1. Introduction
Lens epithelial cells are said to “transdifferentiate” into myofibroblasts by a process that includes epithelial-to-mesenchymal transition (EMT). Myofibroblast formation often occurs in response to an injury to the lens epithelium. Alpha-smooth muscle actin (α-SMA) is commonly used as a marker of myofibroblast formation (Nagamoto et al., 2000). Cataract surgery, which is a major injury to epithelial cells, leads to myofibroblast formation and these “transdifferentiated” lens epithelial cells can form secondary cataracts (for review see Wormstone, 2002). We noted that α-SMA transcripts were detected on microarrays probed with mRNA from freshly-isolated rat lens epithelial cells (not shown). Further studies revealed that α-SMA is normally present in the lens epithelia of several species.
2. Materials and methods
Animal use conformed to the ARVO Policy for the Use of Animals in Research. Bovine eyes were kept on ice and lenses were dissected within four hours postmortem. Human eyes were also kept cold after death and lenses were dissected six to 12 h postmortem. Rabbit and mouse lenses were dissected immediately after death. Lens epithelial cell explants were made by placing the anterior surface of the lens down onto a plastic coverslip. An incision was made in the posterior lens capsule, the capsule was torn with fine forceps, flattening the anterior epithelium onto the coverslip. The lens fiber mass was removed and the posterior capsule and the edge of the anterior capsule were cleaned to remove adhering fiber cells. For western blots, explants were collected in RIPA buffer containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and sodium orthovana-date (0.2 mg/ml, Sigma-Aldrich Chemical, St. Louis, MO). Samples were freeze-thawed and then centrifuged for 15 min. Protein concentration was determined using the Bio-Rad Dc Protein assay (Bio-Rad, Hercules, CA). From most species, aliquots containing 20 μg of protein were run on the western blot; only 10 μg of protein was used for mouse lens epithelia. For 4 day cultures, lens explants were cultured in DMEM with 0.1% BSA. Explants were collected in RIPA buffer as described above and DNA was determined using the dsDNA Pico Green kit (Molecular Probes, Eugene, OR). The DNA assay was used to assure that proteins from equal numbers of cells were used for western blotting. Each lane was loaded with 0.6 pg of DNA for the mouse samples and 1.8 pg for the bovine samples. Blots were probed with a mouse monoclonal antibody to α-SMA (clone 1A4, 1:1000, Sigma-Aldrich); bands were visualized using a horse-radish peroxidase-conjugated anti-mouse secondary antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) and chemiluminescence detection (SuperSignal, Westdura, Pierce, Rockford, IL). For immunohistochemistry, lens explants were fixed in 10% formalin, stained with the antibody to α-smooth muscle actin (1:300) at 4 °C overnight and then with an Alexafluor-labeled anti-mouse secondary antibody (Molecular Probes); TOTO-1 (Molecular Probes) was used to label nuclei. Explants were viewed and photographed using a Zeiss 510 confocal microscope. Pictures were taken along the diameter of the explant at 500 μm intervals.
3. Results
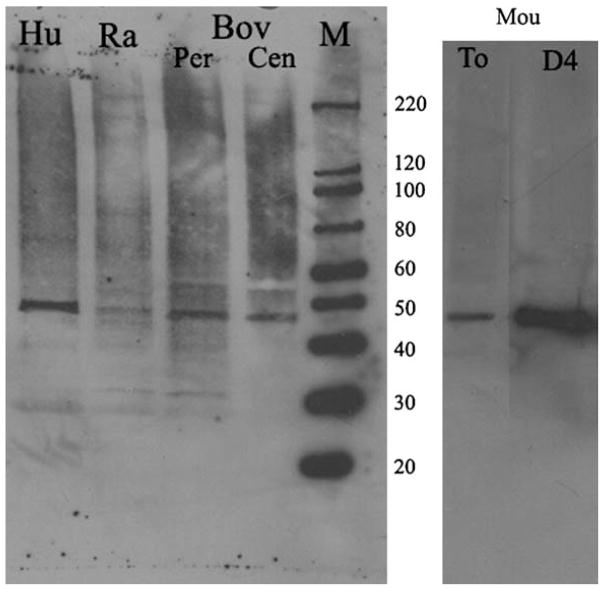
Western blots of lens epithelial cells from fresh bovine, rabbit, human and mouse lenses detected α-SMA in protein extracts of lens epithelial cells (Fig. 1). Fiber cells did not express detectable levels of α-SMA (data not shown). Mouse lens epithelial cells expressed more α-SMA than the other species tested.
Fig. 1.
α-SMA is expressed in lens epithelial cells in vivo. Western blot of α-SMA from lens epithelial cells isolated from fresh lenses of different species. An equal amount of protein (20 μg) was loaded for human, rabbit and bovine samples and 10 μg of protein was loaded for the mouse sample. M, marker; Hu, human; Ra, rabbit; Bov, bovine; Per, peripheral lens epithelia; Cen, central lens epithelia; Mou, mouse, To, fresh lens epithelial cells; D4, lens epithelial cells explants cultured for four days in basal media.
We performed immunohistochemical studies to determine the distribution of α-SMA expression in lens epithelial cell explants from all species examined. In bovine explants, pictures were taken every 500 μm along the diameter of the explant. α-SMA staining was strong and diffusely distributed in all peripheral lens epithelial cells (Fig. 2). Toward the center of the epithelium, labeling became undetectable in most cells. However, occasional single cells were strongly labeled for α-SMA (Fig. 2 D,E). In rabbit and human lens epithelia, the distribution of α-SMA was similar to bovine lens epithelia. Most cells at the periphery of the explants were labeled with the antibody to α-SMA; the number of labeled cells decreased toward the center of the epithelium. In mice, however, the distribution of α-SMA appeared to be uniform across the explant (Fig. 3). When bovine and mouse epithelial explants were cultured for four days, the levels of α-SMA increased markedly, compared to freshly dissected lens epithelial cells (Fig. 4).
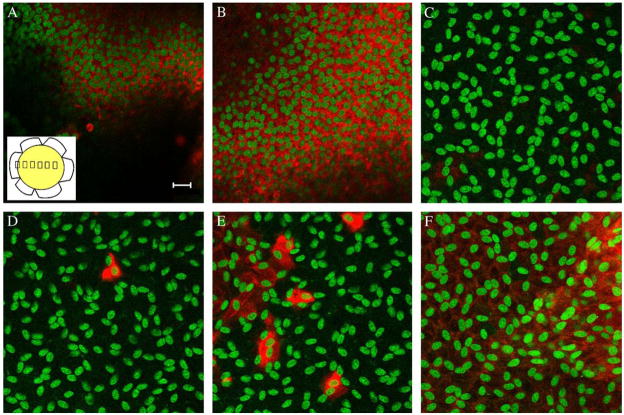
Fig. 2.
Bovine peripheral lens epithelial cells express more α-SMA. Immunohistochemistry of α-SMA in bovine explants, α-SMA is labeled red and nuclei are labeled green. Pictures were taken every 500 μm (inset in A) starting at the periphery of the explant (A). The unstained area is due to folds in the explant. There was no detectable α-SMA expression in the center of the explant (C). Individual cells or small clusters of cells expressed α-SMA near the center of the explant (D and E) and expression increased again towards the other side of the explant (F). Bar in A represents 20 μm.
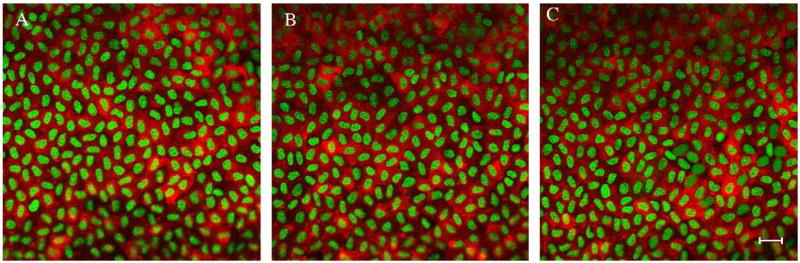
Fig. 3.
α-SMA is expressed in most epithelial cells in the mouse. Immunohistochemistry of mouse lens explants; α-SMA is labeled red and the nuclei are green. There was no difference in the expression of α-SMA in the peripheral (A and C) and central (B) lens epithelial cells in the explants. The mouse was the only species studied that had α-SMA expression in all epithelial cells. Bar in C represents 20 μm.
Fig. 4.
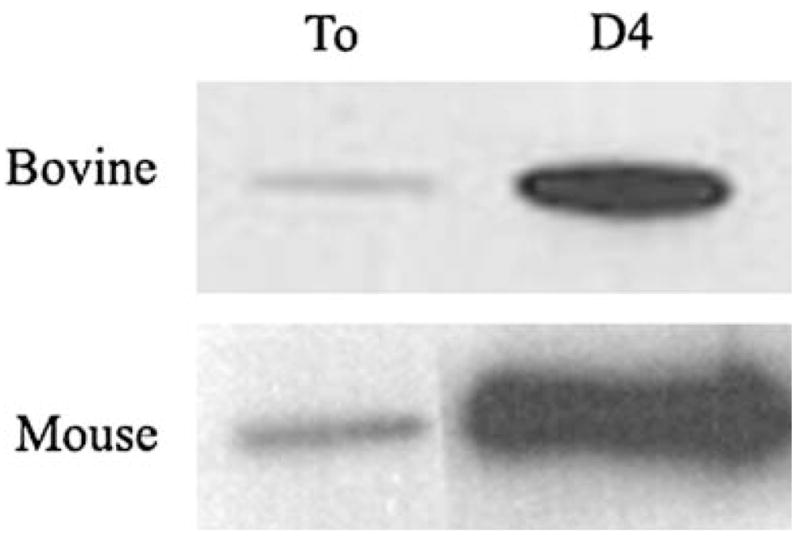
α-SMA expression increases in culture. Western blot of bovine and mouse lens explants isolated immediately postmortem (To) or cultured for four days (D4) in basal media (0.1% BSA in DMEM). There is an increase in α-SMA expression after four days in culture. As in Fig. 1, there is more α-SMA expression in the mouse lens explants. Equal number of cells, determined by DNA content, were used for the To and D4 samples. 0.6 pg of DNA was loaded for the mouse samples and 1.8 pg of DNA for the bovine samples.
4. Discussion
In the lens, α-SMA is often used as a marker for myofibroblasts. However, we show that α-SMA is present in vivo in normal lens epithelial cells of bovine, rabbit, human and mouse lenses, suggesting that α-SMA is a normal constituent of lens epithelial cells. The α-SMA antibody used in our studies is a mouse monoclonal antibody, clone 1A4, which is widely used as a marker to study smooth muscle differentiation and myofibroblast formation (Skalli et al., 1986). This antibody is commonly used to identify myofibroblast formation in the lens (Lovicu et al., 2002; Nagamoto et al., 2000; Srinivasan et al., 1998). Culturing epithelial explants in defined medium, a treatment that leads to myofibroblast formation, caused a large increase in α-SMA accumulation. Therefore, it seems more appropriate to consider α-SMA as a quantitative, rather than a qualitative indicator of myofibroblast formation.
It was notable that scattered cells or small groups of cells near the center of the bovine, rabbit and human lens epithelia were stained for α-SMA. Similar cells were previously reported by Rungger-Brandle and colleagues in human lens epithelia, where their presence was not obviously associated with age or disease (Rungger-Brandle et al., 2005). In ours and this previous study, these cells were present in freshly-isolated epithelia. Therefore, they must be normal residents of the lens epithelium. This observation suggests that myofibroblast-like cells may always be present in some lens epithelia. Rather than evidence of the expression of a new state of differentiation or “transdifferentiation”, the formation of myofibroblasts may be more accurately described as a modulation of the normal state of the lens epithelial cell.
Mouse lens epithelial cells were remarkable for the high, uniform distribution of α-SMA throughout the epithelium. We cannot offer an explanation for the abundant basal expression of α-SMA in cells that are not obviously fibrotic. In other systems, such as the newly-regenerated lens of the newt, α-SMA is also uniformly expressed in cells that do not appear to be fibrotic (Sander and Tsonis, personal communication). These observations offer further evidence that α-SMA is a normal constituent of lens epithelial cells.
Another protein that is considered to be an invariant “marker” of mesodermal cells, the intermediate filament vimentin, is also constitutively expressed by lens cells (Ramaekers et al., 1980). The reason that these well-characterized “mesodermal markers” are expressed in the lens may have to await a more complete understanding of the pathways that regulate their expression in mesodermal and lens cells.
References
- Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, McAvoy JW. TGF{beta} induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002;86:220–226. doi: 10.1136/bjo.86.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto T, Eguchi G, Beebe D. Alpha-smooth muscle actin expression in cultures lens epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1122–1129. [PubMed] [Google Scholar]
- Ramaekers FC, Osborn M, Schimid E, Weber K, Bloemendal H, Franke WW. Identification of the cytoskeletal proteins in lens-forming cells, a special epitheloid cell type. Exp Cell Res. 1980;127:309–327. doi: 10.1016/0014-4827(80)90437-1. [DOI] [PubMed] [Google Scholar]
- Rungger-Brandle E, Conti A, Leuenberger PM, Rungger D. Expression of [alpha]smooth muscle actin in lens epithelia from human donors and cataract patients. Exp Eye Res. 2005;81:539–550. doi: 10.1016/j.exer.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta 1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998;101:625–634. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002;74:337–347. doi: 10.1006/exer.2001.1153. [DOI] [PubMed] [Google Scholar]