5, B8, and B21.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Antic et al. 2000.Antic S, Wuskell JP, Loew L, Zecevic D. Functional profile of the giant metacerebral neuron of Helix aspersa: temporal and spatial dynamics of electrical activity in situ. J Physiol 527: 55–69, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikov et al. 2000.Borovikov D, Evans CG, Jing J, Rosen SC, Cropper EC. A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia. J Neurosci 20: 1990–2002, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox et al. 2000.Cox CL, Denk W, Tank DW, Svoboda K. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc Natl Acad Sci USA 97: 9724–9728, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper et al. 1996.Cropper EC, Evans CG, Rosen SC. Multiple mechanisms for peripheral activation of the peptide-containing radula mechanoafferent neurons B21 and B22 of Aplysia. J Neurophysiol 76: 1344–1351, 1996. [DOI] [PubMed] [Google Scholar]
- Debanne 2004.Debanne D Information processing in the axon. Nat Rev Neurosci 5: 304–316, 2004. [DOI] [PubMed] [Google Scholar]
- Evans et al. 2000.Evans CG, Jing J, Cropper EC. Phase-specific gating of afferent activity in an Aplysia mechanosensory neuron. Soc Neurosci Abstr 26: l74, 2000. [Google Scholar]
- Evans et al. 2003a.Evans CG, Jing J, Proekt A, Rosen SC, Cropper EC. Frequency-dependent regulation of afferent transmission in the feeding circuitry of Aplysia. J Neurophysiol 90: 3967–3977, 2003a. [DOI] [PubMed] [Google Scholar]
- Evans et al. 2003b.Evans CG, Jing J, Rosen SC, Cropper EC. Regulation of spike initiation and propagation in an Aplysia sensory neuron: gating-in via central depolarization. J Neurosci 23: 2920–2931, 2003b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans et al. 2007.Evans CG, Ludwar BC, Cropper EC. Mechanoafferent neuron with an inexcitable somatic region: consequences for the regulation of spike propagation and afferent transmission. J Neurophysiol 97: 3126–3130, 2007. [DOI] [PubMed] [Google Scholar]
- Evans et al. 2005.Evans CG, Romero A, Cropper EC. Inhibition of afferent transmission in the feeding circuitry of aplysia: persistence can be as important as size. J Neurophysiol 93: 2940–2949, 2005. [DOI] [PubMed] [Google Scholar]
- Goldstein and Rall 1974.Goldstein SS, Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J 14: 731–757, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard and Hartline 1991.Graubard K, Hartline DK. Voltage clamp analysis of intact stomatogastric neurons. Brain Res 557: 241–254, 1991. [DOI] [PubMed] [Google Scholar]
- Luscher et al. 1994.Luscher C, Streit R, Quadroni R, Luscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation proterties. J Neurophysiol 72: 622–633, 1994. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1994.Miller MW, Rosen SC, Schissel SL, Cropper EC, Kupfermann I, Weiss KR. A population of SCP-containing neurons in the buccal ganglion of Aplysia are radula mechanoafferents and receive excitation of central origin. J Neurosci 14: 7008–7023, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton and Chiel 1993.Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol 173: 519–536, 1993. [DOI] [PubMed] [Google Scholar]
- Orekhova et al. 2001.Orekhova IV, Jing J, Brezina V, DiCaprio RA, Weiss KR, Cropper EC. Sonometric measurements of motor neuron evoked movements of an internal feeding structure (the radula) in Aplysia. J Neurophysiol 86: 1057–1061, 2001. [DOI] [PubMed] [Google Scholar]
- Raastad and Shepherd 2003.Raastad M, Shepherd GM. Single-axon action potentials in the rat hippocampal cortex. J Physiol 548: 745–752, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen et al. 2000.Rosen SC, Miller MW, Evans CG, Cropper EC, Kupfermann I. Diverse synaptic connections between peptidergic radula mechanoafferent neurons and neurons in the feeding system of Aplysia. J Neurophysiol 83: 1605–1620, 2000. [DOI] [PubMed] [Google Scholar]
- Segev and Schneidman 1999.Segev I, Schneidman E. Axons as computing devices: basic insights gained from models. J Physiol Paris 93: 263–270, 1999. [DOI] [PubMed] [Google Scholar]
- Shapiro et al. 1980.Shapiro E, Castellucci VF, Kandel ER. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci USA 77: 629–633, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetreat-Klein and Cropper 2004.Shetreat-Klein AN, Cropper EC. Afferent-induced changes in rhythmic motor programs in the feeding circuitry of aplysia. J Neurophysiol 92: 2312–2322, 2004. [DOI] [PubMed] [Google Scholar]
- Tauc 1962.Tauc L Site of origin and propagation in spike in the giant neuron of Aplysia. J Gen Physiol 45: 1077–1097, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc and Hughes 1963.Tauc L, Hughes GM. Modes of initiation and propagation of spikes in the branching axons of molluscan central neurons. J Gen Physiol 46: 533–549, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss et al. 1986.Weiss KR, Chiel HJ, Kupfermann I. Sensory function and gating of histaminergic neuron C2 in Aplysia. J Neurosci 6: 2416–2426, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]