Abstract
Apoptosis triggered by death receptors proceeds after defined signal-transduction pathways. Whether signaling at the receptor level is regulated by intracellular messengers is still unknown. We have investigated the role of two messengers, ceramide and nitric oxide (NO), on the apoptotic pathway activated in human monocytic U937 cells by tumor necrosis factor-α (TNF-α) working at its p55 receptor. Two transduction events, the receptor recruitment of the adapter protein, TRADD, and the activation of the initiator caspase, caspase 8, were investigated. When administered alone, neither of the messengers had any effect on these events. In combination with TNF-α, however, ceramide potentiated, whereas NO inhibited, TNF-α-induced TRADD recruitment and caspase 8 activity. The effect of NO, which was cGMP-dependent, was due to inhibition of the TNF-α-induced generation of ceramide. Our results identify a mechanism of regulation of a signal-transduction pathway activated by death receptors.
Death receptors [e.g., CD95; the p55 receptor for tumor necrosis factor-α (TNF-α), from hereon indicated as TNF-R1; death receptors 3–5] are a family of transplasmalemma proteins sharing a homologous cytoplasmic domain, the death domain, responsible for initiation of the apoptotic-signaling cascade (1). Activation of death receptors triggers the assembly of DISC, composed of multiple cytosolic proteins with different functional roles (1). Of these cytosolic proteins, TRADD, which is recruited by some receptors (e.g., TNF-R1 and death receptor 3), serves as an adapter to the binding of FADD and other transductional proteins (1). Once associated, FADD binds a specific pro-protease, procaspase 8, whose proteolytic activation at the receptor level opens the way to the cascade of apoptotic-signaling events leading to cell death (2, 3). The functional regulation of the downstream events is understood in some detail (3). In contrast, regulation by intracellular messengers of the events leading to caspase 8 activation has not yet been reported.
To address this, we have investigated the regulation of the early events that follow the activation of TNF-R1, one of the first recognized and best known members of the death receptor family. We have analyzed the effects of two intracellular messengers, the sphingomyelin-derived lipid metabolite ceramide and the short-lived radical nitric oxide (NO), by using a cell model well characterized for TNF-R1 studies, the human monocytic line U937 (4–6). In a previous study, ceramide was suggested to potentiate apoptosis induced by TNF-α (7). Furthermore, NO has been shown to inhibit TNF-α-induced apoptosis acting via mechanisms both dependent on and independent of guanylyl cyclase activation and cGMP formation (8–11). The mechanism independent of cGMP has been suggested to be due to S-nitrosylation and consequent inhibition of various active caspases (8, 9, 12, 13). The cGMP-dependent mechanism remains undefined.
We now demonstrate that exogenous as well as endogenous ceramide facilitates TNF-R1-activated signal transduction by increasing TRADD recruitment to the DISC complex and caspase 8 activation. In contrast, exogenous NO promotes an early inhibition of the apoptotic response that is mediated by cGMP-dependent inhibition of TNF-α-triggered generation of ceramide.
Materials and Methods
Materials.
The following reagents were purchased as indicated: goat polyclonal anti-TNF-R1 (N20), anti-TRADD (C-20), and horseradish peroxidase (HRP)-conjugated anti-goat IgG antibodies (Abs) from Santa Cruz Biotechnology; monoclonal anti-caspase 8 Abs from Upstate Biotechnology (Lake Placid, NY); [14C]thymidine, 125I-TNF-α, [γ-32P]ATP and the enhanced chemiluminescence (ECL) kit from Amersham Pharmacia; goat anti-mouse IgG-HRP Ab from Bio-Rad; recombinant TNF-α and H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) from Alexis Italia (Florence, Italy); FITC-labeled annexin V from Boehringer Mannheim; diacylglycerol (DAG) kinase from Biomol (Plymouth Meeting, PA); protein G-linked Sepharose beads from Amersham Pharmacia; N-acetyl-IETD-7-amino-4-trifluoromethylcoumarin from Eppendorf; dl-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), tricyclodecan-9-yl xanthate (D609), C2 ceramide, and S-nitroso-acetylpenicillamine (SNAP) from Calbiochem. The reagents for tissue culture were from GIBCO except for fetal clone III serum, obtained from HyClone. All other reagents were from Sigma.
Cell Culture and Treatments.
U937 cells were routinely grown at 37°C, 5% CO2, in RPMI medium 1640 supplemented with 10% heat-inactivated fetal clone III serum, 2 mM glutamine, 100 units/ml streptomycin, and 100 units/ml penicillin and used within the 10th week from thawing. Apart from 125I-TNF-α binding to TNF-R1, the experiments were carried out in the above culture medium. Cell incubations with SNAP (300 μM), ODQ (1 μM), 8 Br-cGMP (3 mM), C2 ceramide (3–100 μM), PDMP (10 μM), and D609 (25 μg/ml) were carried out together with the protein synthesis inhibitor cycloheximide (CHX; 1 μg/ml) added 10 min before TNF-α. Fumonisin B1 (3 μM) was added 5 h before TNF-α/CHX. SNAP was used at a concentration that does not induce cytotoxic effects in U937 cells and D609 was used under conditions that do not stimulate ceramide generation (14).
Apoptosis Detection.
Secondary DNA fragmentation was quantified by using the filter-binding assay as described (15). Briefly, the cells were labeled overnight with [14C]thymidine (0.05 μCi/ml) and incubated for a further 6 h in a medium containing unlabeled thymidine (1 μg/ml) before incubation with or without the various compounds and treatment with TNF-α plus CHX (1 μg/ml). At the indicated time points, the cells were sedimented (1,000 rpm, 5 min at 4°C) and the pellet was resuspended in a saline containing 140 mM NaCl, 5 mM KCl, 5 mM glucose, 5 mM EDTA, 4 mM NaHCO3, pH 8.3. Aliquots of 0.5 × 106 cells then were loaded onto protein-adsorbing (polyvinylchloride) filters (25 mm, 2-μm pore; Nuclepore), washed with the same buffer, and lysed with a solution containing 0.2% sarkosyl, 2 M NaCl, and 40 mM EDTA, pH 10.1. Filters were rinsed with EDTA (20 mM, pH 10.1) and then removed from the filter holders, which were washed twice with 0.4 M NaOH. Radioactivity was counted in the lysates, EDTA washes, filters, and filter-holder washes. For each sample, the DNA fragmentation was determined as the percentage of the 14C-labeled DNA recovered in the lysate plus the EDTA washes.
Programmable, autonomously controlled electrode (PACE) electrophoresis was carried out as described previously (15) on cell samples embedded after the various treatments into agarose plugs (final cell density, 1 × 106 cells per plug) by using a Bio-Rad DRIII variable angle system.
Phosphatidyl serine exposure at the external surface of the plasma membrane was detected by analysis of aliquots of 6 × 104 cells, stained for 15 min with FITC-labeled annexin V (1 μg/ml), by using a fluorescence-activated cell sorter (FACStar Plus; Becton Dickinson) as described previously (16).
125I-TNF-α Binding.
Cells were washed and resuspended in a medium containing 10% fetal clone III serum, 125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2 mM CaCl2, 6 mM glucose, and 25 mM Hepes, pH 7.4. After equilibration at 37°C for 15 min, 1-ml aliquots containing 4 × 106 cells were incubated with or without the various compounds for 10 min and then labeled for 5 min with 1.0 ng/ml 125I-TNF-α (30 μCi/μg) diluted with increasing concentrations of unlabeled TNF-α (0–100 ng/ml). Bound and free counts were separated by filtration through glass-fiber filters (Whatman), which were washed twice with cold buffer. Specific binding was estimated as the radioactivity displaceable by the unlabeled cytokine.
Immunoprecipitation and Western Blot Analysis.
For immunoprecipitation of the TNF-R1-TRADD complex, aliquots of 4 × 107 cells, incubated as described above, were treated with TNF-α (50 ng/ml) plus CHX (1 μg/ml) for the indicated times, washed rapidly twice with cold buffer (150 mM NaCl/1 mM EDTA/ 2 mM Na2P2O6/30 mM NaF/20 mM Tris⋅HCl, pH 7.5), and lysed for 30 min in the same buffer containing 1% Triton X-100, 0.1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Lysates were centrifuged at 3,000 rpm for 15 min, and the supernatants were incubated overnight at 4°C with 4 μg of anti-TNF-R1 Ab. Protein G-Sepharose beads then were added to the lysates, which were incubated for a further 2 h and then washed three times with buffer containing 0.2% Triton X-100. Protein concentration in the samples was assayed by using the bicinchoninic acid procedure (Pierce).
Western blot analyses of TRADD and procaspase 8 were carried out with either the immunoprecipitates or 30 μg of total protein, separated on 12% SDS/PAGE, and transferred onto nitrocellulose membranes. Nonspecific sites were blocked before immunolabeling by incubation for 1 h at room temperature with blotting buffer (PBS containing 0.1% Tween and 5% nonfat milk). The membranes were incubated overnight in blotting buffer with the appropriate Ab and washed five times for 1 h with blotting buffer. The secondary Ab (anti-goat or anti-mouse-HRP for TRADD and procaspase detection, respectively) then was added for 30 min. After several washes of the membranes in blotting buffer, the antigens were revealed with ECL according to the manufacturer's instructions.
Measurement of Caspase 8 Activity.
Aliquots of 2 × 106 cells, incubated as described above, were treated with TNF-α (50 ng/ml) in the presence of CHX (1 μg/ml), washed in 150 mM NaCl/1 mM EDTA/2 mM Na2P2O6/30 mM NaF/20 mM Hepes, pH 7.5, and lysed at the indicated time points in the same buffer containing 1% Triton X-100, 0.1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Soluble proteins (200 μg) were diluted in a reaction buffer containing a final concentration of 25 mM Hepes, 100 mM NaCl, 5 mM DTT, 1 mM EDTA, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and 10% sucrose. Caspase 8 activity was assayed in a Perkin–Elmer LS50 fluorometer, equipped with 400-nm excitation and 505-nm emission filters, by measuring for 1 h at 37°C the fluorescence emitted by the cleaved caspase 8 substrate, N-acetyl-IETD-7-amino-4-trifluoromethylcoumarin (25 μM), calibrated with respect to an AFC concentration curve.
Measurement of Ceramide Concentration.
Cells were incubated as described above, and aliquots were mixed for 5 min at 4°C with TNF-α (50 ng/ml) plus CHX (1 μg/ml) and then rapidly transferred and incubated at 37°C. The DAG kinase assay was carried out as described previously (16) on the phospholipids extracted after 5 min from 2 × 106 cell samples, which were incubated for 1 h at room temperature with 100 microunits of DAG kinase in the presence of 5 mg/ml cardiolipin/7.5% glucopyranoside/1 mM diethylenetriamine pentaacetic acid/10 μCi [γ-32P]ATP (10 mCi/ml). Under these conditions, DAG kinase is not rate-limiting and full conversion of ceramide to ceramide phosphate thus is to be expected (17). The ceramide phosphates produced were separated on a one-dimensional TLC by using chloroform/methanol/acetic acid (65:15:5; vol/vol/vol) as solvent. The relevant spots were identified by autoradiography, and their radioactivity was estimated by microdensitometry using a Molecular Dynamics Imagequant apparatus. To determine the concentration of ceramide per sample, a curve with known amounts of ceramide standard, encompassing the range of ceramide expected in the samples, was processed and loaded in parallel.
Statistical Analysis.
The results are expressed as means ± SEM; n represents the number of individual experiments. Statistical analysis was carried out by using Student's t test for unpaired variables (two-tailed). The asterisks (*, **, and ***) in the figures represent P of <0.05, <0.01, and <0.001, respectively, measured for the cells treated in the various experimental conditions vs. the cells treated with TNF/CHX alone.
Results
Effects of Ceramide and NO/cGMP on TNF-Induced Apoptosis.
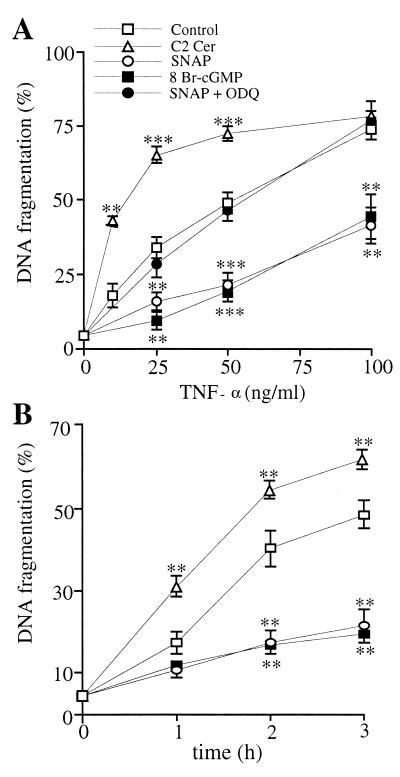
When administered to U937 cells together with the protein synthesis inhibitor CHX, TNF-α is known to induce apoptosis via activation of its specific receptor, TNF-R1 (18). To investigate the effects of NO, cGMP, and ceramide on the early steps of the TNF-α-induced apoptosis, U937 cells were incubated for 10 min with or without the NO donor SNAP (300 μM), alone or together with the guanylyl cyclase inhibitor, ODQ (1 μM); the membrane-permeant cGMP analogue, 8 Br-cGMP (3 mM); or the membrane-permeant ceramide, C2 ceramide (50 μM), and then exposed to increasing concentrations of TNF-α, which was administered for 3 h at 37°C together with a fixed concentration of CHX (1 μg/ml). Cells then were lysed, and apoptosis was studied by measuring DNA fragmentation. At all concentrations of the cytokine tested, SNAP and 8 Br-cGMP decreased significantly the TNF-α-induced DNA fragmentation, whereas coincubation with ODQ reversed the effect of the NO donor (Fig. 1A). Conversely, C2 ceramide increased the effect of TNF-α (Fig. 1A). The effect of ceramide was dependent on its concentration: apoptosis induced by TNF-α (25 ng/ml, 3 h) was increased by 69.2 ± 4.6, 114.5 ± 11.3, 130.4 ± 4.2, 146.3 ± 13.6, and 147.9 ± 9.1% at 3, 10, 30, 50, and 100 μM C2 ceramide, respectively. Apoptosis induction by ceramide alone was not detected during the period investigated (n = 6).
Figure 1.
Effects of exogenous ceramide, NO, and cGMP on TNF-α-induced apoptosis. (A) U937 cells were treated for 3 h with increasing concentrations of TNF-α plus CHX (1 μg/ml) (control) with or without SNAP (300 μM), SNAP + ODQ (1 μM), 8 Br-cGMP (3 mM), and C2 ceramide (C2 Cer, 50 μM). Here and in B, the secondary DNA fragmentation is expressed as the percentage of the 14C-labeled DNA recovered in the lysates plus EDTA washes. (B) Cells were treated with TNF-α (50 ng/ml)/CHX (1 μg/ml) (control) with or without SNAP, 8 Br-cGMP, and C2 Cer (concentrations as in A) for the indicated time points before DNA fragmentation analysis. Statistical probability, calculated vs. TNF-α/CHX-treated control, is shown here and in the following figures as described in Materials and Methods (n = 8).
The time dependence of the effects of SNAP, 8 Br-cGMP, and C2 ceramide was investigated next. SNAP and 8 Br-cGMP delayed whereas C2 ceramide increased the kinetics of onset of apoptosis induced by TNF-α (50 ng/ml)/CHX (1 μg/ml) as measured by DNA fragmentation (Fig. 1B). Similar results were obtained when apoptosis was studied by measuring the kinetics of appearance of both phosphatidyl serine residues at the cell surface (by annexin V staining) and high-molecular-weight DNA fragments (by PACE electrophoresis) (not shown). These results demonstrate that the potentiation by ceramide and the inhibition by NO take place within the first few hours of the TNF-α-induced apoptosis. That ODQ prevented the inhibition by SNAP confirmed that the effect of NO is mediated via formation of cGMP.
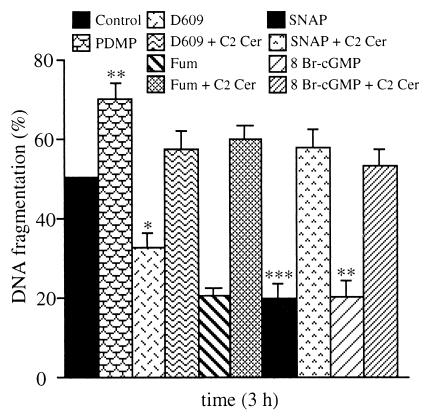
Further experiments were carried out to investigate the possible interactions between ceramide and NO. Cells were treated with PDMP (10 μM), an inhibitor of glucosylceramide synthase that leads to increased ceramide concentrations (19); D609 (25 μg/ml), which inhibits acidic sphingomyelinase activity via inhibition of the phosphatidylcholine-specific phospholipase C (20); or fumonisin B1 (3 μM), an inhibitor of ceramide synthase and TNF-α-induced apoptosis (21). None of these compounds had any effect when given alone; however, PDMP increased whereas D609 and fumonisin B1 decreased the TNF-α-induced DNA fragmentation (Fig. 2). The effect of D609 and fumonisin B1 was prevented by coincubation of the cells with C2 ceramide. Similarly, cell treatment with C2 ceramide reversed the inhibition of TNF-α-induced DNA fragmentation observed with either SNAP or 8 Br-cGMP (Fig. 2).
Figure 2.
Effects of endogenous ceramide and interaction between NO/cGMP and ceramide on TNF-α-induced apoptosis. Cells were treated for 3 h with TNF-α (50 ng/ml)/CHX (1 μg/ml) (control) with or without PDMP (10 μM), D609 (25 μg/ml), fumonisin B1 (Fum, 3 μM), SNAP (300 μM), 8 Br-cGMP (3 mM), and C2 Cer (50 μM) in various combinations, as indicated in the key. DNA fragmentation analysis is expressed as described in Fig. 1 (n = 5).
Treatment with SNAP, 8 Br-cGMP, or C2 ceramide did not induce any appreciable change in TNF-α binding to TNF-R1 (not shown, n = 3).
Effects of Ceramide and NO/cGMP on TRADD Recruitment and Caspase 8 Activation.
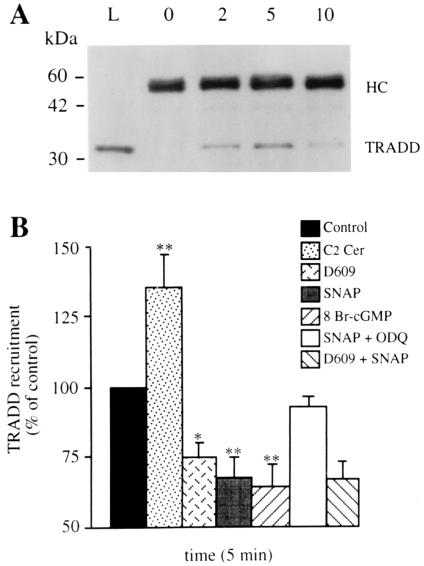
Establishment of the TNF-R1/TRADD complex was already detectable by immunoprecipitation at 2 min after treatment with TNF-α alone, peaked at 5 min, and decreased thereafter (Fig. 3A), as already described (22). Incubation with C2 ceramide induced an increase whereas incubation with D609 induced a decrease of the TNF-α-induced TRADD recruitment (Fig. 3B). Significant decreases also were observed after cell incubation with either SNAP or 8 Br-cGMP. The effect of SNAP could be inhibited by coincubating the cells with ODQ. In the cells exposed to either SNAP or 8 Br-cGMP together with D609, the inhibition of TNF-α-induced TRADD recruitment was not greater than that observed with either SNAP or D609 alone (P > 0.02) (Fig. 3B and not shown). When the cells were exposed to SNAP, 8 Br-cGMP, C2 ceramide, or D609 but not treated with TNF-α/CHX, no TRADD recruitment was detected (not shown). These results indicate that both endogenously formed and exogenous ceramide potentiates whereas NO, via cGMP formation, inhibits TRADD recruitment to the TNF-R1. That in the presence of D609 no further effect of NO/cGMP was observed suggests that the action of these messengers is mediated via inhibition of ceramide generation.
Figure 3.
Effects of ceramide, NO, and cGMP on TNF-α-induced TRADD recruitment to TNF-α-R1. (A) Time course of TRADD recruitment induced by TNF-α (50 ng/ml)/CHX (1 μg/ml) revealed by Western blotting of the precipitates with an anti-TRADD Ab. The gel shown is representative of four individual experiments: 0, 2, 5, and 10 indicate the time (min) of cell exposure to TNF-α before immunoprecipitation. Thirty micrograms of whole-cell homogenate was loaded in lane L as an internal control. Migration of the Ig heavy chain (HC) and TRADD is specified on the right. (B) Cells were treated with TNF-α (50 ng/ml)/CHX (1 μg/ml) (control) with or without SNAP (300 μM), 8 Br-cGMP (3 mM), ODQ (1 μM), D609 (25 μg/ml) and C2 Cer (50 μM) in various combinations, as indicated in the key. The specific bands recognized by the anti-TRADD Ab were analyzed by densitometry, and values are expressed as a percentage of those observed in the TNF-α/CHX-treated control (n = 5).
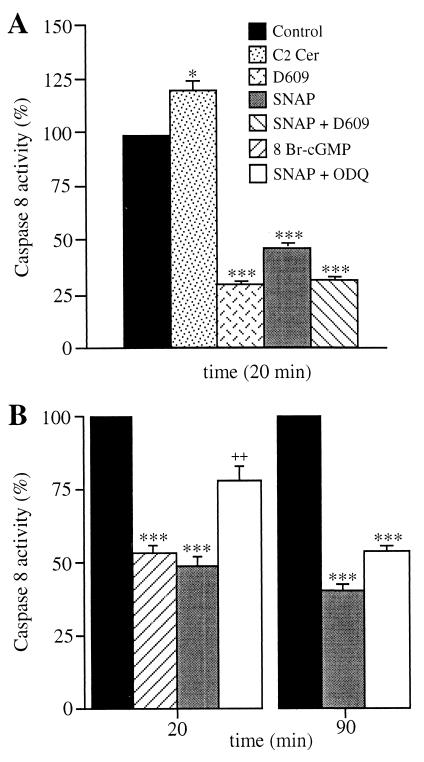
The time course of the activation of procaspase 8 by TNF-α was studied by both the cleavage of the specific substrate, N-acetyl-IETD-7-amino-4-trifluoromethylcoumarin, and the proteolytic cleavage of the proenzyme (by Western blotting; not shown). Caspase 8 activity was 10.1 ± 0.5 pmol/min per mg at time 0 and increased by 46 ± 3.1% after 20 min of cell incubation with TNF-α (n = 5). Cell treatment with C2 ceramide increased TNF-α-induced caspase 8 activity, whereas D609 and SNAP decreased it (Fig. 4A). The inhibition induced by the combination of D609 and SNAP did not exceed that induced by D609 alone, suggesting that the effect of NO was mediated through inhibition of ceramide generation.
Figure 4.
Effects of TNF-α, ceramide NO, and cGMP on caspase 8 activity. Cells were treated for the indicated times with TNF-α (50 ng/ml)/CHX (1 μg/ml) (control) with or without SNAP (300 μM), D609 (25 μg/ml), C2 Cer (50 μM), 8 Br-cGMP (3 mM), and ODQ (1 μM) as indicated in the key. Caspase 8 activity was measured in cell lysates as described in Materials and Methods. Values are expressed as a percentage of those observed in the control. Analysis of values measured in the cell samples treated with TNF-α/CHX + SNAP vs. those measured in cell samples treated with TNF-α/CHX + SNAP + ODQ showed a statistically significant difference at 20 min (P < 0.01, indicated by the symbol ++) but not at 90 min (n = 5).
The role of cGMP in the NO-induced inhibition of caspase 8 activity was investigated next. At 20 min, 8 Br-cGMP inhibited caspase 8 activity to an extent similar to that observed with SNAP (Fig. 4B). This inhibition by SNAP was largely prevented when ODQ was added to the incubation. By contrast, at 90 min, ODQ did not reverse the effect of SNAP, unless administered together with the reducing agent DTT (2 mM) (caspase 8 activity was restored to 75.9 ± 3.3% of that observed in cells treated with TNF-α and DTT, n = 3). Inhibition of caspase 8 by NO therefore appears to be cGMP-dependent early on, whereas it becomes predominantly cGMP-independent later. Reversal by DTT is consistent with an enzyme S-nitrosylation mechanism sustaining the delayed effect of NO (13).
NO and cGMP Inhibit the TNF-α-Induced Generation of Endogenous Ceramide.
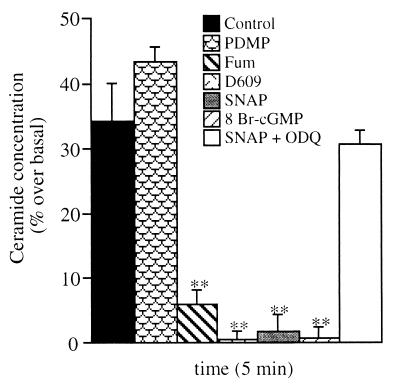
The intracellular concentration of ceramide increased at 5 min after TNF-α treatment, an effect enhanced by incubation of the cells with PDMP and prevented by incubation with D609 or fumonisin B1 (Fig. 5). Incubation with either SNAP or 8 Br-cGMP abolished ceramide increase induced by TNF-α. In contrast, when SNAP was used together with ODQ, it had no effect (Fig. 5). When administered alone, SNAP and 8 Br-cGMP did not modify the concentrations of ceramide (values were 140.4 ± 8.2, 129.0 ± 9.1, and 138.1 ± 6.9 pmol/mg proteins for controls and SNAP- and 8 Br-cGMP-treated cells, respectively, n = 5). Moreover, in cells treated with either D609 or fumonisin B1, the ceramide concentrations were not decreased further by SNAP or 8 Br-cGMP (not shown).
Figure 5.
Effects of NO and cGMP on TNF-α-induced ceramide generation. Cells were treated with TNF-α (50 ng/ml)/CHX (1 μg/ml) (control) with or without PDMP (10 μM), fumonisin B1 (Fum, 3 μM), D609 (25 μg/ml), SNAP (300 μM), 8 Br-cGMP (3 mM), and ODQ (1 μM) as indicated in the key. Cell aliquots were collected and lysed, and ceramide content was measured by TLC as described in Materials and Methods. Ceramide concentrations are expressed as a percentage of those measured in cell samples run in parallel but not treated with TNF-α/CHX (140.4 ± 8.2 pmol/mg proteins, with no significant change over time) (n = 5).
Discussion
We have investigated the events that follow the activation of TNF-R1 in the monocytic cell line U937. Specifically, we have studied the induction of apoptosis by TNF-α, analyzing DNA fragmentation and phosphatidyl serine exposure at the plasma membrane. We also have studied two early signaling events known to take place after TNF-R1 activation, namely, recruitment of the DISC component TRADD and activation of caspase 8.
The messengers investigated as possible TNF-R1 regulators were ceramide and NO, neither of which induced changes in binding of TNF-α to the receptor. Ceramide is a group of neutral sphingolipids produced by sphingomyelinases, whose activation after stimulation of death receptors and other apoptotic treatments is well known (23). The role of ceramide in the apoptotic process is supported by the resistance to TNF-α observed in various cells, including U937, when their ability to stimulate sphingomyelinases is impaired (4, 24–27). In those cells, restoration of sphingomyelinase activity was reported to restore the sensitivity to TNF-α (25, 27). Based on the apoptotic effects of its membrane-permeant analogues, ceramide has been considered to be a downstream effector of death receptors (28). Recently, however, such a role has been questioned because the action of TNF-α is distinctly faster than that of exogenous ceramide (5). Moreover, although the generation of ceramide in TNF-α-treated cells commences within a few minutes after addition of the cytokine, the intracellular accumulation of the lipid messenger was found to be greater at later times (hours) (5, 28). In addition, the ceramide species found in the early stages after TNF-α addition were different from those detected later during the apoptotic process (6).
Our results confirm that induction of apoptosis by TNF-α is rapid, with clear signs detectable within 1 h, whereas apoptosis induced by exogenous ceramide was not detected in the period investigated (3 h). However, we found that activation of TNF-R1 induced a rapid increase in endogenous ceramide, already appreciable within a few minutes. Furthermore, increased concentrations of ceramide, obtained by either addition of C2 ceramide or inhibition of glucosylceramide synthase by PMPD, were able to induce a clear, early potentiation of the TNF-α-induced apoptotic response. We also found that inhibition of the activities of acidic sphingomyelinase and ceramide synthase by D609 and fumonisin B1, respectively, which abrogates TNF-α-induced ceramide formation, clearly reduced the apoptotic effect of TNF-α. When administered alone, C2 ceramide did not induce any TRADD recruitment to TNF-R1. TRADD recruitment and activation of caspase 8 induced by TNF-α, however, were both potentiated rapidly in the presence of C2 ceramide and inhibited by D609. The effect of ceramide and its proapoptotic-signaling pathways appear, therefore, to be different depending on whether or not the TNF-R1 is activated (29). In particular, our results demonstrate a novel effect of ceramide, i.e., the positive modulation of signal transduction at TNF-R1, which contributes to the rapid apoptotic effect of the cytokine, suggesting a role for the lipid messenger as an amplifying factor in death receptor signaling. It should be emphasized that ceramide is generated not only in response to activation of TNF-R1 and other death receptors, but also after a variety of other stimuli, including oxidative stress, hydrogen peroxide, and IL-1β, commonly present during inflammatory processes (23). These stimuli, therefore, may synergize with TNF-α, via ceramide generation, to increase apoptosis by potentiation of TNF-R1 signaling.
Nitric oxide protection from TNF-α-induced apoptosis is known to be mediated by both cGMP-dependent and independent mechanisms (8–11). Using the NO donor SNAP and the membrane-permeant cGMP analogue 8 Br-cGMP, administered alone or together with the guanylyl cyclase inhibitor ODQ, we found that shortly after administration of TNF-α, the inhibition by NO of TRADD recruitment and caspase 8 activity occurred via a cGMP-dependent mechanism and that this effect was overcome by incubation of the cells with exogenous ceramide. Moreover, exposure to NO or cGMP abolished the early increase in endogenous ceramide induced by TNF-α. In addition, after inhibition of ceramide generation by D609, no further inhibition of TRADD recruitment and caspase 8 activity by NO/cGMP was observed. This excludes the possibility of a direct effect of NO/cGMP on these parameters. Thus, the cGMP-dependent inhibition of apoptosis by NO is due to blockade of ceramide generation induced by TNF-α. These results identify a molecular mechanism to explain the inhibition by NO/cGMP of the TNF-α-induced apoptosis observed in various cell systems (10, 11). Whether the cGMP action is mediated by direct inhibition of sphingomyelinase(s) or by indirect effects remains to be investigated.
NO was found to inhibit apoptosis both at an early and at a later stage. The late-stage protection was found to be cGMP-independent and to depend on the oxidative state of the cells, as suggested by its inhibition by the reducing agent DTT. Such an action is consistent with previous observations showing that NO is able to induce redox-dependent S-nitrosylation of activated caspases (8, 9, 12, 13). S-nitrosylation might be facilitated by the decrease in the cell-reducing potential that occurs in the course of TNF-α-induced apoptosis (17, 30) as well as during prolonged exposure to NO (31). The diversity in timing and targets of the two mechanisms of protection by NO suggests that they have different functional significance.
Our results demonstrate that signal transduction at TNF-R1 may be operating under a control dependent on the equilibrium between ceramide and NO. The signaling systems participating in the generation and function of these two messengers are expressed variously among different cell types and regulated (or induced) by multiple mechanisms (1, 22, 28, 32). Thus, the sensitivity of a given cell type to TNF-α-induced apoptosis may depend at least in part on its ability to generate ceramide and NO. However, this may not be the case in hepatocytes in which, although NO is known to protect from TNF-α-induced apoptosis acting in a cGMP-dependent way before proteolytic cleavage of procaspases (33), ceramide appears to have no role in TNF-α-induced apoptosis (34).
Apoptosis induced by TNF-α plays a role in diseases such as atherosclerosis, vasculitis, graft rejection, and gastric damage as well as various forms of hepatitis (11, 35–37). In view of this, the regulation of TNF-R1 described here, together with the cGMP-dependent protective action by NO in hepatocytes, could be of importance not only to clarify the consequences of activating this receptor under different conditions but also to understand why the delivery of NO in vivo may have a protective effect against liver and gastric damage (11, 38).
Acknowledgments
We thank Annie Higgs and Ennio Ongini for their critical revision of the manuscript and Maria Grazia Cifone for her help with the DAG kinase assay. This work was supported by grants from Schering Plough Italia; the Italian Association for Cancer Research; Consiglio Nazionale delle Ricerche, Target Project Biotechnology; and the Armenise–Harvard Foundation. C.D.N., J.P.L., and C.S. were supported by fellowships from the Association pour la Recherche contre le Cancer, the Institut National de la Santé et de la Recherche Medicale and Schering Plough Italia, respectively.
Abbreviations
- TNF-α and TNF-R1
tumor necrosis factor-α and its p55 receptor
- ODQ
H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one
- PDMP
dl-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- D609
tricyclodecan-9-yl xanthate
- SNAP
S-nitroso-acetylpenicillamine
- CHX
cycloheximide
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070062397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070062397
References
- 1.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Green D R. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 3.Borner C, Monney L, Olivier R, Rossé T, Häcki J, Conus S. Cell Death Differ. 1999;6:201–206. doi: 10.1038/sj.cdd.4400478. [DOI] [PubMed] [Google Scholar]
- 4.Schütze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse M-L, Heinrich M, Wickel M, Krönke M. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 5.Karasavvas N, Zakeri Z. Cell Death Differ. 1999;6:115–123. doi: 10.1038/sj.cdd.4400482. [DOI] [PubMed] [Google Scholar]
- 6.Watts J D, Gu M, Patterson S D, Aebersold R, Polverino A J. Cell Death Differ. 1999;6:105–114. doi: 10.1038/sj.cdd.4400472. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi M, Singh S, Jaffrezou J-P, Aggarwal B B. J Immunol. 1996;156:297–304. [PubMed] [Google Scholar]
- 8.Dimmeler S, Haendeler J, Nehls M, Zeiher A M. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y-M, Talanian R, Billiar T R. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y H, Wang X L, Wilcken D E L. FEBS Lett. 1998;433:125–131. doi: 10.1016/s0014-5793(98)00844-8. [DOI] [PubMed] [Google Scholar]
- 11.Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, Mannucci R, Del Soldato P, Morelli A. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 12.Mannick J B, Asano K, Izumi K, Kieff E, Stamler J S. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Billiar T R, Talanian R V, Kim Y M. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 14.Pörn-Ares M I, Chow S C, Slotte J P, Orrenius S. Exp Cell Res. 1997;235:48–54. doi: 10.1006/excr.1997.3641. [DOI] [PubMed] [Google Scholar]
- 15.Sestili P, Cattabeni F, Cantoni O. FEBS Lett. 1996;396:337–342. doi: 10.1016/0014-5793(96)01130-1. [DOI] [PubMed] [Google Scholar]
- 16.Sciorati C, Rovere P, Ferrarini M, Heltai S, Manfredi A A, Clementi E. J Biol Chem. 1997;272:23211–23215. doi: 10.1074/jbc.272.37.23211. [DOI] [PubMed] [Google Scholar]
- 17.Perry D K, Hannun Y A. Trends Biochem Sci. 1999;24:226–227. doi: 10.1016/s0968-0004(99)01407-3. [DOI] [PubMed] [Google Scholar]
- 18.Cossarizza A, Franceschi C, Monti D, Salvioli S, Bellesia E, Rivabene R, Biondo L, Rainaldi G, Tinari A, Malorni W. Exp Cell Res. 1995;220:232–240. doi: 10.1006/excr.1995.1311. [DOI] [PubMed] [Google Scholar]
- 19.Rani C S, Abe A, Chang Y, Rosenzweig N, Saltiel A R, Radin N S, Shayman J A. J Biol Chem. 1995;270:2859–2867. doi: 10.1074/jbc.270.6.2859. [DOI] [PubMed] [Google Scholar]
- 20.Cifone M G, Roncaioli P, De Maria R, Camarda G, Santoni A, Ruberti G, Testi R. EMBO J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laouar A, Glesne D, Huberman E. J Biol Chem. 1999;274:23526–23534. doi: 10.1074/jbc.274.33.23526. [DOI] [PubMed] [Google Scholar]
- 22.Jones S J, Ledgerwood E C, Prins J B, Galbraith J, Johnson D R, Pober J S, Bradley J R. J Immunol. 1999;162:1042–1048. [PubMed] [Google Scholar]
- 23.Mathias S, Peña L A, Kolesnick R N. Biochem J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright S C, Zheng H, Zhong J. FASEB J. 1996;10:325–332. doi: 10.1096/fasebj.10.2.8641566. [DOI] [PubMed] [Google Scholar]
- 25.Cai Z, Bettaieb A, Mahdani N E, Legres L G, Stancou R, Masliah J, Chouaib S. J Biol Chem. 1997;272:6918–6926. doi: 10.1074/jbc.272.11.6918. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid L M, Hannun Y A. J Biol Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- 27.Bezombes C, Maestre N, Laurent G, Levade T, Bettaieb A, Jaffrezou J-P. FASEB J. 1998;12:101–109. doi: 10.1096/fasebj.12.1.101. [DOI] [PubMed] [Google Scholar]
- 28.Hannun Y A. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 29.Belaud-Rotureau M-A, Lacombe F, Durrieu F, Vial J-P, Lacoste L, Bernard P, Belloc F. Cell Death Differ. 1999;6:788–795. doi: 10.1038/sj.cdd.4400552. [DOI] [PubMed] [Google Scholar]
- 30.Higuchi M, Proske R J, Yeh E T H. Oncogene. 1998;17:2515–2524. doi: 10.1038/sj.onc.1202485. [DOI] [PubMed] [Google Scholar]
- 31.Clementi E, Brown G C, Feelisch M, Moncada S. Proc Natl Acad Sci USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuehr D J. Biochim Biophys Acta. 1999;1410:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Bombeck C A, Yang S, Kim Y-M, Billiar T R. J Biol Chem. 1999;274:17325–17333. doi: 10.1074/jbc.274.24.17325. [DOI] [PubMed] [Google Scholar]
- 34.Arora A S, Jones B J, Patel T C, Bronk S F, Gores G J. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- 35.Appleyard C B, McCafferty D-M, Tigle A W, Swain G, Wallace J L. Am J Physiol. 1996;270:G42–G48. doi: 10.1152/ajpgi.1996.270.1.G42. [DOI] [PubMed] [Google Scholar]
- 36.Beutler B, Bazzoni F. Blood Cells Mol Dis. 1998;24:216–230. doi: 10.1006/bcmd.1998.0187. [DOI] [PubMed] [Google Scholar]
- 37.Bradham C A, Plümpe J, Manns M P, Brenner D A, Trautwein C. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 38.Saavedra J E, Billiar T R, Willimas D L, Kim Y-M, Watkins S C, Keefer L K. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]