Abstract
Opioids depress the activity of brain stem respiratory-related neurons, but it is not resolved whether the mechanism at clinical concentrations consists of direct neuronal effects or network effects. We performed extracellular recordings of discharge activity of single respiratory neurons in the caudal ventral respiratory group of decerebrate dogs, which were premotor neurons with a likelihood of 90%. We used multibarrel glass microelectrodes, which allowed concomitant highly localized picoejection of opioid receptor agonists or antagonists onto the neuron. Picoejection of the μ receptor agonist [d-Ala2, N-Me-phe4, gly-ol5]-enkephalin (DAMGO, 1 mM) decreased the peak discharge frequency (mean ± SD) of expiratory neurons to 68 ± 22% (n = 12), the δ1 agonist d-Pen2,5-enkephalin (DPDPE, 1 mM) to 95 ± 11% (n = 15), and δ2 receptor agonist [d-Ala2] deltorphin-II to 86 ± 17% (1 mM, n = 15). The corresponding values for inspiratory neurons were: 64 ± 12% (n = 11), 48 ± 30% (n = 12), and 75 ± 15% (n = 11), respectively. Naloxone fully reversed these effects. Picoejection of morphine (0.01–1 mM) depressed most neurons in a concentration dependent fashion to maximally 63% (n = 27). Picoejection of remifentanil (240–480 nM) did not cause any significant depression of inspiratory (n = 11) or expiratory neurons (n = 9). 4. Intravenous remifentanil (0.2–0.6 μg·kg−1·min−1) decreased neuronal peak discharge frequency to 60 ± 12% (inspiratory, n = 7) and 58 ± 11% (expiratory, n = 11). However, local picoejection of naloxone did not reverse the neuronal depression. Our data suggest that μ, δ1, and δ2 receptors are present on canine respiratory premotor neurons. Clinical concentrations of morphine and remifentanil caused no local depression. This lack of effect and the inability of local naloxone to reverse the neuronal depression by intravenous remifentanil suggest that clinical concentrations of opioids produce their depressive effects on mechanisms upstream from respiratory bulbospinal premotor neurons.
INTRODUCTION
Opioid agonists such as morphine, fentanyl, and remifentanil are potent clinical analgesics and respiratory depressants. The respiratory depressant effects are thought to be mainly due to μ receptor activation although depression by δ opioid receptor activation has also been implicated (Sales et al. 1985). Neurons expressing opioid receptors have been identified throughout the respiratory system. For example, μ and δ opioid receptors have been pharmacologically (Denavit-Saubie et al. 1978; Morin-Surun et al. 1984) and immunohistochemically (Haji et al. 2003b) identified on feline respiratory neurons, where they are differentially distributed either predominantly on the soma, e.g., in most bulbospinal premotor neurons, or on the dendrites (Haji et al. 2003b). Several studies have shown that clinical (Haji et al. 2003a; Lalley 2003) and/or pharmacological (Denavit-Saubie et al. 1978; Lalley 2003; Morin-Surun et al. 1984) concentrations of fentanyl and morphine cause depression of various types of respiratory neurons in vivo via both direct and indirect mechanisms. However, it remains unresolved whether clinically relevant concentrations of opioids exert their effect via network effects or via direct effects on specific respiratory neurons in the brain stem.
We investigated this question for respiratory bulbospinal neurons in a decerebrate dog model that allows us to study the effects of synaptic neurotransmitters and pharmacological agonists or antagonists on functionally identified single respiratory neurons in vivo under physiologically relevant conditions without confounding background anesthesia(Tonkovic-Capin et al. 1998). In this model, inspiratory and expiratory bulbospinal premotor neurons are the major source of excitatory drive (Dogas et al. 1995; Krolo et al. 1999, 2000) for inspiratory and expiratory motoneurons, which directly activate the respiratory musculature and may thus be direct or indirect targets of clinical opioid effects.
As a first step we sought to determine whether μ and δ opioid receptors were present on canine respiratory premotor neurons. We investigated whether selective μ and δ opioid agonists had direct and reversible depressant effects on these neurons in vivo as well as the maximum effect for each agonist. Once the presence of functional opioid receptors was established, we investigated with similar protocols whether picoejection of the clinical μ opioid agonists morphine and remifentanil had a direct depressant effect on premotor neurons and whether the doses necessary to achieve neuronal depression were in a plasma concentration range that can be expected during clinical use. Morphine was chosen because of its frequent clinical use and to allow comparison of our model with multiple studies in other in vivo and in vitro preparations. Remifentanil was chosen to allow direct comparison with the subsequent protocol using intravenous remifentanil. With the last protocol, we investigated whether the significant depression of respiratory premotor neurons that is caused by clinical dose rates of intravenous remifentanil resulted at least in part from a direct opioid effect. We used the clinical μ opioid agonist remifentanil because the pharmacokinetics of this drug allowed achievement of a steady-state global respiratory depression with intravenous infusion and then a quick recovery to baseline conditions for postremifentanil control runs. The fast and complete metabolism of the drug also allowed multiple protocols in the same animal without confounding effects of residual systemic opioids.
METHODS
This research was approved by the subcommittee on animal studies of the Zablocki Veterans Affairs Medical Center, Milwaukee, WI, in accordance with provisions of the Animal Welfare Act, the PHS Guide for the Care and Use of Laboratory Animals, and VA policy. Experiments were performed on mongrel dogs of either sex, weighing from 8 to 16 kg. Inhalational anesthesia was induced by mask and maintained with isoflurane at 1.5–2.5% end-tidal concentration. The animals were monitored for signs of inadequate anesthesia such as salivation, lacrimation, and increases in blood pressure and heart rate. If required, anesthetic depth was increased immediately.
Surgical procedures
The trachea of dogs was intubated with a cuffed endotracheal tube, and their lungs were mechanically ventilated with an air-O2-isoflurane mixture. The surgical procedures, monitoring, and maintenance of body homeostasis have been previously described in detail (Dogas et al. 1998). Briefly, after cannulating the femoral artery for blood pressure recording and blood gas sampling and femoral vein for continuous infusion of maintenance fluids and administration of drugs, a bilateral pneumothorax was performed to reduce motion artifacts. The animal was then decerebrated by midcollicular transection (Tonkovic-Capin et al. 1998), and isoflurane was discontinued. After decerebration, the animal was ventilated with an air-O2 mixture and maintained in hyperoxic normocapnia (FiO2 >0.6). The dorsal surface of the medulla oblongata was exposed by an occipital craniotomy for neuronal recording. Phrenic nerve activity was recorded from the desheathed right C5 rootlet. The phrenic neurogram (PNG) was obtained from the moving-time average (100 ms) of the amplified phrenic nerve activity and was used to produce timing pulses corresponding to the beginning and end of the inspiratory phase. Continuous neuromuscular block was achieved with pancuronium (0.1 mg·kg−1·h−1) to reduce motion artifacts during neuronal recordings.
Picoejection technique and neuronal recording
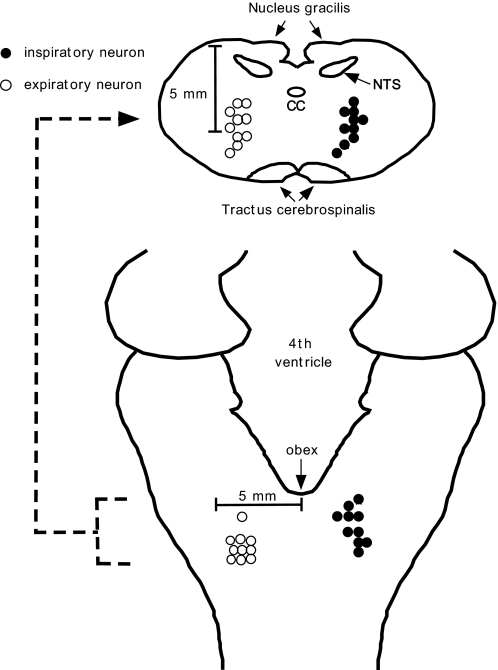
A minimum of 1 h was allowed for preparation stabilization before data collection. Extracellular single-unit recordings from caudal ventral respiratory group (VRG) neurons were obtained using multibarrel micropipettes (10–30 μm composite tip diameter) consisting of a recording barrel containing a 7-μm-thick carbon filament and three drug barrels. We have shown in previous studies that >90% of antidromically tested inspiratory (Bajic et al. 1992; Stuth et al. 1994) and expiratory (Bajic et al. 1992) neurons in the caudal and intermediate VRG from where we sampled neurons were bulbospinal premotor neurons. The microelectrodes allowed extracellular recordings of neuronal action potentials before and during pressure ejection of picomolar amounts of opioid agonists or the opioid antagonist naloxone onto the neuron. The ejected solutions consisted of artificial cerebrospinal fluid (ACSF) and one of the following agents that was dissolved in ACSF at a 1 mM concentration: the μ receptor agonist [d-Ala2, N-Me-Phe4, Gly-ol5]-enkephalin (DAMGO), the δ1 agonist d-Pen2,5-enkephalin (DPDPE), the δ2 agonist [d-Ala2]-deltorphin II (deltorphin II), or the nonselective opioid receptor antagonist naloxone. The ACSF consisted of (in mM) 124 NaCl, 2 KCl, 2 MgCl, 1.3 KH2PO4, 0.9 CaCl2, 26 NaHCO3, and 11 glucose. Similarly, the μ receptor agonist morphine sulfate was dissolved in ACSF in concentrations of 10 μM, 100 μM, and 1 mM, and the μ agonist remifentanil was dissolved in ACSF in concentrations of 240 and 480 nM. Because remifentanil was only available from one source, the final solutions also contained 10 and 20 μM glycine. The picoejected dose rate was measured via height changes of the meniscus in the pipette barrel with a ×50 magnification microscope equipped with a reticule (resolution: ∼2 nl). The picoejection technique has been described in detail by Dogas et al. (1998) and Krolo et al. (1999). The location of the recorded neurons was in a region extending from 1 to 5 mm caudal to the obex, 2.5–4.5 mm lateral to the midline and 2–4.5 mm below the dorsal medullary surface. A distance of ≥500 μm was required between picoejection sites to ensure that results were not confounded by residual drug effects (<0.3% of barrel concentration for a 10 nl injection) (Nicholson 1985). A time-amplitude window discriminator was used to generate a standard logic pulse for each spike. Neuronal discharge frequency, Fn, was continuously determined by the number of discharges in each 100-ms time increment.
Protocols
1. Picoejection of subtype specific opioid receptor agonists onto single neurons: determination of the presence of opioid receptor subtypes and maximum effect
After establishing a stable recording of single neuron activity, the neuronal control activity was recorded for ≥2–4 min. Then picoejection was started and the dose rate was gradually increased until a neuronal response was observed. Thereafter the dose rates were increased stepwise until the effects saturated, i.e., an additional increase in dose-rate did not produce any further change in neuronal activity. To avoid potential cross-contamination from multiple barrels. only one selective agonist was tested for each neuron protocol. In a subset of neurons, after maximal effects had been obtained, picoejection of the nonselective opioid antagonist naloxone was used to ensure that the agonist effects were reversible. In a separate group of neurons, this antagonist was picoejected without prior agonist application to determine whether the neurons received any endogenous input through opioid receptors.
2. Picoejection of morphine onto single neurons: assessment of the threshold concentration for a direct morphine effect and of the maximum direct morphine effect
In the second set of animals, the prototypic clinical μ agonist morphine was picoejected in increasing dose rates until a ceiling effect was reached for each of the three concentrations tested. Because the experimental setup has a limit of three drug barrels, we initially tested only the higher morphine concentrations (100 μM and 1 mM). When we found that the 100 μM concentration already led to a significant neuronal depression, we added experiments with 10 μM morphine to more closely assess the threshold concentration for neuronal depression. In a subset of neurons, reversibility of the morphine effect was tested with naloxone. In addition, i.e., at least once per animal, picoejection of the ACSF vehicle was used to verify that the ACSF constituents and ejected volumes were without effect on single neurons.
3. Picoejection of remifentanil onto single neurons: assessment of a direct depressant effect of clinically relevant concentrations
In the third set of animals, the clinical μ agonist remifentanil was picoejected in increasing dose rates until a ceiling effect was reached for each of the two concentrations tested. Because remifentanil is only commercially available as a mixture with glycine, we only tested two concentrations of remifentanil (240 and 480 nM). These concentrations are ∼10–20 times higher than published peak plasma concentrations encountered clinically during high-dose intravenous remifentanil infusions. The final glycine concentrations in the picoejected solution were 10 and 20 μM, which is below the effective concentration for in vivo picoejection (0.5–1 mM) (Zuperku, unpublished observations from glycine picoejection experiments). In a subset of neurons, reversibility of the remifentanil effect was tested with naloxone. In addition, i.e., at least once per animal, picoejection of the ACSF vehicle was used to verify that the ACSF constituents and ejected volumes were with out effect on neuronal frequency.
4. Picoejection of the opioid antagonist naloxone onto single neurons during intravenous remifentanil infusion: does direct opioid receptor activation on premotor neurons contribute to the respiratory depression by systemic remifentanil?
In the last protocol, a stable recording from a single respiratory bulbospinal neuron was obtained, and baseline activity was recorded. Then an intravenous infusion of the μ agonist remifentanil was started and increased until peak phrenic nerve activity was depressed by ≈50%. The required doses (0.2–0.6 μg·kg−1·min−1) were in the clinically used dose range for dogs, which is similar to humans. Once a steady-state depression of peak phrenic activity and neuronal discharge frequency was established for ≥5 min, the nonselective opioid antagonist naloxone was picoejected onto the neuron in increasing dose rates. Dose rates were chosen that had previously completely reversed the depressant effect of 1 mM DAMGO picoejected onto a different premotor neuron in the same animal. Any reversal of the remifentanil induced neuronal depression by local naloxone was considered evidence of direct postsynaptic neuronal depression of the neuron. In addition, to assess any endogenous activation of neuronal opioid receptors, which may confound the determination of direct remifentanil effect, naloxone was again ejected onto the same neuron after remifentanil was stopped and neuronal and phrenic nerve activity had recovered to control levels.
Data analysis
Cycle-triggered histograms (CTHs; bin width: 50 ms), triggered from the onset of either the inspiratory or expiratory phase as defined by the PNG, and based on 5–20 respiratory cycles were used to quantify the neuronal discharge pattern, peak Fn and time-averaged Fn during the active neuronal phase. Peak Fn correlates closely with the peak excitatory drive transmitted to the phrenic motoneurons, whereas the average Fn allows a better interpretation of the drug effects on the overall neuronal discharge pattern (Eldridge 1975). For peak and average Fn, the values obtained during the preejection control period were used to normalize the values during the maximum effect of the respective drug. The main a priori goals of these studies were to determine the maximum drug effect for each agonist and to determine whether there were differential effects between inspiratory and expiratory neurons.
Statistical procedures were carried out using SigmaStat 3.5 (Systat Software, Richmond, CA). For all data sets, tests for normality of the normalized data (Kolmogorov-Smirnov test) were performed before parametric procedures were used. A two-way ANOVA, with type of drug and type of neuron as main factors, was used to determine statistically significant differences for those studies involving the μ and δ agonists. For the morphine studies with up to three concentrations per neuron, a two-way repeated-measures ANOVA (1-factor repetition) was used. A general linear model was automatically used to provide least-squares estimates of the means for cells with missing data and unbalanced data sets. The Holm-Sidak method was used for pairwise multiple comparisons with a family-wide error rate of 0.05. For one data set that failed the normality test, a Kruskal-Wallis one-way ANOVA on ranks was used with Dunn's method for pairwise multiple comparisons.
For protocol 4, the effects of intravenous remifentanil and local naloxone antagonism on peak Fn were analyzed using a two-way repeated-measures ANOVA (1-factor repetition) with type of neuron and type of treatment (e.g., remifentanil/naloxone) as main factors. Differences were considered significant for P < 0.05. Values are expressed as means ± SE.
RESULTS
Picoejection of subtype specific opioid receptor agonists onto single neurons: determination of the presence of opioid receptor subtypes and maximum effect
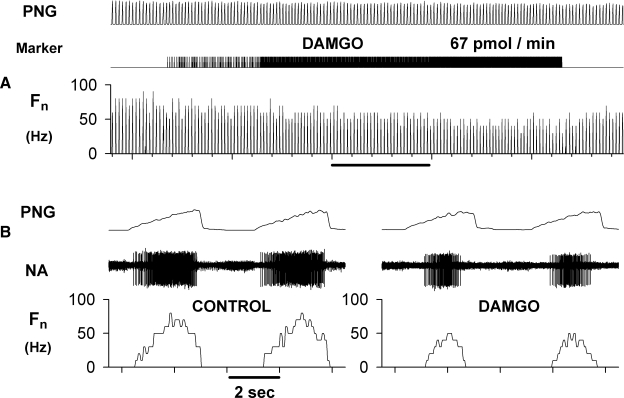
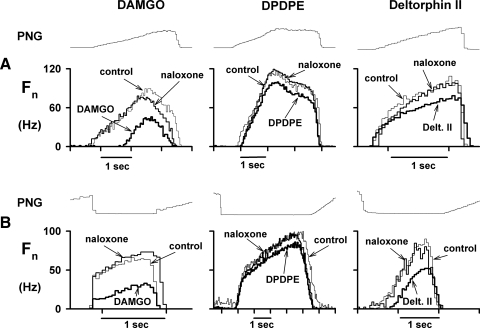
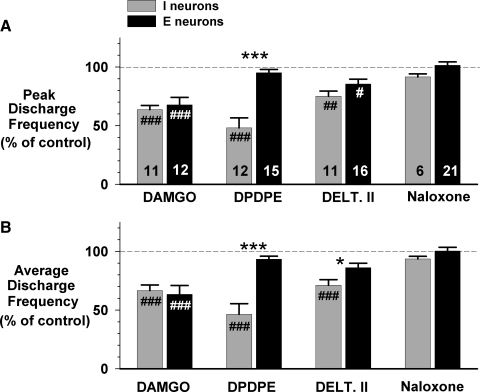
Fifteen animals were used for this protocol. A representative example of the response to picoejection of increasing dose rates of the selective μ agonist DAMGO (1 mM) is shown in Fig. 1 for an inspiratory premotor neuron. The inspiratory neuronal peak discharge frequency Fn was depressed from 75 to 50 Hz at maximally effective dose-rates of DAMGO of 67 pmol/min. Examples of the CTHs of inspiratory and expiratory neuronal responses to DAMGO, DPDPE, deltorphin II, and naloxone are shown in Fig. 2, A and B, respectively. Figure 2A shows that all three agonists depressed the peak discharge frequency of three different inspiratory neurons at maximally effective dose rates. Naloxone fully reversed these effects. Figure 2B suggests that DAMGO and deltorphin II are more depressant than the δ1 agonist DPDPE (middle CTH) in expiratory neurons. The pooled data for both inspiratory and expiratory neurons are shown in Fig. 3, A and B. In inspiratory neurons, peak Fn was decreased by DAMGO to 64 ± 4%, by DPDPE to 48 ± 9%, and by deltorphin II to 75 ± 4% of control. The corresponding values for time-averaged Fn were: 67 ± 5, 46 ± 9, and 71 ± 5% of control (Fig. 3B). In six inspiratory neurons, naloxone was used and was able to fully reverse the agonist-induced depression.
FIG. 1.
Response of an inspiratory premotor neuron to the selective μagonist [d-Ala2, N-Me-phe4, gly-ol5]-enkephalin (DAMGO). A: the extracellularly recorded activity of the neuron was dose-dependently depressed by 1 mM DAMGO, which was ejected at increasing dose rates onto the neuron until maximal effects were reached at the peak dose rate of 67 pmol/min. DAMGO application was highly localized and phrenic nerve activity (PNG) was not affected. B: time-expanded views of the neuron activity during control and at maximum DAMGO effect. Note that the neuron discharged in phase with the phrenic nerve and was thus an inspiratory neuron. PNG: time averaged phrenic neurogram (arbitrary units), Marker: picoejection marker, Fn: neuronal rate-meter recording of the action potential discharge frequency in Hz, NA: extracellular neuron activity.
FIG. 2.
Cycle-triggered histograms (CTHs) of the effects of three selective opioid agonists at maximally effective dose rates on peak respiratory neuron activity and the neuronal discharge pattern. A: responses of 3 inspiratory neurons (see text for details). B: responses of 3 expiratory neurons (see text for details). All agonist effects were reversed with local picoejection of the nonselective opioid antagonist naloxone. Fn, CTHs of the neuronal action potential discharge frequency pattern in Hz during various conditions (10–30 cycles/CTH).
FIG. 3.
Pooled data for the depressant effects of 3 selective opioid agonists at maximally effective dose rates and naloxone reversal on neuron activity, normalized to preejection control. A: effect on peak discharge frequency. B: effect on average discharge frequency. #, significant difference compared with control (100%); *, significant difference between type of neuron (#P < 0.05, ##P < 0.01, ###P < 0.001, *P < 0.05, **P < 0.01, ***P < 0.001).
In expiratory neurons, peak Fn was decreased by DAMGO to 68 ± 6%, by DPDPE to 95 ± 3%, and by deltorphin II to 86 ± 4% of control (Fig. 3A, top). The corresponding values for time-averaged Fn were 63 ± 8, 93 ± 3, and 86 ± 4% of control (Fig. 3B, bottom). Naloxone produced full reversal of all agonist-induced depressions in those expiratory neurons that were tested (n = 21).
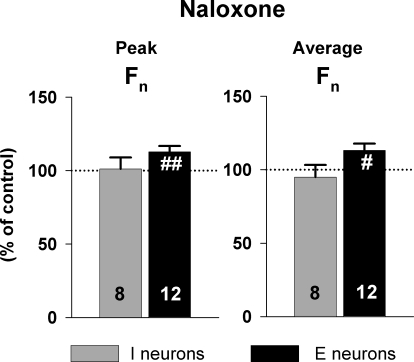
In a separate protocol, naloxone was picoejected onto 8 inspiratory and 12 expiratory neurons to assess the presence of endogenous opioid receptor activation (Fig. 4). Naloxone had no significant effect on inspiratory neurons but produced a small, yet statistically significant increase of 13 ± 4% in the peak Fn and 13 ± 5% in average Fn of expiratory neurons.
FIG. 4.
Pooled data of the effect of the nonselective opioid antagonist naloxone on control neuron activity at maximally effective dose rates. In this protocol, naloxone was given to assess any endogenous opioid effects on premotor neurons that may be present during the control condition. Left: effect on peak discharge frequency. Right: effect on average discharge frequency. Data (means ± SE) were normalized to preejection control values. Naloxone caused a small but statistically significant excitation in expiratory neurons (see text for details). #, significant difference compared with control (#P < 0.05, ##P < 0.01).
Picoejection of morphine onto single neurons: assessment of the threshold concentration for a morphine effect and of the maximum morphine effect
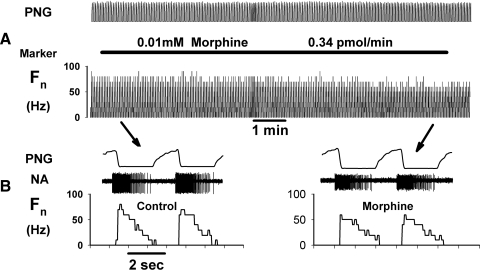
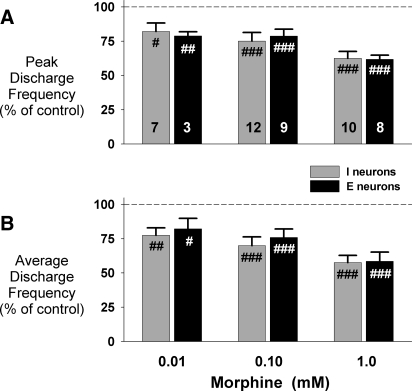
In this protocol, 27 bulbospinal neurons (15 inspiratory and 12 expiratory) were studied in 13 animals. The anatomical location of these neurons, based on stereotaxic coordinates, is shown in Fig. 5 and is typical for all of the neuron protocols in this report. We used up to three concentrations of morphine in different pipette barrels. The barrel concentrations ranged from 0.01 to 1.0 mM to more precisely delineate the full range of the dose-dependent effects of morphine on neuronal activity. A representative example of the effects of 0.01 mM morphine on an expiratory neuron is shown in Fig. 6, which illustrates the approximate lower threshold for morphine-induced neuronal depression. At the 0.01 mM morphine barrel concentration, neuronal depression becomes apparent only at the highest picoejection dose rates when the tissue concentration in the vicinity of the neuron is close to the barrel concentration. The peak and average neuronal activity was only minimally depressed at this concentration. The pooled data for the depressant effects of maximal dose rates of morphine at each of the three barrel concentrations are shown in Fig. 7. In inspiratory neurons, peak Fn was maximally decreased by 0.01, 0.1, and 1 mM morphine to 82 ± 6, 75 ± 6, and 62 ± 5% of control (Fig. 7A). The corresponding values for time-averaged Fn were: 78 ± 5, 70 ± 6, and 58 ± 5% of control (Fig. 7B). For expiratory neurons, peak Fn was decreased to 79 ± 3, 79 ± 5, and 62 ± 3% of control (Fig. 7A). The corresponding values for time-averaged Fn in expiratory neurons were 82 ± 8, 76 ± 6, and 58 ± 7% of control (Fig. 7B). Our sample of expiratory neurons included six with an augmenting pattern and six with a decrementing pattern. There was no difference between the two types of neurons in the amount of morphine-induced depression of peak Fn for the 1 mM concentration (P = 0.08, t-test).
FIG. 5.
Schematic diagram showing the anatomical location of 10 inspiratory and 10 expiratory neurons studied in protocol 2. Locations are based on stereotaxic coordinates relative to the obex, midline, and dorsal surface. To improve legibility neurons with identical locations are not marked as double. CC, central canal; NTS, nucleus tractus solitarii.
FIG. 6.
Response of an expiratory premotor neuron to the clinical μ-agonist morphine at the 0.01 mM barrel concentration shows threshold effects. A: the extracellularly recorded activity of the neuron was modestly depressed by morphine only at the highest effective dose rate where the local concentration approaches the barrel concentration. Morphine application was highly localized and phrenic nerve activity (PNG) was not affected. B: time-expanded views of the neuron activity during control and at peak morphine dose rate. Note that the expiratory neuron discharged out of phase with the phrenic nerve.
FIG. 7.
Pooled data for the effects of 3 concentrations of morphine at maximally effective dose rates on neuron activity, normalized to control (100%). A: effect on peak discharge frequency. B: effect on average discharge frequency. #, significant difference compared with control (#P < 0.05, ##P < 0.01, ###P < 0.001). Increasing concentrations of morphine caused a dose-dependent depression of respiratory premotor neurons, but there was no statistically significant difference between the 2 neuron types.
The depressant effects of morphine were completely reversed with local application of naloxone in the three neurons that we tested with naloxone in this protocol. Of note, a small number of neurons (n = 6), which were analyzed separately, did not show any depression but were excited at the highest morphine concentration. These neurons were not depressed by morphine but showed a maximum increase in peak Fn to 140 ± 9% of control with 1 mM morphine. These excitatory effects could not be reversed with naloxone and thus do not seem to have been opioid receptor mediated.
Picoejection of remifentanil onto single neurons: assessment of a direct depressant effect at clinically relevant concentrations
Five animals were used for this protocol. Neither of the two remifentanil barrel concentrations (240 and 480 nM) used had a depressant effect on neuronal activity. We studied 11 inspiratory and 9 expiratory neurons, respectively, and there were no significant differences from control activity. In inspiratory neurons, peak and average Fn during picoejection of 480 nM remifentanil solution at maximum dose rates was 96 ± 3 and 94 ± 4% of control, respectively. The corresponding values for expiratory neurons were 95 ± 3 and 101 ± 6% of control, respectively.
Picoejection of the opioid antagonist naloxone onto single neurons during systemic intravenous remifentanil infusion at clinically relevant dose rates
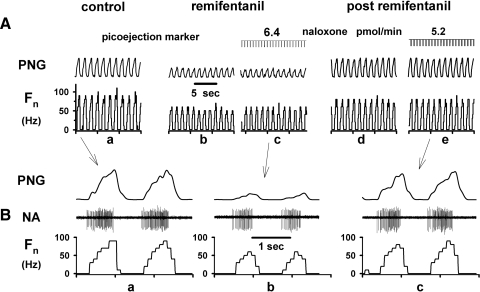
Nine animals were used for this protocol to determine whether opioid receptor activation on premotor neurons contributes to the respiratory neuronal depressant effects of systemic intravenous remifentanil. Figure 8 shows a typical remifentanil protocol. Peak phrenic nerve activity and single neuron activity were depressed by systemic remifentanil infusion by ∼50% (Fig. 8, a and b). During steady-state infusion, naloxone was picoejected onto the neuron to attempt local reversal of the depressant remifentanil effects (Fig. 8c). Because neuronal activity did not increase with maximal local naloxone rates, direct neuronal postsynaptic opioid receptor activation did not appear to contribute to the depression of neuronal activity. Pooled data show that systemic infusions of remifentanil depressed phrenic nerve activity by ∼50% and decreased the peak inspiratory and expiratory premotor neuron discharge frequency to 60 ± 4% (n = 7) and 58 ± 3% (n = 11), respectively (see Fig. 9). Local naloxone ejection did not reverse any of the remifentanil-induced neuronal depression (peak Fn of inspiratory and expiratory neurons: 62 ± 4 and 56 ± 3%, respectively; Fig. 9 REMI and naloxone; REMI: Fn during intravenous remifentanil infusion). These data confirm that clinically relevant infusions of remifentanil do not depress respiratory premotor neurons via postsynaptic opioid receptor activation on these neurons but rather via upstream effects. After discontinuation of the remifentanil infusion, inspiratory neuronal activity recovered to baseline. Control ejection of naloxone had no effect on inspiratory neurons. However, expiratory neuronal activity showed a small but significant increase in Fn compared with control (Fig. 9, end control and naloxone).
FIG. 8.
Systemic remifentanil protocol: response of an inspiratory premotor neuron and phrenic nerve activity to the continuous systemic infusion of remifentanil (≈0.5 μg·kg−1·min−1): A: extracellularly recorded neuronal activity and the peak phrenic nerve activity during control conditions (a) and at ≈50% depression by the systemic remifentanil infusion (b). During continuous remifentanil infusion, the opioid antagonist naloxone was picoejected directly onto the neuron (c). Despite high naloxone dose rates, which had previously been shown to fully reverse the depressant effects of a locally applied μ agonist (DAMGO), the remifentanil induced neuronal depression was not reversed. After termination of remifentanil neuronal discharge activity recovered to control levels (d), and repeat picoejection of naloxone had no further effect on neuronal discharge frequency (e). B: time-expanded views of the neuronal activity during control, during remifentanil induced depression and maximal naloxone picoejection and during recovery from remifentanil and maximal naloxone picoejection.
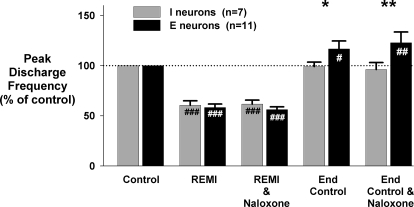
FIG. 9.
Systemic remifentanil protocol: pooled data summarizes the effects of systemic remifentanil (REMI) on peak neuron discharge frequency. Data are normalized to baseline control condition (100%). Local naloxone (REMI and naloxone) did not reverse any of the depression that was caused by intravenous infusion of remifentanil (REMI). After recovery from remifentanil (end control), use of local naloxone (end control and naloxone) suggests that there was no endogenous opioid induced depression of inspiratory neurons present during end control conditions. Expiratory neurons showed a small but statistically significant increase above baseline after recovery from remifentanil and with naloxone similar to our findings in a previous naloxone protocol (Fig. 4). *, significant difference compared with control (*P < 0.05, **P < 0.01).
DISCUSSION
The main findings of these studies show that opioid receptor activation on functionally well-defined bulbospinal respiratory neurons in an in vivo canine preparation depresses neuronal activity, but that neuronal depression with morphine occurs only at local concentrations above the clinical range. In addition, clinical doses of intravenous remifentanil, which depress the activity of these neurons to a similar extent as maximally effective local agonist concentrations, do not act via direct opioid receptor activation. Thus opioid-induced effects on presynaptic neurons appear to be responsible for the depression of these neurons at clinical concentrations.
Inspiratory premotor neurons respond to μ, δ1, and δ2 opioid receptor activation, whereas expiratory neurons only respond to μ and δ2 activation.
We found that both μ and δ agonists depressed the activity of respiratory premotor neurons but that the δ1 receptor agonist DPDPE was ineffective for expiratory neurons yet highly effective for inspiratory neurons. Local antagonism with the nonselective antagonist naloxone fully reversed any depressant opioid effects. These results match radioactive labeling studies that show μ as well as δ opioid receptors distributed in the respiratory centers of the brain stem (Sales et al. 1985). Haji et al. (2003b) showed with immunoreactive labeling that μ receptors were widely distributed on functionally identified bulbospinal respiratory neurons. Our study provides additional evidence that δ receptor activation can contribute to depression of respiratory neurons, and it is the first to demonstrate a depression of functionally identified respiratory premotor neurons by δ agonists. Interestingly, there was a ceiling effect of 40–50% depression for all agonists and even maximally effective agonist dose rates were not able to completely silence any of these neurons. Indeed, many neurons were only moderately depressed, i.e., much <50% at maximally effective local agonist dose rates.
Only supraclinical doses of morphine have a direct depressant effect on respiratory premotor neurons
The μ receptor agonist morphine has been shown to depress respiratory neurons in vivo. Using iontophoresed morphine (50 mM) onto single bulbar respiratory neurons in spinalized cats, Denavit-Saubie et al. (1978) found that 35 of 58 neurons were dose-dependently depressed, whereas 14 showed no effect and 9 showed dose-dependent excitation that, unlike the depressive effects, was not naloxone-reversible. More recently, Haji et al. (2003b) iontophoresed morphine (50 mM) onto feline bulbar respiratory neurons and found membrane hyperpolarization and a decrease in action potential discharge frequency in most neurons. These effects were consistent with postsynaptic opioid receptor activation on these neurons. Our studies show that at a 10 μM barrel concentration of morphine, premotor neurons are only slightly depressed (≈20%). This seems to be the threshold concentration for postsynaptic receptor activation that our preparation can discern. However, this concentration is already ∼250 times larger than the plasma concentration of morphine that causes adequate analgesia in dogs (KuKanich et al. 2005; Lucas et al. 2001). We expect that the iontophoresed (50 mM) morphine in the preceding studies most likely resulted in concentrations at the neuronal level that were similar to or even exceeded the highest concentration at maximal picoejection dose rates in our studies (1 mM).
The level of opioid tissue concentration is an important consideration in determining the sites of action that produce depression and slowing within the respiratory control neural network. Our finding that clinically relevant concentrations, which are in the nanomolar range, have no direct effect on respiratory premotor neurons is consistent with those of Lalley (2006), who used intracellular recordings from ventrolateral respiratory column neurons in barbiturate-anesthetized or decerebrate adult cats. Intravenous doses of the μ agonist fentanyl in the clinical dose range (3–7 μg/kg) led to significant slowing of the respiratory rhythm as well as an increase in duration of discharge in inspiratory and expiratory bulbospinal and propriobulbar neurons. However, there were no changes in the intrinsic properties of the studied neurons; that is, there was no evidence from measurements of input resistance or action potential threshold, shape, and afterhyperpolarization that the doses of fentanyl that slowed rhythm acted on membrane conductances. Lalley (2006) suggested that slowing was evidently generated through effects on other neurons that control the onset and termination of inspiratory and expiratory neuron discharges, such as those in the pons and pre-Bötzinger complex.
Clinically relevant concentrations of remifentanil do not have a direct depressant effect on respiratory premotor neurons
We used two barrel concentrations of remifentanil (240 and 480 nM), which were 20 and 40 times higher than the peak plasma concentration reached during intravenous infusions at high clinical dose rates. At the steady-state picoejection rates that were used, the drug concentration within 100–200 μm of the pipette tip will eventually approach the barrel concentration. To obtain adequate signal strength, the neuron is typically <100 μm from the tip, so we are fairly confident that our neuronal remifentanil target concentrations during picoejection exceeded peak plasma concentrations during intravenous remifentanil by ≥20-fold. Due to the presence of glycine in the remifentanil product, we did not test higher remifentanil concentrations to avoid the confounding effects of glycine-mediated inhibition. The glycine concentrations corresponding to the 240 and 480 nM remifentanil concentrations were 10 and 20 μM, respectively. We have shown that glycine concentrations in the range of 0.5–1 mM produce a marked inhibition of respiratory neuronal activity, including premotor neurons. Neither of the two remifentanil/glycine concentrations had an appreciable effect on premotor neuronal activity. These data suggested that remifentanil at clinically relevant concentrations does not directly depress respiratory bulbospinal neurons, which was confirmed by the systemic remifentanil protocol.
Intravenous remifentanil depresses respiratory premotor neuron activity but not through direct neuronal opioid receptor activation
We explored the effects of clinical plasma concentrations further by using an infusion of the ultra-short acting μ agonist remifentanil, which allowed steady-state depression of neuronal activity as well as end controls without residual opioid activity. The results show that remifentanil at clinically relevant infusion rates, which caused significant depression of phrenic nerve activity (∼50%), also significantly depressed respiratory premotor neuron activity (∼40%). We conclude that none of this depression is due to the direct action of the opioid on inspiratory and expiratory bulbospinal neurons due to the evidence from our two approaches. 1) During intravenous remifentanil, the difference in neuronal peak discharge frequency immediately before and during picoejection of naloxone was <1% (mean ± SD: 0.86 ± 6.5%, paired t-analysis; P = 0.579) for 18 neurons. 2) Picoejection of remifentanil at concentrations 20–40 times greater than the expected peak plasma concentration during intravenous infusions at high clinical dose rates did not alter the discharge frequency of these neurons. In addition, the sensitivity of our technique was able to detect a 13% increase in expiratory neuronal activity produced by local picoejection of naloxone indicating a small but significant level of endogenous opioidergic activity. Thus we conclude that intravenous remifentanil had no direct effect on the respiratory bulbospinal neurons and that their depression was due to presynaptic effects.
We believe that the discrepancy between the direct, reversible depressant effect of morphine on single neurons that we and other investigators observed and the lack of local naloxone reversibility of the intravenous remifentanil results from the difference of the attained agonist concentrations at the neuronal level. The remifentanil infusion rates we used (≈0.5 μg·kg−1·min−1) are expected to result in plasma concentrations of ≤10 ng/ml or ≤24 nanomolar (Michelsen et al. 1996) in dogs, which is ≈40,000 times less than the maximal concentrations of DAMGO or morphine that were used in the local picoejection experiments to cause a similar depression of neuronal activity. Furthermore, picoejected remifentanil at concentrations >20 times larger than reported peak plasma concentrations during systemic remifentanil infusions had no effect on the discharge frequency of the bulbospinal neurons. Taken together our data strongly suggest that clinical doses of therapeutic μ opioid agonists depress respiratory premotor neurons by affecting mechanisms upstream from these neurons and not via direct opioid receptor activation on these neurons.
With regard to the nature of the presynaptic depression by intravenously administered opioids at clinical doses, our experimental approach is unable to pinpoint the exact mechanism. However, we speculate that the remifentanil-induced depression of the bulbospinal premotor neurons by ∼40% is likely due to effects on specific presynaptic neurons that are extremely sensitive to the opioid at clinical concentrations. An alternative explanation is that the opioid is producing a small amount of postsynaptic depression throughout the network and the summation of these small effects produces the 40% depression of the bulbospinal neurons. For this latter explanation to be true, it would require these small effects to be propagated in a cascade manner to summate to a greater level of depression. For example, to produce a 40% depression from cascaded 5% depressions would require 10 synaptically coupled neurons or groups of neurons in series (0.9510 = 0.6). Depressions by 10% would require five cascaded effects. Most network models of the central respiratory system typically show many convergent connections to a given VRG neuron but those connections are not made by fourth- or higher-order neurons.
Evidence in support of specific presynaptic neurons that may be very sensitive to low clinical concentrations of opioids is the finding of high concentrations of endomorphin-containing varicose fibers in brain stem regions that are intimately involved with the control of breathing such as the nucleus tractus solitarius (NTS) and pontine parabrachial region (Martin-Schild et al. 1999). These endomorphin-immunoreactive fibers have been found to be in close proximity to high densities of μ opioid receptors (Zadina et al. 1999). Not only would the number of μ opioid receptors on a neuron contribute to its sensitivity but also the location of the receptors on the neuron. If the receptors are strategically located on the axon terminals similar to the primary afferent fibers in the dorsal horn of the spinal cord, which appear to inhibit the release of neurotransmitters via inhibition of voltage-dependent Ca2+ channels, the effect of the opioid could be markedly enhanced compared with a location of μ opioid receptors near or on the soma. Thus there are possible candidates for extremely opioid-sensitive presynaptic neurons within the pathways that relay excitation to the bulbospinal neurons.
The main source of excitation of canine bulbospinal premotor neurons consists of synaptic release of glutamate (Dogas et al. 1995; Krolo et al. 1999, 2000). The level of excitation of these neurons is also related to the level of chemodrive. Thus it is likely that opioid-induced depression of the chemosensory system is involved. High densities of endomorphin (endogenous μ opioid agonist) immunoreactive fibers and cell bodies have been found in the dorsal medial portion of the caudal NTS, a region where second-order neurons of the carotid body chemoreceptors are located (Martin-Schild et al. 1999). Anatomical data at the light and electron microscopic level and electrophysiological observations showed that μ opioid receptors are present at both pre- and postsynaptic sites in the dorsolateral NTS, often at the same synapse (Poole et al. 2007). Thus it is possible that opioids affect the peripheral chemoreflex pathway by depressing synaptic input to the second-order neurons. In addition, there appear to be multiple chemosensing areas in the brain stem (Feldman et al. 2003) with the retrotrapezoid nucleus playing a dominant role and having inputs to the ventral respiratory column and the dorsal pons (Guyenet et al. 2008). The role of the lateral pons in the control of breathing is well established (Rybak et al. 2004; St-John and Paton 2004) and the fact that it receives chemodrive inputs and is densely populated with endomorphin-immunoreactive fibers (Martin-Schild et al. 1999) suggests that the pontine input to bulbospinal neurons is also depressed.
Another potential source of opioid-induced presynaptic depression are neurons within the pre-Bötzinger complex (preBC). In a neonatal rat preparation, Gray et al. (1999) suggested that the distribution of neurokinin-1 (NK1) receptor neurons anatomically defined the preBC and that a subpopulation of neurons expressing both NK1 receptors and μ opioid receptors generate respiratory rhythm and modulate respiratory frequency. Furthermore, NK1 receptor-expressing cells within the preBC are predominantly glutamatergic (Guyenet et al. 2002). Correlation analysis between rostral and caudal VRG inspiratory neurons suggests that the more rostral preBC neurons drive the more caudal bulbospinal neurons (Rekling and Feldman 1998; Segers et al. 1987). Thus any opioid-induced depression of preBC neurons may reduce excitatory drive to the bulbospinal neurons.
Methodological considerations
This is the first study to use a continuous infusion of remifentanil to test for opioid effects on respiratory neurons in vivo. The establishment of a clinically relevant steady-state opioid concentration, as judged from the steady-state ≈50% depression of phrenic nerve activity, is important to achieve conclusive results. For example, Haji injected intravenous morphine (0.3–3.0 mg/kg) into decerebrate cats and showed a biphasic effect on bulbar respiratory neurons with an initial membrane hyperpolarization and decrease in input resistance indicating postsynaptic effects and subsequent elevation of input resistance indicating presynaptic effects only (Haji et al. 2003a). Possibly due to pharmacokinetic effects, the initial changes resulted from a transient increase in morphine blood concentration after the bolus, whereas the later changes were observed during lower blood and effect-site concentrations. Such transient concentration effects make interpretation more difficult.
Possible physiological role of neuronal opioid receptors
We have discussed that the agonist plasma concentrations reached during the remifentanil infusion are not sufficient to directly affect respiratory premotor neuronal opioid receptors. This leaves the question whether any physiological opioidergic/enkephalinergic input could be of sufficient magnitude to have effects on these premotor neuronal receptors. Based on our data we expect this to require release of at least micromolar concentrations of endogenous opioid agonists into the synaptic cleft. It is well established that synaptic concentrations of various endogenous neurotransmitters will transiently approach the concentrations of neurotransmitters stored in presynaptic vesicles. No data are available for synaptic concentrations of opioidergic neuropeptides, but release of the opioidergic neuropeptide met-enkephalin from synaptosomes has been shown to be similar to classical neurotransmitters (Verhage et al. 1992). For excitatory neurotransmitters, concentrations in presynaptic vesicles range from 1 to 5 mM for glutamate (Clements 1996; Clements et al. 1992) to 270 mM for serotonin in Retzius cells (Bruns et al. 2000). Thus it is possible that transient synaptic concentrations of endogenous opioids from the fiber varicosities adjacent to μ opioid receptors (Zadina et al. 1999) can reach the micromolar and possibly millimolar range in vivo.
Lack of endogenous opioid activity at the premotor neuronal level
In our preparation, naloxone did not have any effect on inspiratory neuron activity indicating that no endogenous opioid activation was present. However, a small but statistically significant increase in neuronal frequency by ∼10% was observed in expiratory neurons in both, substudies 2 and 3. Haji also described small excitatory effects of iontophoresed naloxone due to membrane depolarization in 5 of 18 bulbar respiratory neurons in decerebrate cats (Haji et al. 2003a). However, the systemic administration of large doses of naloxone (0.1 mg/kg) did not alter resting ventilation in healthy human volunteers (Bianchi et al. 1995; Tabona et al. 1982). The physiological role of opioidergic/enkephalinergic inputs on the respiratory system has yet to be clarified.
In conclusion, our current studies on bulbospinal respiratory premotor neurons in decerebrate dogs provide evidence that these neurons express functional μ and δ opioid receptors. However, clinically relevant concentrations of the μ agonist remifentanil that depress phrenic nerve activity and respiratory premotor neuron activity by ≈ 50% appear to exert their effects entirely upstream from these premotor neurons, i.e., at presynaptic neurons.
GRANTS
Support was received from the Department of Anesthesiology, Medical College of Wisconsin, Children's Hospital of Wisconsin and the Zablocki Veterans Administration Medical Center, Milwaukee, WI. This work also was supported by grant GM59234-06A1 “Volatile Anesthetics and Respiratory Neurotransmission” from the National Institutes of Health, Bethesda, MD, to E. A. Stuth and VA Medical Research Funds, Washington, DC, to E. J.Zuperku.
Acknowledgments
The authors thank J. Tomlinson, Biological Laboratory Technician, Zablocki VA Medical Center, Milwaukee, WI, for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bajic et al. 1992.Bajic J, Zuperku EJ, Tonkovic-Capin M, Hopp FA. Expiratory bulbospinal neurons of dogs. I. Control of discharge patterns by pulmonary stretch receptors. Am J Physiol Regulatory Integrative Comp Physiol 262: R1075–R1086, 1992. [DOI] [PubMed] [Google Scholar]
- Bianchi et al. 1995.Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. PhysiolRev 75: 1–45, 1995. [DOI] [PubMed] [Google Scholar]
- Bruns et al. 2000.Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron 28: 205–220, 2000. [DOI] [PubMed] [Google Scholar]
- Clements 1996.Clements JD Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci 19: 163–171, 1996. [DOI] [PubMed] [Google Scholar]
- Clements et al. 1992.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science 258: 1498–1501, 1992. [DOI] [PubMed] [Google Scholar]
- Denavit-Saubie et al. 1978.Denavit-Saubie M, Champagnat J, Zieglgansberger W. Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res 155: 55–67, 1978. [DOI] [PubMed] [Google Scholar]
- Dogas et al. 1998.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol 80: 2368–2377, 1998. [DOI] [PubMed] [Google Scholar]
- Dogas et al. 1995.Dogas Z, Stuth EAE, Hopp FA, McCrimmon DR, Zuperku EJ. NMDA receptor-mediated transmission of carotid body chemoreceptor input to expiratory bulbospinal neurones in dogs. J Physiol 487: 639–651, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge 1975.Eldridge FL Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol 39: 567–574, 1975. [DOI] [PubMed] [Google Scholar]
- Feldman et al. 2003.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray et al. 1999.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286: 1566–1568, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al. 2002.Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22: 3806–3816, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al. 2008.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji et al. 2003a.Haji A, Okazaki M, Ohi Y, Yamazaki H, Takeda R. Biphasic effects of morphine on bulbar respiratory neuronal activities in decerebrate cats. Neuropharmacology 45: 368–379, 2003a. [DOI] [PubMed] [Google Scholar]
- Haji et al. 2003b.Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neuroscience letters 351: 37–40, 2003b. [DOI] [PubMed] [Google Scholar]
- Krolo et al. 1999.Krolo M, Stuth EA, Tonkovic-Capin M, Dogas Z, Hopp FA, McCrimmon DR, Zuperku EJ. Differential roles of ionotropic glutamate receptors in canine medullary inspiratory neurons of the ventral respiratory group. J Neurophysiol 82: 60–68, 1999. [DOI] [PubMed] [Google Scholar]
- Krolo et al. 2000.Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Relative magnitude of tonic and phasic synaptic excitation of medullary inspiratory neurons in dogs. Am J Physiol Regulator Integrative Comp Physiol 279: R639–R649, 2000. [DOI] [PubMed] [Google Scholar]
- KuKanich et al. 2005.KuKanich B, Lascelles BD, Papich MG. Pharmacokinetics of morphine and plasma concentrations of morphine-6-glucuronide following morphine administration to dogs. J Vet Pharmacol Ther 28: 371–376, 2005. [DOI] [PubMed] [Google Scholar]
- Lalley 2003.Lalley PM Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regulatory Integrative Comp Physiol 285: R1287–1304, 2003. [DOI] [PubMed] [Google Scholar]
- Lalley 2006.Lalley PM Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am J Physiol Regulatory Comp Physiol 290: R1387–1396, 2006. [DOI] [PubMed] [Google Scholar]
- Lucas et al. 2001.Lucas AN, Firth AM, Anderson GA, Vine JH, Edwards GA. Comparison of the effects of morphine administered by constant-rate intravenous infusion or intermittent intramuscular injection in dogs. J Am Vet Med Assoc 218: 884–891, 2001. [DOI] [PubMed] [Google Scholar]
- Martin-Schild et al. 1999.Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405: 450–471, 1999. [PubMed] [Google Scholar]
- Michelsen et al. 1996.Michelsen LG, Salmenpera M, Hug CC, Jr, Szlam F, VanderMeer D. Anesthetic potency of remifentanil in dogs. Anesthesiology 84: 865–872, 1996. [DOI] [PubMed] [Google Scholar]
- Morin-Surun et al. 1984.Morin-Surun MP, Boudinot E, Gacel G, Champagnat J, Roques BP, Denavit-Saubie M. Different effects of mu and delta opiate agonists on respiration. Eur J Pharmacol 98: 235–240, 1984. [DOI] [PubMed] [Google Scholar]
- Nicholson 1985.Nicholson C Diffusion from an injected volume of a substance in the brain tissue with arbitrary volume fraction and tortuosity. Brain Res 333: 325–329, 1985. [DOI] [PubMed] [Google Scholar]
- Poole et al. 2007.Poole SL, Deuchars J, Lewis DI, Deuchars SA. Subdivision-specific responses of neurons in the nucleus of the tractus solitarius to activation of mu-opioid receptors in the rat. J Neurophysiol 98: 3060–3071, 2007. [DOI] [PubMed] [Google Scholar]
- Rekling and Feldman 1998.Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–404, 1998. [DOI] [PubMed] [Google Scholar]
- Rybak et al. 2004.Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Morschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol 143: 307–319, 2004. [DOI] [PubMed] [Google Scholar]
- Sales et al. 1985.Sales N, Riche D, Roques BP, Denavit-Saubie M. Localization of mu- and delta-opioid receptors in cat respiratory areas: an autoradiographic study. Brain Res 344: 382–386, 1985. [DOI] [PubMed] [Google Scholar]
- Segers et al. 1987.Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol 57: 1078–1100, 1987. [DOI] [PubMed] [Google Scholar]
- St-John and Paton 2004.St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol 143: 321–332, 2004. [DOI] [PubMed] [Google Scholar]
- Stuth et al. 1994.Stuth EAE, Tonkovic-Capin M, Kampine JP, Bajic J, Zuperku EJ. Dose-dependent effects of halothane on the carbon dioxide responses of expiratory and inspiratory bulbospinal neurons and the phrenic nerve activities in dogs. Anesthesiology 81: 1470–1483, 1994. [DOI] [PubMed] [Google Scholar]
- Tabona et al. 1982.Tabona MV, Ambrosino N, Barnes PJ. Endogenous opiates and the control of breathing in normal subjects and patients with chronic airflow obstruction. Thorax 37: 834–839, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkovic-Capin et al. 1998.Tonkovic-Capin M, Krolo M, Stuth EAE, Hopp FA, Zuperku EJ. Improved method of canine decerebration. J Appl Physiol 85: 747–750, 1998. [DOI] [PubMed] [Google Scholar]
- Verhage et al. 1992.Verhage M, Ghijsen WE, Wiegant VM. Characterization of the release of Met-enkephalin from isolated nerve terminals: release kinetics and cation-dependence. Brain Res 598: 294–301, 1992. [DOI] [PubMed] [Google Scholar]
- Zadina et al. 1999.Zadina JE, Martin-Schild S, Gerall AA, Kastin AJ, Hackler L, Ge LJ, Zhang X. Endomorphins: novel endogenous mu-opiate receptor agonists in regions of high mu-opiate receptor density. Ann NY Acad Sci 897: 136–144, 1999. [DOI] [PubMed] [Google Scholar]