Abstract
Development of the embryonic nervous system requires thyroid hormone. However, the underlying mechanisms and targets of thyroid hormone action are not well defined. To identify embryonic roles for thyroid hormone we tested for effects on a key neuronal trait, voltage-gated sodium current (INa), in the zebrafish model system. We recorded from Rohon–Beard sensory neurons (RBs) using whole cell voltage-clamp methods. Here, we provide in vivo evidence for thyroid hormone regulation of INa. Chronic thyroid hormone application increased RB peak INa density 1.4-fold. However, INa density showed a similar increase within 5 min of an acute hormone application, a time course not expected for a genomic mechanism. Tetraiodothyroacetic acid (tetrac), a thyroid hormone blocker, blocked both chronic and acute effects. Further, the thyroid hormone precursor thyroxine (T4) affected INa, yet the traditionally active form triiodothyronine did not. Consequently, we tested for a nonconventional T4 receptor. LM609, a selective antagonist of integrin αVβ3, occluded the rapid effect of T4, implicating a specific integrin dimer as a T4 receptor. Chronic application of either tetrac or LM609 significantly reduced sodium conductance, demonstrating an in vivo requirement for T4-integrin regulation of INa. Further, removing endogenous T4 levels via yolkectomy reduced sodium conductance, an effect that was partially rescued by T4 supplementation following surgery. Because RBs mediate the embryonic touch response, we tested for behavioral effects. Tetrac and LM609 significantly reduced the percentage of touch trials eliciting a normal touch response. T4's rapid effect on RB INa highlights the importance of embryonic T4 availability and nongenomic T4 signaling.
INTRODUCTION
Maternally supplied thyroid hormones are present during embryonic stages before onset of fetal thyroid hormone production and play essential roles in vertebrate nervous system development (Calvo et al. 2002; Contempre et al. 1993; Ferreiro et al. 1988; James et al. 2007; Vulsma et al. 1989). For example, lack of maternal thyroid hormone exposure during embryonic development causes irreversible mental retardation (Hetzel 2000) and maternal hypothyroidism results in developmental delays and behavioral deficits after birth (Francis and Riley 1987; Mirabella et al. 2000; Pacaud et al. 1995; Rovet and Hepworth 2001; Smit et al. 2000). Conversely, maternal hyperthyroidism poses risks to fetal development by significantly increasing miscarriage rates (Anselmo et al. 2004; Kriplani et al. 1994). The clinical symptoms observed following altered maternal thyroid hormone states during embryonic stages indicate that thyroid hormones regulate neuronal properties during early development.
Previous data show that thyroid hormones modulate voltage-gated sodium current (INa) in neonatal cardiac muscle (Huang et al. 1999) and neurons (Potthoff and Dietzel 1997). However, the effects of thyroid hormone on embryonic neurons are not well studied. INa not only underlies rapid signaling in neurons but also regulates many aspects of neurodevelopment, including programmed cell death (Svoboda et al. 2001), axonal morphology (Pineda et al. 2006), and behavior (Ribera and Nüsslein-Volhard 1998). If thyroid hormone regulated voltage-gated sodium channel function during embryonic stages, then rapid signaling as well as neurodevelopment would be altered by thyroid disorders.
Conventional thyroid hormone action involves triiodothyronine (T3) binding to nuclear thyroid hormone receptors and modulation of gene expression, producing cellular effects after considerable delay. However, emerging evidence supports an alternate signaling pathway that requires plasma membrane receptors, occurs rapidly within minutes, and shows increased selectivity for thyroxine (T4), the T3 precursor (Davis et al. 2004). In vitro studies with cultured fibroblasts or chick chorioallantoic membranes indicate that nongenomic effects require T4 interaction with an αVβ3 integrin to initiate rapid effects (Bergh et al. 2005; Mousa et al. 2006). The αVβ3 integrin belongs to the Arg-Gly-Asp (RGD)–type integrin family, which influences zebrafish neurodevelopment (Becker et al. 2003). In addition, the RGD protein-recognition site of αVβ3 binds T4 (Davis et al. 2005). Whether T4 integrin-dependent mechanisms also play a role in vivo during embryonic neurodevelopment is unknown.
The developing zebrafish (Danio rerio) embryo provides a model to test for in vivo roles of thyroid hormone in regulation of neuronal function during embryogenesis. We assayed physiological function of embryonic Rohon–Beard cells (RBs) using both whole cell voltage-clamp and behavioral assays. We focused on INa because of the important roles this current plays in neuronal signaling (Pineda et al. 2006; Svoboda et al. 2001). Moreover, the macho mutant has demonstrated that the embryonic touch response requires a critical RB INa density to initiate swimming behavior (Granato et al. 1996; Ribera and Nüsslein-Volhard 1998), allowing for a behavioral assay of RB INa modulation. In addition, the zebrafish embryo allows manipulation of thyroid hormone signaling in vivo by adding exogenous or decreasing endogenous T4 levels and by blocking endogenous T4 receptors. Using these approaches, we obtained evidence for an integrin-dependent nongenomic T4 mechanism in vivo that rapidly modulates INa in embryonic sensory neurons.
METHODS
Animals
Zebrafish (Danio rerio) adults were bred according to guidelines outlined in The Zebrafish Book (Westerfield 1995). Embryos were incubated at 28.5°C in embryo media (EM: 130 mM NaCl, 0.5 mM KCl, 0.02 mM Na2HPO4, 0.04 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.4 mM NaH2CO3) and staged according to external morphology (Kimmel et al. 1995).
Electrophysiology
Whole cell voltage-clamp recordings were obtained from zebrafish spinal cord RBs at 50- to 55-h postfertilization (hpf) (Pineda et al. 2005; Ribera and Nüsslein-Volhard 1998). Zebrafish were immobilized in Ringer solution [(in mM):145 NaCl, 3 KCl, 1.8 CaCl2, and 10 HEPES, pH 7.2] containing 0.02% tricaine (Sigma) and glued to glass coverslips with Vetbond (3M, St. Paul, MN) tissue adhesive. Glass dissecting needles sufficed for removal of the skin, thus avoiding use of proteolytic enzymes. After dissection, the preparation was washed with ≥40 mL extracellular recording solution for 15 min, providing a semiintact preparation for recordings. We used a reduced extracellular sodium bath solution [(in mM): 30 NaCl, 97 N-methyl glucamine, 20 tetraethylammonium, 3 KCl, 2 CoCl2, and 10 HEPES] to limit INa amplitudes and reduce potential series resistance voltage errors (Pineda et al. 2005). Glass electrodes (2.0–3.5 MΩ) were filled with solution containing (in mM): 10 NaCl, 135 CsCl, 10 EGTA, and 10 HEPES. We subtracted passive leak currents and capacitive transients from recordings of voltage-gated sodium and potassium currents using a P/8 protocol. Data were recorded using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and data were analyzed with Clampfit8 (Axon Instruments) and Origin software (OriginLab, Northhampton, MA). Results are presented as means ± SE. Statistical analysis was performed with Origin v7.0 software (OriginLab). Except for the behavioral data presented in the following text, statistical comparisons of means were performed using unpaired two-tailed t-tests or one-way ANOVAs with Bonferroni correction for multiple comparisons.
Hormone and drug application
Solutions of T4 (3,3′,5,5′-tetraiodo-l-thyronine; Sigma), T3 (3,3′,5-triiodolthyronine; Sigma), and tetrac (3,3′,5,5′-tetraiodothyroacetic acid; Sigma) were diluted to final concentrations in solutions of embryo media for chronic recordings and extracellular recording solution for acute recordings. During chronic application, hormone solutions were replaced daily to minimize deiodination effects. Tetrac (10 μM) was applied to embryos by addition to embryo media. The LM609 antibody (Millipore, Danvers, MA) was microinjected directly into the yolk sac of 24-hpf embryos. LM609 (50 μg/mL) was diluted with H2O and 1% Fast Green dye to visually confirm distribution of microinjections throughout the zebrafish embryo. Vehicle controls consisted of 1% Fast Green injections.
Behavioral studies
We assayed touch sensitivity of 48-hpf embryos as described by Pineda et al. (2005). We performed touch sensitivity assays with the observer impartial to the treatment groups. To focus on sensory versus motor function, we verified that embryos could swim spontaneously with a normal pattern. Next, we gave each embryo ten touch trials with a 3-s rest between trials; each trial entailed gentle application of a metal probe to the dorsal trunk. For each trial, the response outcome was scored as none, abnormal (e.g., segmentally restricted trunk bend or twitching movement), or normal (i.e., swimming response). Results are presented as histograms that display the percentage of trials generating each response outcome for each treatment group. For each outcome, we compared different treatment groups with nonparametric statistical tests (Mann–Whitney or Kruskal–Wallis followed by Dunn's test for multiple comparisons) using InStat3 software (GraphPad) and present data as means ± SE.
Yolkectomy
To reduce endogenous T4 stores, we partially removed (50%) the yolk sac by suction using a mouth pipette from 23- to 25-hpf wildtype embryos. Following surgery, embryos were placed in either normal EM or EM supplemented with 30 nM T4 for recovery experiments and returned to a 28.5°C incubator. Sham yolkectomies consisted of yolk sac puncture only without suction.
RESULTS
Chronic exposure to T4, but not T3, increases peak INa density in zebrafish embryonic sensory neurons
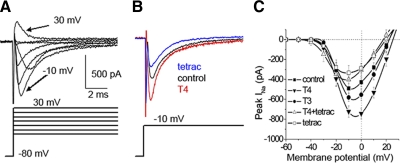
In initial experiments, we raised zebrafish embryos beginning with the first hpf in embryo media containing either 30 nM T4, 30 nM T3, or no hormone. To test for thyroid hormone effects on RB INa in 50- to 55-hpf embryos, we elicited sodium currents with depolarizing pulses (Fig. 1A). Chronic T4 exposure increased peak INa by 46% over control (P < 0.01) without changing cell capacitance (control: 3.2 ± 0.3 pF; n = 21; T4: 3.2 ± 0.2 pF; n = 22; P = 0.87). In contrast, chronic application of the thyroid hormone blocker tetrac reduced peak INa by 37% compared with control (P < 0.05) (Fig. 1B), demonstrating that T4 interactions with a thyroid hormone receptor modulated INa. Tetrac application did not significantly affect cell capacitance compared with controls (tetrac 3.3 ± 0.2 pF; n = 17; P = 0.89). Chronic application of 30 nM T3 did not significantly alter peak INa compared with controls. The current–voltage plots demonstrated that peak amplitudes for controls occurred near 0 mV and reversal potentials averaged near +22 mV, close to the calculated reversal potential of +27.7 mV. In response to T4 incubation, INa amplitude increased at all test potentials without changing the reversal potential (Fig. 1C).
FIG. 1.
Chronic T4 (3,3′,5,5′-tetraiodo-lthyronine) treatment increased sodium current (INa) amplitude. A: INa was elicited by depolarizing voltage steps ranging between −30 and +30 mV in 30 mM extracellular sodium solution from a holding potential of −80 mV. Traces represent recordings obtained from Rohon–Beard sensory neurons (RBs) in 50- to 55-h postfertilization (hpf) embryos raised in media alone (control). B: chronic incubation in 30 nM T4 increased peak INa amplitude (red) in comparison to control (black). Conversely, chronic incubation with the thyroid hormone antagonist 3,3′,5,5′-tetraiodothyroacetic acid (tetrac; blue) reduced INa amplitude. C: average current–voltage (I–V) relationships for INa recorded from embryos incubated in control (▪; n = 18), T4 (▾; n = 8), T3 (3,3′,5-triiodo-l-thyronine, •; n = 8), T4 + tetrac (▵; n = 6), and tetrac (□; n = 8) media. Chronic exposures to T4 but not T3 increased peak INa amplitude vs. control, respectively (P < 0.01). Effects of T4 were prevented by coapplication of the antagonist tetrac. Moreover, tetrac alone reduced INa amplitude, suggesting in vivo modulation of INa by endogenous T4 (P < 0.05).
To test whether the chronic T4 effects were specific to INa or affected multiple ion currents, we recorded both leak and voltage-gated potassium currents (IKv) from RB cells in either control or 30 nM T4-treated embryos. Leak currents were assessed between −100 and −60 mV, a range not expected to activate voltage-dependent nonohmic conductances. We found that leak currents were not affected by chronic T4 treatment (Fig. 2, A and B). Similarly, chronic T4 exposure had no effect on steady-state IKv compared with control (Fig. 2, C and D) and there were no consistent effects observed on IKv kinetics after application of T4. Taken together, the results suggest a selective action of T4 on INa.
FIG. 2.
Chronic T4 treatment did not affect steady-state potassium current (IKv). A: background currents were recorded in the absence of leak subtraction and elicited with hyperpolarizing and depolarizing stimuli in the range of −100 to −60 mV from a holding potential of −80 mV. B: leak currents activated between −100 and −60 mV displayed linear (ohmic) I–V relationships for both control (•; n = 4) and T4 ( ; n = 4) treated RB recordings. The leak conductance, evaluated from the slope of the I–V curve, was unaffected by T4 treatment. C: potassium currents were elicited by voltage steps from −70 to +30 mV for 30 ms from a holding potential of −80 mV. D: steady-state IKv was measured as the mean current elicited by +30 mV for the last 5 ms of depolarization. Mean current was normalized for cell size and T4 treatment did not alter cell capacitance while recording IKv. Chronic incubation of 30 nM T4 (n = 5) had no effect on steady-state IKv compared with controls (n = 17).
; n = 4) treated RB recordings. The leak conductance, evaluated from the slope of the I–V curve, was unaffected by T4 treatment. C: potassium currents were elicited by voltage steps from −70 to +30 mV for 30 ms from a holding potential of −80 mV. D: steady-state IKv was measured as the mean current elicited by +30 mV for the last 5 ms of depolarization. Mean current was normalized for cell size and T4 treatment did not alter cell capacitance while recording IKv. Chronic incubation of 30 nM T4 (n = 5) had no effect on steady-state IKv compared with controls (n = 17).
We next examined whether T4 modulated sodium maximal conductance (Gmax) and voltage-dependent properties of steady-state activation by determining the effects of T4 on the sodium conductance–voltage relationship. We accounted for differences in cell size by normalizing conductance to cell capacitance, yielding conductance density. Chronic incubation in 30 nM T4 increased RB sodium Gmax by 43% from the control value (P < 0.05), whereas chronic incubation in 30 nM T3 had no significant effect on sodium Gmax (Fig. 3A). Coincubation of T4 with tetrac, a competitive thyroid hormone antagonist, blocked the effect of T4 on Gmax, consistent with involvement of a specific thyroid hormone receptor. The conductance–voltage plots (Fig. 3, B and C) showed no significant changes in either midpoint of activation (V1/2) or slope factor (k) following chronic application or blockade of thyroid hormones, suggesting that T4 does not alter INa amplitudes by shifting the voltage dependence of steady-state activation.
FIG. 3.
Chronic T4 and tetrac altered RB sodium maximal conductance (Gmax). A: mean sodium RB Gmax was increased by 30 nM T4 (n = 12) and decreased by either 30 nM T4 + 10 μM tetrac (n = 6) or 10 μM tetrac alone (n = 9) compared with controls (n = 18) at 50–55 hpf (*P < 0.05, ANOVA). B: average voltage dependence of steady-state activation relationships for embryos incubated in control (▪), T4 (▾), T3 (•), T4 + tetrac (▵), and tetrac (□) media. C: chronic hormone incubation did not affect either the midpoint of activation (V1/2) or slope factor (k) for steady-state activation curves.
T4 shows physiologically relevant regulation of RB INa
The data presented earlier indicate that exogenous T4 modulates RB INa. However, application of exogenous hormone does not address whether endogenous T4 regulates RB INa in vivo. Maternally derived stores present in the embryonic yolk sac provide T4 to the embryo prior to function of the zebrafish thyroid gland at 72 hpf (Brown 1997). We pharmacologically blocked endogenous T4 by incubating embryos in 10 μM tetrac chronically and recorded RB INa at 50–55 hpf. Incubation in 10 μM tetrac significantly reduced sodium Gmax by 46% (P < 0.05) (Fig. 3A), consistent with blockade of endogenous T4 and an in vivo role for T4 INa regulation.
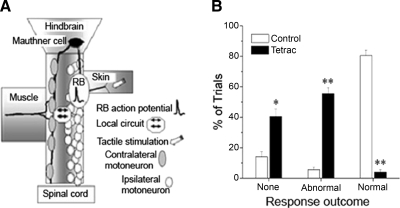
Application of tactile stimuli to RB cell free nerve endings in the skin triggers the touch response. After tactile stimulation RBs fire an action potential that recruits involvement of many cell types (neurons, muscle) and synapses (Fig. 4A). Study of the macho touch-insensitive mutant has shown that reduction of RB INa density can prevent the behavioral response to touch without affecting spontaneous swimming behavior (Granato et al. 1996; Ribera and Nüsslein-Volhard 1998). Because tetrac treatment produced such a large reduction in the RB sodium Gmax we hypothesized that RBs might not be able to mediate the touch response (Fig. 4A).
FIG. 4.
Embryonic touch response behavior is affected by chronic tetrac application. A: RB cells serve as primary sensory neurons for the zebrafish embryo (Clarke and Roberts 1984; Clarke et al. 1984; Nissanov et al. 1990). In the diagram, the right and left sides of the cord present dorsal and ventral views, respectively. The touch response is triggered by tactile stimulation of mechanosensitive channels in cutaneous RB free nerve endings. Activation of mechanosensitive channels leads to depolarization and consequent generation of an action potential. RBs contact the hindbrain Mauthner cell via an ascending axon. Activation of the ipsilateral Mauthner cell activates contralateral motor neurons, resulting in a rapid swim response. In addition, RB stimulation leads to a segmentally restricted response via activation of local circuits. B: 48-hpf embryos raised in 10 μM tetrac displayed a reduction in the percentage of trials that elicited normal responses, but an increase in percentage of trials showing no or abnormal responses (*P < 0.0005 vs. control; **P < 0.0001 vs. control).
We tested the touch responses of spontaneously swimming tetrac-treated embryos. Embryos were tested for normal swimming behavior in response to touch, an abnormal response to touch such as a single trunk bend without swimming movement, or no response to touch. Normal responses indicate propagation of an action potential from the RB cell to the ipsilateral Mauthner neuron, which in turn initiates a swimming pattern among contralateral motoneurons, whereas an abnormal response may suggest activation of local interneuron circuits by RB cells, but not activation of the Mauthner neuron (Fig. 4A). We found that 10 μM tetrac significantly decreased the percentage of trials that elicited normal touch responses of 48-hpf embryos compared with clutch-matched controls (P < 0.01) (Fig. 4B), suggesting that endogenous T4 is required in vivo for normal development of the RB-mediated touch response by 48 hpf.
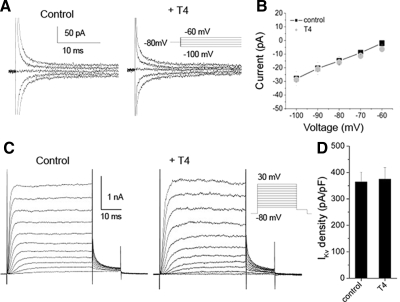
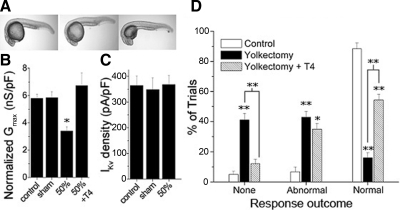
As an independent test of the in vivo requirement for endogenous T4, we surgically removed about 50% of the yolk sac, the location of maternal deposits of thyroid hormone present prior to function of the embryo's thyroid gland (Fig. 5A) (Tagawa et al. 1990; Walpita et al. 2007). Compared with control embryos and sham yolkectomies we found that 50% yolkectomy reduced RB sodium Gmax by 41% (P < 0.05) (Fig. 5B). Addition of 30 nM T4 to the embryo media immediately following a 50% yolkectomy rescued sodium Gmax to control values (Fig. 5B). Despite rescue by T4 of yolkectomy effects on INa, other developmental signals within the yolk may have had general nonspecific effects. To control for nonspecific effects on ion currents, we tested whether yolkectomy altered IKv. In contrast to INa, RB IKv steady-state amplitude was not significantly different from controls in 50% yolkectomized embryos (Fig. 5C).
FIG. 5.
T4 rescued effects of yolkectomy on INa density. A: at 24 hpf, yolkectomy was performed to reduce the yolk volume by 50% (right). Sham treatment consisted of only piercing embryos with the yolkectomy needle and not applying suction (middle). B: mean sodium Gmax in RBs was reduced by yolkectomy (n = 10) compared with control (n = 31) and sham (n = 9) treatments. Supplementing embryos with 30 nM T4 (n = 4) immediately after surgery rescued the effects of yolkectomy (*P < 0.05, ANOVA). C: 50% yolkectomy (n = 4) and sham yolkectomy (n = 5) had no effect on IKv elicited by a +30-mV voltage step compared with controls (n = 17). D: 50% yolkectomy reduced the percentage of touch trials eliciting normal responses (n = 35) relative to controls (n = 8). The effect of yolkectomy, however, was partially rescued by addition of 30 nM T4 to embryos after yolkectomy (n = 35), as indicated by the increase in the percentage of trials showing normal responses (*P < 0.01; **P < 0.001).
Consistent with the reduction in RB INa density, yolkectomized embryos displayed a significant decrease in the percentage of trials that elicited normal touch responses at 48 hpf versus control (P < 0.001) (Fig. 5D). When yolkectomized embryos were supplemented with 30 nM T4, the percentage of trials showing a normal touch response increased significantly compared with those that had been yolkectomized but not supplemented with T4 (P < 0.001), indicating that some of the yolkectomy effects were due to thyroid hormone deficiency. However, yolkectomized embryos that received T4 treatment still showed a decrease in normal touch responses versus control (P < 0.01), suggesting that some of the effects of yolk sac reduction on behavior were independent of T4. Overall, the results indicate that endogenous thyroid hormone acts in vivo to maintain a sufficient INa density for RBs to initiate touch response behavior.
T4 rapidly modulates RB INa
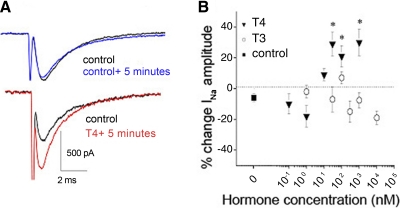
Because the nongenomic thyroid hormone pathway displays a faster time course than that of the conventional genomic pathway (Davis and Davis 1996), we tested whether T4 would rapidly regulate INa. We acutely added either T4 or T3 to the bath solution and recorded effects on peak INa amplitudes within 5 min of application (Fig. 6). We present the data as the percentage change in peak INa amplitude that occurred within 5 min of drug application. Positive values indicate that the drug treatment rapidly increased INa amplitude, whereas negative values reflect a decrease in peak current over time. We found that acute T4 application rapidly increased RB INa peak amplitude by 39% (Fig. 6A). The rapid effect of T4 on INa amplitude permitted a dose–response analysis with T4 concentrations varying between 100 pM and 1 μM (Fig. 6B). Because T3 activates the nongenomic pathway in some systems (Bergh et al. 2005; Sarkar et al. 2006), we also tested for T3 effects over a broad concentration range (1 nM to 10 μM). Addition of 30 nM, 100 nM, and 1 μM T4 significantly increased INa peak amplitude over changes in control amplitudes (P < 0.05). In contrast, addition of 1 to 100 nM T3 did not significantly affect INa peak amplitude. Overall, the results demonstrate rapid modulation of RB INa by T4 but not T3.
FIG. 6.
Acute T4 rapidly increased peak INa amplitude; acute T3 did not affect INa amplitude. A: peak INa amplitude did not significantly change over 5 min in control solution (top), but application of 30 nM T4 to an RB cell rapidly increased peak INa within 5 min (bottom). INa was elicited by a depolarizing voltage step to −10 mV. B: dose–response relationships demonstrated that T4's effect on RB INa peak amplitude (▾) (0.1 nM, n = 4; 1 nM, n = 4; 10 nM, n = 6; 30 nM, n = 11; 100 nM, n = 6; 1 μM, n = 3) was not mimicked by T3 (○) (1 nM, n = 4; 30 nM, n = 3; 100 nM, n = 5; 300 nM, n = 2; 1 μM, n = 5; 10 μM, n = 3), over a wide range of concentrations. Acute application of T4 to RBs increased peak INa at concentrations of ≥30 nM, whereas T3 did not significantly increase INa at any test concentration compared with control cells (▪) (n = 9) (*P < 0.05, ANOVA).
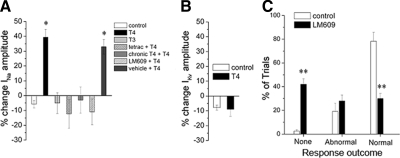
Next we tested whether the thyroid hormone antagonist tetrac blocked the acute effects of T4. Coapplication of tetrac prevented the acute effect of T4 on INa. Because chronic T4 application increased RB INa amplitude by 46% (Fig. 1C), we tested whether the acute and chronic effects of T4 involved the same pathway. We reasoned that if the acute and chronic effects occurred by independent mechanisms, then their effects would be additive. In contrast, if the acute and chronic effects involved the same mechanism, pretreatment with chronic T4 would prevent acute T4 application from having additional effects. We found that chronic T4 incubation occluded the effects of acute T4 treatment (Fig. 7A), suggesting that both the rapid and chronic effects of T4 are mediated by the same pathway. In contrast to INa, IKv was not affected by acute application of 30 nM T4 (Fig. 7B), similar to the results for chronic application of T4 on IKv.
FIG. 7.
The acute affects of T4 are blocked by tetrac and LM609. A: acute application of T4 increased peak INa amplitude (n = 11) within 5 min compared with controls (n = 9) at 50–55 hpf. In contrast, acute T3 did not change INa amplitude over 5 min (n = 3). Acute T4 effects were blocked by coapplication of 10 μM tetrac (n = 3), chronic incubation in 30 nM T4 (n = 7), or injection of 50 μg/mL LM609 but not vehicle (H2O) (n = 3) (*P < 0.05, ANOVA). B: acute T4 application (n = 5) did not affect IKv density over 5 min of application compared with controls (n = 6). C: compared with controls, the αVβ3 antibody LM609 (50 μg/mL) increased the percentage of trials eliciting no touch response for 48-hpf embryos, whereas LM609 decreased the percentage of trials eliciting a normal response (*P < 0.01 vs. control; **P < 0.001 vs. control).
T4 modulates INa via integrin αVβ3
The rapid and selective effect of T4 versus T3 on INa density suggested a nontraditional thyroid hormone signaling pathway. Because the RGD-type integrin dimer αVβ3 has been implicated in vitro as a plasma membrane receptor for T4 signaling (Bergh et al. 2005), we tested whether the function-blocking αVβ3 antibody LM609 (Cheresh and Spiro 1987) could affect RB cell sodium current and embryonic behavior. We injected 50 μg/mL LM609 into 24-hpf zebrafish embryos and recorded RB INa at 50–55 hpf. LM609 injection significantly reduced RB peak INa amplitudes by 46% (557 ± 32 vs. 303 ± 43 pA; n = 13 and 9, control and LM609 treated, respectively; P < 0.005). Further, LM609 significantly reduced the percentage of touch response trials eliciting a normal response (Fig. 7C; P < 0.001), consistent with αVβ3 serving as a T4 receptor.
If the acute effects of T4 were mediated by αVβ3, then LM609 injection would be expected to block acute T4 effects on INa. We found that LM609 injection prior to acute T4 application occluded the rapid increase in RB INa amplitude produced by T4. In contrast, T4 elicited a rapid increase in INa amplitude in RBs of control vehicle-only injected embryos (Fig. 7A). These findings suggest that the αVβ3 integrin serves as a mediator for T4's rapid modulation of RB cell INa amplitude.
DISCUSSION
Despite evidence that changes in thyroid hormone concentration alter development of the nervous system, there is little information about mechanisms that underlie T4 action during embryonic stages. The majority of research has focused on genomic mechanisms whereby thyroid hormones signal via nuclear receptors. However, the discovery of a plasma membrane αVβ3 integrin thyroid hormone receptor (Bergh et al. 2005) led us to test for nongenomic mechanisms during embryonic stages. Our results indicate that T4 rapidly modulates sensory neuron INa in embryonic zebrafish. The time course of T4 action is not consistent with genomic mechanisms. Moreover, we find that integrin αVβ3 is required for T4's rapid modulation of embryonic RB INa.
In both humans and zebrafish, the embryo does not begin to synthesize thyroid hormone until late gestation or larval stages, respectively. Consequently, in both species, maternal thyroid hormone provides the early embryo with access to thyroid hormone stores. We found that T4 initiated a rapid signaling pathway at concentrations ≥30 nM, a range similar to maternal contributions of total thyroid hormone during fetal development (30–70 nM; Vulsma et al. 1989). These considerations underscore the importance of maintaining maternal euthyroid status and placental function.
Our data show changes in both RB sodium current density and touch sensitivity in response to T4. All treatments that significantly reduced Gmax also produced a significant increase in the percentage of trials eliciting no touch response (Figs. 4, 5, and 7). This high level of agreement was not expected because other neurons, in addition to RBs, play a role in generation of the touch response (Fig. 4A). Further, T4 may affect other excitable membrane properties, even though we did not observe changes in voltage-gated or leak potassium currents. Nonetheless, the strong relationship between RB INa amplitude and touch sensitivity was observed regardless of whether we reduced T4-integrin signaling by reducing the effective levels of T4 (tetrac, yolkectomy) or blocking the novel T4 plasma membrane integrin receptor (LM609). In this regard, we obtained similar but slightly less robust results with echistatin, a blocker of the RGD-type integrin dimer family, of which αVβ3 is a member (data not shown). Taken together, the data suggest a strong relationship between RB INa amplitude and the touch response. However, because T4 may have undiscovered effects on other conductances or cells, it is not possible to conclude that T4's effect on touch response behavior is due solely to reduction of RB INa amplitude.
Our data implicate maternally provided T4 as an important regulator of RB INa. Reducing endogenous stores of T4 from the yolk sac significantly reduced peak sodium current and the percentage of touch response trials eliciting a normal response. Addition of 30 nM T4 to yolkectomized embryos immediately after surgery completely recovered effects on peak sodium current density and partially rescued the behavioral effects. Although the behavioral rescue promoted by T4 supplementation was statistically significant (Fig. 5D; P < 0.001), the percentage of normal touch responses was still reduced compared with that of control. The partial recovery suggests that the embryonic yolk sac houses factors other than T4 that affect the touch response. Overall, the data indicate that endogenous T4 stores play a role in maintaining RB cell INa and exert an influence on embryonic touch response behavior.
The touch response allows zebrafish embryos to flee from predators and thereby promotes survival. In addition to maintaining an appropriate physiological RB INa density, T4 modulated the embryonic touch response. The touch response swim reflex was compromised by either reducing thyroid hormone concentrations (surgically or pharmacologically) or blocking function of the αVβ3 integrin. We interpret the effects of T4 on behavior as a consequence of T4's effects on RB INa density. However, it is possible that T4 reduction, or αVβ3 blockade, had additional effects on other elements of the circuit mediating the touch response (Fig. 4A). Nevertheless, the results demonstrate that blockade of T4-αVβ3 signaling influenced a behavior critical for embryonic zebrafish survival.
Previous work has demonstrated that two different sodium channel α-subunit isotypes, nav1.6 and nav1.1L, underlie RB INa (Pineda et al. 2005): nav1.6 channels carry the majority of RB INa, whereas nav1.1L channels carry a much smaller fraction. Further, nav1.6 channels pass current that reaches peak amplitude more rapidly than nav1.1L channels can. Our studies did not address whether T4 affects current in both channel types or in only one. Interestingly, although not studied in detail here, we observed that T4 consistently reduced time to peak INa (e.g., Fig. 1B). Due to the substantial and consistent increase in INa density and possible effects on kinetics, the data suggest that T4 may have selective effects on nav1.6 channels. Future studies will address this possibility.
In addition to the specific effects INa has on electrical excitability, INa also influences other aspects of neuronal differentiation such as axon morphology and cell death (Pineda et al. 2006; Svoboda et al. 2001). Disruption of maternal thyroid hormone status during early development would potentially alter INa and its effects on nervous system development. The long-term consequences of altered nongenomic T4 signaling during embryonic stages are unknown.
Our studies have focused on thyroid hormone action during embryonic development. However, thyroid hormone regulation of ion currents has been reported during other developmental stages through both conventional and rapid time courses (Huang et al. 1999; Potthoff and Dietzel 1997; Wang et al. 2003; Zinman et al. 2006). Whether T4's rapid regulation of INa via integrin αVβ3 is limited to early development or is cell specific to RBs is unknown. Nonetheless, our findings raise the possibility that rapid T4 signaling mechanisms and effects on INa could contribute to nervous system deficits associated with altered thyroid hormone status. Altogether, the identification of rapid thyroid hormone effects on neurons and the involvement of integrin αVβ3 in acute INa regulation support a nongenomic mechanism of action for T4 during embryonic stages and provide new insights into thyroid hormone mechanisms in the embryonic nervous system.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants F30NS-059147 to M. A. Yonkers and R01NS-038937 and P30NS-048154 to A. B. Ribera.
Acknowledgments
We thank S. Stroh and M. Perales for care of zebrafish, Dr. A. D. Robertson for statistical advice, and Dr. K. Beam and members of the Ribera lab for discussion.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Anselmo et al. 2004.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. J Am Med Assoc 292: 691–695, 2004. [DOI] [PubMed] [Google Scholar]
- Becker et al. 2003.Becker T, McLane MA, Becker CG. Integrin antagonists affect growth and pathfinding of ventral motor nerves in the trunk of embryonic zebrafish. Mol Cell Neurosci 23: 54–68, 2003. [DOI] [PubMed] [Google Scholar]
- Bergh et al. 2005.Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146: 2864–2871, 2005. [DOI] [PubMed] [Google Scholar]
- Brown 1997.Brown DD The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci USA 94: 13011–13016, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo and Jauniaux et al. 2002.Calvo RM, Jauniaux E, Gulbis B, Asuncion M, Gervy C, Contempre B, Morreale de Escobar G. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 87: 1768–1777, 2002. [DOI] [PubMed] [Google Scholar]
- Cheresh and Spiro 1987.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von-Willebrand factor. J Biol Chem 262: 17703–17711, 1987. [PubMed] [Google Scholar]
- Clarke et al. 1984.Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol 348: 511–525, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke and Roberts 1984.Clarke JD, Roberts A. Interneurones in the Xenopus embryo spinal cord: sensory excitation and activity during swimming. J Physiol 354: 345–362, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contempre et al. 1993.Contempre B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab 77: 1719–1722, 1993. [DOI] [PubMed] [Google Scholar]
- Davis et al. 2004.Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94: 1500–1506, 2004. [DOI] [PubMed] [Google Scholar]
- Davis and Davis 1996.Davis PJ, Davis FB. Nongenomic actions of thyroid hormone. Thyroid 6: 497–504, 1996. [DOI] [PubMed] [Google Scholar]
- Davis et al. 2005.Davis PJ, Davis FB, Cody V. Membrane receptors mediating thyroid hormone action. Trends Endocrinol Metab 18: 429–435, 2005. [DOI] [PubMed] [Google Scholar]
- Ferreiro et al. 1988.Ferreiro B, Bernal J, Goodyer CG, Branchard CL. Estimation of nuclear thyroid hormone receptor saturation in human fetal brain and lung during early gestation. J Clin Endocrinol Metab 67: 853–856, 1988. [DOI] [PubMed] [Google Scholar]
- Francis and Riley 1987.Francis G, Riley W. Congenital familial transient hypothyroidism secondary to transplacental thyrotropin-blocking autoantibodies. Am J Dis Child 141: 1081–1083, 1987. [DOI] [PubMed] [Google Scholar]
- Granato et al. 1996.Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123: 399–413, 1996. [DOI] [PubMed] [Google Scholar]
- Hetzel et al. 2000.Hetzel BS Iodine and neuropsychological development. J Nutr 130: 493S–495S, 2000. [DOI] [PubMed] [Google Scholar]
- Huang et al. 1999.Huang CJ, Geller HM, Green WL, Craelius W. Acute effects of thyroid hormone analogs on sodium currents in neonatal rat myocytes. J Mol Cell Cardiol 31: 881–893, 1999. [DOI] [PubMed] [Google Scholar]
- James et al. 2007.James SR, Franklyn JA, Kilby MD. Placental transport of thyroid hormone. Best Pract Res Clin Endocrinol Metab 21: 253–264, 2007. [DOI] [PubMed] [Google Scholar]
- Kimmel et al. 1995.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995. [DOI] [PubMed] [Google Scholar]
- Kriplani et al. 1994.Kriplani A, Buckshee K, Bhargava VL, Takkar D, Ammini AC. Maternal and perinatal outcome in thyrotoxicosis complicating pregnancy. Eur J Obstet Gynecol Reprod Biol 54: 159–163, 1994. [DOI] [PubMed] [Google Scholar]
- Mirabella et al. 2000.Mirabella G, Feig D, Astzalos E, Perlman K, Rovet JF. The effect of abnormal intrauterine thyroid hormone economies on infant cognitive abilities. J Pediatr Endocrinol Metab 13: 191–194, 2000. [DOI] [PubMed] [Google Scholar]
- Mousa et al. 2006.Mousa SA O'Connor L, Davis FB, Davis PJ. Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology 147: 1602–1607, 2006. [DOI] [PubMed] [Google Scholar]
- Nissanov et al. 1990.Nissanov J, Eaton RC, DiDomenico R. The motor output of the Mauthner cell, a reticulospinal command neuron. Brain Res 517: 88–98, 1990. [DOI] [PubMed] [Google Scholar]
- Pacaud et al. 1995.Pacaud D, Huot C, Gattereau A, Brown RS, Glorieux J, Dussault JH, Van Vliet G. Outcome in three siblings with antibody-mediated transient congenital hypothyroidism. J Pediatr 127: 275–277, 1995. [DOI] [PubMed] [Google Scholar]
- Pineda et al. 2005.Pineda RH, Heiser RA, Ribera AB. Developmental, molecular, and genetic dissection of INa in vivo in embryonic zebrafish sensory neurons. J Neurophysiol 93: 3582–3593, 2005. [DOI] [PubMed] [Google Scholar]
- Pineda and Svoboda et al. 2006.Pineda RH, Svoboda KR, Wright MA, Taylor AD, Novak AE, Gamse JT, Eisen JS, Ribera AB. Knockdown of Nav1.6a Na+ channels affects zebrafish motoneuron development. Development 133: 3827–3836, 2006. [DOI] [PubMed] [Google Scholar]
- Potthoff and Dietzel 1997.Potthoff O, Dietzel ID. Thyroid hormone regulates Na+ currents in cultured hippocampal neurons from postnatal rats. Proc Biol Sci 264: 367–373, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera and Nüsslein-Volhard 1998.Ribera AB, Nüsslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. J Neurosci 18: 9181–9191, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet and Hepworth 2001.Rovet JF, Hepworth S. Attention problems in adolescents with congenital hypothyroidism: a multicomponential analysis. J Int Neuropsychol Soc 7: 734–744, 2001. [DOI] [PubMed] [Google Scholar]
- Sarkar et al. 2006.Sarkar PK, Durga ND, Morris JJ, Martin JV. In vitro thyroid hormone rapidly modulates protein phosphorylation in cerebrocortical synaptosomes from adult rat brain. Neuroscience 137: 125–132, 2006. [DOI] [PubMed] [Google Scholar]
- Smit et al. 2000.Smit BJ, Kok JH, Vulsma T, Briet JM, Boer K, Wiersinga WM. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr 89: 291–295, 2000. [PubMed] [Google Scholar]
- Svoboda et al. 2001.Svoboda KR, Linares AE, Ribera AB. Activity regulates programmed cell death of zebrafish Rohon-Beard neurons. Development 128: 3511–3520, 2001. [DOI] [PubMed] [Google Scholar]
- Tagawa et al. 1990.Tagawa M, Tanaka M, Matsumoto S, Hirano T. Thyroid hormones in eggs of various freshwater, marine and diadromous teleosts and their changes during egg development. Fish Physiol Biochem 8: 515–520, 1990. [DOI] [PubMed] [Google Scholar]
- Vulsma et al. 1989.Vulsma T, Gons MH, Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to organification defect or thyroid agenesis. N Engl J Med 321: 13–16, 1989. [DOI] [PubMed] [Google Scholar]
- Walpita et al. 2007.Walpita CN, Van der Geyten S, Rurangwa E, Darras VM. The effect of 3,5,3′-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen Comp Endocrinol 152: 206–214, 2007. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2003.Wang YG, Dedkova EN, Fiening JP, Ojamaa K, Blatter LA, Lipsius SL. Acute exposure to thyroid hormone increases Na+ current and intracellular Ca2+ in cat atrial myocytes. J Physiol 546: 491–499, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield 1995.Westerfield M The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: Univ. of Oregon Press, 1995.
- Zinman et al. 2006.Zinman T, Shneyvays V, Tribulova N, Manoach M, Shainberg A. Acute, nongenomic effect of thyroid hormones in preventing calcium overload in newborn rat cardiocytes. J Cell Physiol 207: 220–231, 2006. [DOI] [PubMed] [Google Scholar]