Abstract
Using a gaze-following task, the authors assessed whether self-experience with the view-obstructing properties of blindfolds influenced infants’ understanding of this effect in others. In Experiment 1, 12-month-olds provided with blindfold self-experience behaved as though they understood that a person wearing a blindfold cannot see. When a blindfolded adult turned to face an object, these infants gaze followed significantly less than control infants who had either (a) seen and felt the blindfold but whose view had not been obstructed by it or (b) experienced a windowed blindfold through which they could see. In Experiment 2, 18-month-olds experienced either (a) a trick blindfold that looked opaque but could be seen through, (b) an opaque blindfold, or (c) baseline familiarization. Infants receiving trick-blindfold experience now followed a blindfolded adult’s gaze significantly more than controls. The authors propose 3 mechanisms underlying infants’ capacity to use self-experience as a framework for understanding the visual perception of others.
Keywords: social cognition, gaze following, training experience, theory of mind, intention
Gaze following is a key aspect of social interaction. Adults turn their head and eyes to see what another person is seeing. For adults, another person’s turning to look at an object is not merely a physical movement, but an act that binds together the person and object via visual perception. Visual perception is a kind of psychological contact at a distance.
Searle (1983) draws a distinction between spatiotemporal movements (described in physical terms) and acts driven by intention (described in psychological terms). Children with autism have gaze-following deficits and may process adults’ gaze behavior as movements rather than as acts (Baron-Cohen, 1995; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Hobson, 1993; Mundy, Sigman, & Kasari, 1990; Toth, Munson, Meltzoff, & Dawson, 2006). How do typically developing children come to understand adults’ looking behavior as psychologically relevant?
Gaze following in typically developing children has been documented before their first birthday (e.g., Brooks & Meltzoff, 2005; Butterworth & Cochran, 1980; Carpenter, Nagell, & Tomasello, 1998; D’Entremont, Hains, & Muir, 1997; Mundy et al., 2007). There is a debate about the mechanisms underlying this behavior and whether it shows that infants have a primitive grasp of seeing and visual contact in other perceivers (Flavell, 1999; Flom, Lee, & Muir, 2007; Moore & Dunham, 1995).
One view is that young infants initially treat others’ looking behavior as mere movements. In the most conservative version, young infants watch or visually track the adult’s head movement in space and in so doing are pulled into the correct hemi-field where they catch sight of the salient target object by happenstance (Butterworth & Jarrett, 1991). Over time, infants then learn that the adult’s head turn is a reliable cue indicating where an object can be seen (Moore, 1999). On the opposite side of the debate, others have offered a rich, nativist view suggesting that infants have a built-in (or early maturing) module that takes eye gaze as input and automatically makes attributions about seeing and visual experience in others (Baron-Cohen, 1995; Leslie, 1994). A third, developmental view is that understanding others’ vision and trying to see what they see emerges from more skeletal beginnings (Meltzoff & Brooks, 2007, 2009). One potential mechanism of change is infants’ experience with their own vision: Infants may develop an understanding of the vision of others, in part through their own acts of turning in order to see, opening and shutting their eyes to cut off and reinstate visual experience, and peering around occluders to see objects (Meltzoff, 2007a; Meltzoff & Brooks, 2007). Other theories and/or hybrid models have been articulated (Csibra & Gergely, 2006; Johnson, 2003; Moore, 2006; Tomasello, Carpenter, Call, Behne, & Moll, 2005; Woodward, 2003, 2005).
To address these theoretical concerns, researchers have investigated the conditions under which infants do and do not follow gaze and the cues they use (Corkum & Moore, 1995; Frischen, Bayliss, & Tipper, 2007; Johnson, Slaughter, & Carey, 1998; Rakison & Poulin-Dubois, 2001; Tomasello, Hare, Lehmann, & Call, 2007). Experimental results demonstrate that at least by 10 months of age, if not earlier, infants engage in genuine gaze following and are not simply processing the salient head movements of others. For example, 10- to 12-month-olds follow an adult if she turns to a target with open eyes, but not if she performs the identical head movement with closed eyes (Brooks & Meltzoff, 2002, 2005). Thus, beyond head movement alone (which is controlled in this study), 10- to 12-month-olds are sensitive to eye status and line of visual regard.
Eye closure is not the only way to sever visual contact between a perceiver and an object. Opaque barriers and blindfolds also block line of regard, and research has investigated how infants interpret such events (e.g., Brooks & Meltzoff, 2002; Butler, Caron, Brooks, 2000; D’Entremont & Morgan, 2006; Dunphy-Lelii & Wellman, 2004). Research directly comparing infants’ understanding of eye closure versus blindfolds shows that infants understand that eye-closure blocks an adult’s visual access to external objects in advance of their understanding that blindfolds do so (Brooks & Meltzoff, 2002). The adult’s line of sight is blocked by an opaque barrier in both cases; why is there a difference? Eye closure is a biological motion with which infants have extensive first-person experience: Infants can control their own vision by closing their eyes when they do not want to look at something. The experience of turning off and on visual access to the world by eye closing–opening might serve as a framework for understanding such behavior in others. This raises the interesting possibility that if infants are given systematic, novel experience that blindfolds block their own view, they might make different attributions to others. One goal of the current work was to design an experimental manipulation that provided this type of blindfold experience to infants and to test whether it affected their interpretation of the experience of others who donned the same blindfold.
There are at least two bodies of work—imitation and habituation studies—suggesting that infants use their own behavior and experiences as a basis for interpreting the similar behavior of others. The very act of imitating demonstrates that infants can process the equivalences between behavior they see others perform and their own behavior (Meltzoff, 2007b). Strikingly, children’s prior first-person experience with a goal-directed act significantly affects their imitation of others performing that act (Williamson, Meltzoff, & Markman, 2008). Infants also can detect when their own acts are being imitated, showing that they can recognize an external instantiation of their own motor productions generated by another person (Agnetta & Rochat, 2004; Meltzoff, 2007a; Nadel, 2002). Meltzoff and colleagues argued that the cross-modal self-other equivalence manifest in imitation plays a key role in social development (Meltzoff, 2007a, 2007b; Meltzoff & Gopnik, 1993). Others are seen as “like me,” and infants’ own first-person experience influences their interpretation of like behaviors in others.1
Using a visual habituation paradigm, work on reaching and means-ends understanding leads to a similar inference (Sommerville & Woodward, 2005a, 2005b; Woodward & Guajardo, 2002). For example, Sommerville, Woodward, and Needham (2005) gave 3-month-olds experience with Velcro mittens. When infants touched the objects, the mittens increased the success of the reaches because the objects stuck to them. Infants were then habituated to an adult’s mitten-covered hand reaching to one of two objects. For the test, the left–right position of the objects was switched, and looking time was measured to the adult reaching along the same path to grasp a new object or along a different path to grasp the same object. Infants without the mitten training did not respond systematically, but those with training looked longer at the adult grasping the new object. The authors argued that this was because infants had learned about goal directedness or had learned to encode the relationship between the hand and object from their own self-generated experience during training.
However, reaching and touching are concrete acts that link hands and objects. Infants may simply have observed and generalized from those concrete acts. Biro and Leslie (2007) noted that infants see their hand touching the object during training and argued that watching this event may be enough for infants to encode the link between hand and object even if the event had not been self-produced, and indeed even if a nonhuman event was involved. To test this, Biro and Leslie (2007) showed infants a Velcro-tipped rod that contacted and lifted toys. This visual experience led to the same basic effects reported by Sommerville et al. (2005). Biro and Leslie concluded that self-generated activity has no privileged status and was not essential for the reported effects (for similar reasoning, see Király, Jovanovic, Prinz, Aschersleben, & Gergely, 2003; see also Hauf, Aschersleben, & Prinz, 2007).
The current training study moved beyond hand–object relations and instrumental actions and tested infants’ understanding of mental states such as seeing. Moreover, although the sight of the infant’s hand and someone else’s are similar, infants cannot observe themselves wearing the blindfold. They see the other person, but their own face is invisible to them.
We provided infants with first-person experience related to line of regard and vision. We assessed whether experience with visual occluders influenced infants’ interpretation of the looking behavior of others. Infants were randomly assigned to systematic experience with how a blindfold blocked their own view of external objects. They were then tested to see whether this changed their interpretation of the experience of another person wearing a blindfold. If so, it cannot be based on the fact that the self and other look the same while wearing the blindfold. Rather the generalization would be based on the infant’s own experience of the view-obstructing consequences of the blindfold. This generalization would show that first-person experience changes infants’ psychological interpretation of others.
Experiment 1
The hypothesis tested in this study is that self-experience with the properties of blindfolds will influence how infants interpret blindfolds worn by others. In the treatment group, we administered training experience by playing a game in which a blindfold was systematically interposed between the infants and the objects. A control group was merely familiarized with the blindfold laying flat on the table. A second control group had a blindfold interposed between themselves and the objects, but the blindfold had a window cut out so infants could see through it. Infants in all three groups did not witness the adult wearing the blindfold until the gaze-following test. The experimental question was whether infants’ performance on the gaze-following test systematically varied as a function of the randomly assigned training experience.
Method
Participants
The participants were 96 typically developing 12-month-old infants (M = 12.02 months, SD = 0.13 months; range: 357–372 days). Half of the participants were female. Infants were recruited by telephone from the university’s computerized participation pool. Preestablished criteria for admission into the study were that the infants have normal birth weight (2.5–4.5 kg [5.5–9.9 lb]); normal length of gestation (40 ± 3 weeks); and had no known sensory, motor, or mental handicap. According to parental report, the racial–ethnic makeup of the final sample was 80.2% White, 3.1% Asian, 2.1% African American, 1.1% Hispanic, 7.3% other (e.g., more than one race), and 6.2% not disclosed. The sample was primarily middle to upper middle class. Additional infants began testing but were excluded from the final sample due to extreme infant fussiness–tiredness (15), experimental–equipment problems (11), inability to center the infant for trial onset (8), and parent interference (3).
Test Environment and Apparatus
The study took place in a room (3.3 × 3.5 m) with plain blue walls to provide a consistent background for the visual test. Infants sat at a small rectangular table (0.75 × 1.2 m) on their parent’s lap facing the experimenter. Two synchronized video cameras recorded the session, each on a separate recorder. The main camera provided a close-up of the infant’s face and upper body; the other focused on the experimenter (to keep track of the experimental conditions). A character generator added synchronized time codes (30 per second) onto the two recordings.
Test Materials
The blindfolds were made of black cloth. One pair of cloths (a broad and a narrow one) was opaque; the other pair (also broad and narrow) had a rectangular window cut out so that the infants could peer through it—dubbed windowed cloth. The use of two sizes of cloth in each category helped provide variety, keep infants’ interest, and promote generalization. The dimensions of the broad opaque cloth were 29 × 29 cm. The dimensions of the narrow opaque cloth, which also served as the experimenter’s blindfold, were 51 × 6 cm. For the windowed versions, the cloths had the same external dimensions. The window dimensions for the broad and narrow cloths were, respectively, 21 × 21 cm and 32 × 4 cm.
Two colorful objects served as targets in the gaze-following test. They were identical, soundless, plastic objects (9 cm high × 16 cm diameter). During the test they rested on plain pedestals on either side of the infant, 75° off the infant’s midline, at eye level, 135 cm away from the infant. The placement of the targets required that the experimenter make a 70° head turn from her midline in order to face a target (nose lined up with the object).
Design and Procedure
The infants were randomly assigned to one of three groups, with 32 infants per group: experience (opaque cloth), experience (windowed cloth), and baseline familiarization. Half the infants in each group were girls. The procedure involved two phases. In Phase 1 the experimentally designed experience was provided, which differed by group. In Phase 2 the test of gaze following was conducted, which was identical across groups.
Phase 1: Experience
Infants were given an opportunity to explore and manipulate the cloths. To introduce the cloths to the infant, the experimenter initially placed each cloth (broad and then narrow) flat on the table with a toy resting on top of it. Phase 1 was an infant-controlled procedure allowing each infant to play with the cloths for at least 1.5 min and terminated when infants declined to play with them further.
For the baseline familiarization group, infants were not given specific experience that the cloths could serve as a barrier to vision. The goal was simply to acclimate infants to the cloths as they lay flat on the table. Infants played with the broad opaque cloth and then the narrow one. The experimenter encouraged infants to touch each cloth. The cloth was never interposed between the infant’s eyes and an object, but rather objects were placed on the cloth one at a time and were slid across the table. The familiarization period was terminated when the child tired of the game, as expressed by throwing the cloth on the floor, turning around to face the parent, arching his–her back, or other common rejection maneuvers of infants. The mean duration of cloth familiarization was 2.92 min (SD = 0.87).
Infants in the two experience groups (opaque and windowed cloth) played the familiarization games already described, but they also were given the experience of the cloth being interposed between their eyes and an object. The opaque cloth blocked their view of the object and the windowed cloth did not—infants could peer through the window and see the object. In both of the experience groups, the experimenter positioned the cloth near but not touching the infant’s face (about 7.5 cm from the infant’s eyes), minimizing infant avoidance or blinking. As the experimenter raised the cloth, she said, in a gamelike way, “Where’s the [bunny]?” After about 1 s, the experimenter lowered the cloth. After several such cloth-interposition games with the broad cloth, the experimenter repeated the routine with the narrow cloth.
Importantly, we used multiple object exemplars in multiple spatial locations to provide generalized experience that the interposed opaque cloth blocked vision and that the windowed cloth did not. Colorful rubber or plastic toys ranging from 4.5 cm to 16.5 cm high were placed one at a time on different spots on the tabletop at the farthest extent of the infant’s reach. When the infant fixated an object, the cloth was interposed. Infants rapidly learned the routine and seemed to enjoy it in both the windowed and opaque cloth. The mean length of opaque-cloth experience (M = 7.34 min, SD = 1.85) was virtually identical to windowed-cloth experience (M = 7.28 min, SD = 1.41), but of course the content of the experience was different: One group experienced that the interposed cloth was a barrier to vision and the other that it was not.
Phase 2: Gaze-following test
The infants from all groups were treated identically. All saw the adult turn to face one of two objects while wearing the opaque narrow blindfold. As in previous studies (e.g., Brooks & Meltzoff, 2002), infants were familiarized with the experimenter touching and wearing the narrow opaque cloth by seeing her wrap it around her wrists before putting it on top of her head (above the hair line). The experimenter then placed the targets on the peripheral pedestals (counterbalanced left vs. right side for first target placement) and resumed playing with the infant by using a toy at the table.
At the start of a trial, the experimenter removed the toy from the tabletop and made eye contact with the infant to ensure that each infant began each trial looking at midline. The experimenter then pulled the cloth down from the top of her head so that it covered her eyes and silently turned her head to align with one of the two targets. A 6.5-s trial was electronically timed from the onset of the adult head movement (termination signaled to the experimenter via a silent, tactile pad). This trial length was selected because a majority of previous gaze-following studies used trials in the 5- to 7-s range (Brooks & Meltzoff, 2002; Butler et al., 2000; Moore & Corkum, 1998; Scaife & Bruner, 1975) and because pilot work suggested infants became bored or fussy during longer trials. After the trial, the experimenter returned the cloth to the top of her head and resumed the play interaction. Four trials were conducted with approximately a 2-min intertrial interval. The direction in which the experimenter turned followed either a pattern of ABAB or ABBA, with the direction of the first turn counterbalanced and each pattern counterbalanced within experimental group and gender. Four trials were always presented, but infrequently one of the four trials was excluded from analysis as a mistrial (1.3% of the trials), because of infant movement (e.g., looking down) synchronous with the trial onset. This affected only 5 of 384 total possible trials (96 infants × 4 trials = 384), and no infant had two mistrials.
Scoring
The direction of the adult head turn was not visible on the video record scored by the coder, and the coder was kept uninformed about infants’ group assignment and hypotheses of the study. The coder scored the 96 infants in a random order. The coder was informed about the two target locations, although the targets were not visible on the display. The coder identified the time when an infant first looked at a target for each trial.
The operational definition of “look at target” was that an infant lined up his or her eyes with the target for at least 0.33 s (10 video frames). For each trial, an infant’s first target look was scored as either a correct look if it was to the target the experimenter was looking at (+1) or an incorrect look if it was to the opposite target (−1). If infants did not look at either target during the 6.5-s trial (e.g., looked down), they received a score of 0 indicating nonlooking. As standard in the gaze-following literature, the looking score for each infant was the sum of correct looks, incorrect looks, and nonlooks (e.g., Butler et al., 2000; Corkum & Moore, 1995; Dunphy-Lelii & Wellman, 2004). Thus, the possible range for the looking scores across the four trials was from −4 to + 4. (For the rare cases with a mistrial, the score was based on the remaining trials. Results remained identical if scores were converted to proportions or if a mean value was substituted for mistrials.)
Scoring agreement was assessed by having a randomly selected 25% of the trials rescored by an independent observer, who was also naive to all test parameters. Inter- and intraobserver agreement were assessed by Cohen’s (1960) kappa. Both were excellent, exceeding .90.
Results and Discussion
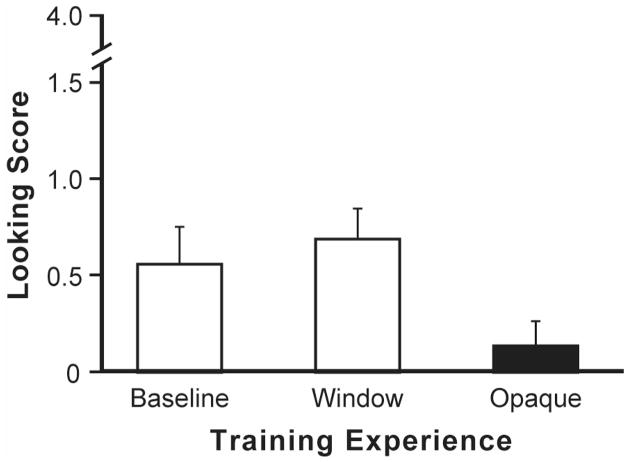
The chief question was whether infants would follow the head turns of the blindfolded adult differentially as a function of their prior experience with the blindfold. The looking scores for the three groups are shown in Figure 1. We used a one-way analysis of variance to assess looking scores as a function of group (opaque cloth, windowed cloth, and baseline). The results showed a significant group effect, F(2, 93) = 3.24, p <.05, . As predicted, planned contrasts showed that infants gaze followed the blindfolded adult significantly less often after experience with the opaque cloth (M = 0.12, SD = 0.75) than after experience with the windowed cloth (M = 0.69, SD = 0.93; p < .01) or baseline familiarization (M = 0.56, SD = 1.07; p < .05).2
Figure 1.
Training with opacity. Infants were randomly assigned three different types of training experiences prior to a gaze-following test (n = 32 per group). Infants who experienced an opaque blindfold subsequently gaze followed the blindfolded adult significantly less than controls. Error bars represent standard error of the means.
This study replicates previous work showing that untrained 12-month-olds follow the gaze of a blindfolded adult (Brooks & Meltzoff, 2002) and mistakenly act as though adults can see objects on the opposite side of a freestanding opaque barrier (Caron, Kiel, Dayton, & Butler, 2002). The new finding concerns the effects of systematically training infants about how blindfolds affect their own perception. In this study, an experimental group was given repeated first-person experience that a blindfold interposed between them and an external object blocked their view. Infants trained on the properties of blindfolds were significantly less likely to follow the gaze of the blindfolded adult than were controls without this experience. The windowed-cloth group is a good control, because it followed exactly the same treatment protocol as the experimental group, save that the blindfold had a window; when it was interposed, infants could see the distal objects. Infants in the experimental and control groups were the same age and were randomly assigned. The difference in their performance is attributable to the training experience.
Experiment 2
Brooks and Meltzoff (2002) reported that 18-month-old infants, unlike 12-month-olds, no longer turn to follow the gaze of blindfolded adults. This finding is compatible with reports by Butler et al. (2000) that 18-month-olds no longer follow the gaze of an adult through freestanding, opaque barriers. Presumably naturally occurring experience with obstructions plays a role in children’s development. The purpose of Experiment 2 was to provide 18-month-olds with a novel training experience. We constructed a trick blindfold that looked opaque from the outside but actually could be seen through when it was interposed between looker and object (cf. Novey, 1975). Infants were randomly assigned to three groups: see-through trick blindfold experience, opaque blindfold experience, and a baseline control. We predicted that experience with the trick blindfold would lead infants to gaze follow when they saw the adult wearing a blindfold.
Method
Participants
The participants were 72 typically developing 18-month-old infants (M = 18.04 months, SD = 0.14 months; range: 540–556 days old). Half of the participants were female. Infants were recruited in the same manner and with the same preestablished inclusion criteria as in Experiment 1. According to parental report, the racial–ethnic makeup of the final sample was 81.9% White, 5.6% Hispanic, 6.9% other (e.g., more than one race), and 5.6% not disclosed. The sample was primarily middle to upper middle class. Additional infants began testing but were excluded from the study due to extreme infant fussiness–tiredness (15), experimental–equipment problems (9), inability to center the infant for trial onset (1), and parent interference (6).
Test Environment and Apparatus
Infants were tested while seated at a small table (0.75 × 1.2 m) with a black top. The test room, cameras, and timing devices were the same as described in Experiment 1.
Materials
As in Experiment 1, there were broad and narrow cloths. One broad–narrow pair was identical to Experiment 1 and was made of the same black opaque material. The other pair (the trick cloths) looked opaque but was not. These trick cloths had the same outside dimensions as the opaque ones; however, a special material was sewn inside the black opaque border. This see-through portion comprised one layer of special black material that was the same color and texture as the opaque cloth; when the cloth lay flat on the black table top, it looked virtually indistinguishable from the real opaque cloth. However, when it was held vertically, one could see through the fine mesh. The dimensions of the see-through area were 21 × 21 cm for the broad cloth and 32 × 4 cm for the narrow one.
Design and Procedure
Infants were randomly assigned to one of three groups, with 24 per group: experience (opaque cloth), experience (trick cloth), and baseline. Each had equal numbers of boys and girls.
Phase 1: Experience
The same general procedure was followed as described in Experiment 1. This consisted of an infant-controlled procedure allowing each infant to play with the cloths for at least 5 min and terminated when infants declined to play with them further. The experience phase was lengthened in comparison to Experiment 1 (previously 1.5 min), because the older infants could tolerate a longer interval, and the information being acquired in the crucial Experience (trick cloth) condition was contradictory to learning prior to the lab visit—that the seemingly opaque blindfold did not block one’s view.
The baseline group was familiarized with the trick cloths as they lay flat on the table. As in Experiment 1, the experimenter put objects on top of them and slid the objects across the tabletop. When a black cloth lay flat on the black tabletop, it looked opaque and therefore this group had no exposure to its trick properties. The mean duration of the baseline familiarization group was 7.53 min (SD = 0.83 min).
The two experience groups (opaque and trick cloth) were given experience with the cloths interposed between themselves and various colorful toys. Multiple object exemplars in multiple spatial locations on the tabletop were used to prompt the understanding that the trick cloth did not block vision (and that the ordinary one did). As in Experiment 1, small objects were placed, one at a time, on different spots on the tabletop. When the infant fixated an object, the cloth was interposed. The infants often experimented with the barriers. After a couple of minutes of experience in which the adult interposed the opaque cloth between infant and toy, many infants lifted the opaque barrier in front of their own eyes, blocking their own view, before lowering it and reaching for the toy. Infants in the trick-cloth group would lean forward carefully peering through the see-through portion; the closer they put their eyes to the cloth, the more transparent it became. Infants played with this relationship, leaning backward and forward, testing the properties of the trick cloth. The mean duration of opaque-cloth experience (M = 8.01 min, SD = 1.28) and trick-cloth experience (M = 8.10 min, SD = 1.42) was virtually identical.
Phase 2
This phase followed the same protocol as in Experiment 1 and was identical across groups. In brief, the experimenter wore the narrow opaque cloth over her eyes as a blindfold and turned to one of two targets. Four blindfold test trials were always presented, but one trial was infrequently excluded from the analysis as a mistrial (2.4% of the trials), because of infant movement (e.g., looking down) at trial onset. No infant had more than one mistrial, and the results remained the same if scores were converted to proportions or if mean-value substitution was used for mistrials.
Scoring
The video records used for scoring contained images of the infants but no record of the direction of the adult’s head turn. Infant looks were scored in the same manner as in Experiment 1 by a coder who remained naive to the direction of the adult’s head turn, the treatment group of the infant, and the hypothesis under study. For a randomly selected 25% of the infants, inter- and intraobserver agreement was assessed using Cohen’s kappa. Agreement was excellent, exceeding .90.
Results and Discussion
The principal question was whether infants differed in how they interpreted the actions of the blindfolded adult as a function of their own prior experience with the blindfold. We used a one-way analysis of variance to assess the infant looking scores (see Figure 2). The results showed a significant group effect, F(2, 69) = 4.21, p < .05, . As predicted, planned contrasts showed that infants followed the blindfolded adult significantly more often after experience that the cloth could be seen through (M = 1.04, SD = 1.37) than after experience that it was opaque (M = 0.12, SD = 1.15; p < .005) or baseline familiarization (M = 0.33, SD = 0.87; p < .05).
Figure 2.
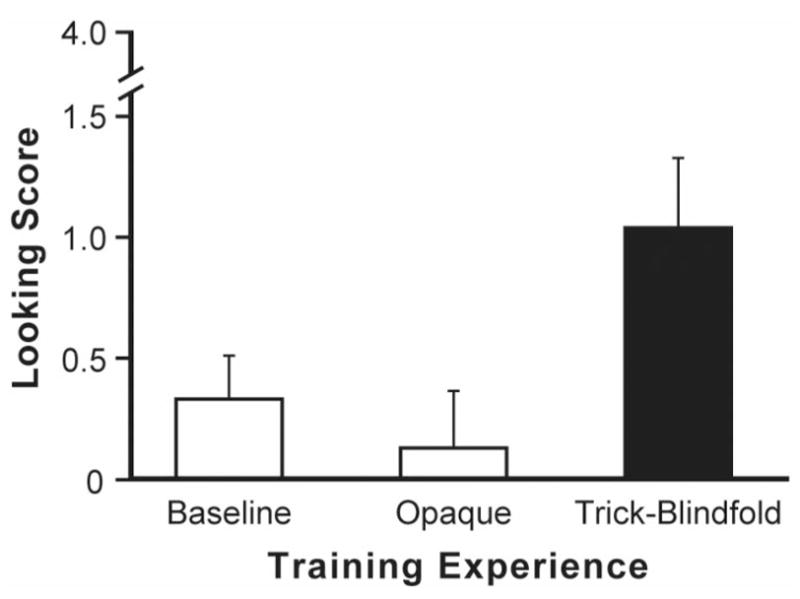
Training with transparency using trick blindfolds. The specially constructed, trick blindfold looked opaque from the outside but could be seen through. Infants were randomly assigned three different types of training experiences prior to a gaze-following test (n = 24 per group). Infants who experienced a see-through, trick blindfold subsequently gaze followed the blindfolded adult significantly more than controls. Error bars represent standard error of the means.
It is noteworthy that previous training studies served to accelerate development that would have occurred naturally—infants were simply moved forward along an expected pathway. The current experiment is different. With the help of a specially constructed trick blindfold, infants were given the novel experience that an opaque-looking barrier did not have opaque properties. Although it looked opaque from the outside, infants could see through it. When infants learned this from first-person experience, they readily used it as a basis for changing their interpretation of the behavior of others who wore the blindfold.
General Discussion
The current experiments show that infants’ first-person experience influences their understanding of others. We find that systematic training on how occluders influence their own visual perception changes infants’ interpretation of the visual behavior of others.
Experiment 1 builds on work showing that 12-month-olds understand that eye-closure blocks vision prior to understanding that blindfolds do so (e.g., Brooks & Meltzoff, 2002). One reason might be that infants open–close their own eyes and have the experience of seeing and not seeing. This predicts that one could accelerate infants’ interpretation of others who are blindfolded by providing infants with self-experience of blindfolds as barriers to their own line of sight. In Experiment 1, 12-month-old infants were randomly assigned to one of three groups, only one of which provided them experience with the perceptual effects of opaque blindfolds. Infants in the baseline control were familiarized with the opaque cloth; infants in the windowed-cloth control had the cloth interposed between their eyes and objects but could see through it. Infants in the opaque-cloth treatment group had the cloth interposed between their eyes and objects, and it blocked their view. All infants then saw a blindfolded adult turn to objects. There was significantly more gaze following in the controls than in the treatment group that had specific experience with the blindfold blocking their own view. Infants learned something during training that was generalized to others wearing the same blindfold.
In the natural course of development, infants change their understanding of visual perception. By 18 months of age, infants do not act as though adults can see through opaque barriers—whether eyelids, blindfolds, screens, or walls (Brooks & Meltzoff, 2002; Butler et al., 2000 Dunphy-Lelii & Wellman, 2004). In Experiment 2, we provided 18-month-old infants with a completely novel experience—one they would not have encountered outside of the laboratory. We constructed a trick blindfold that looked opaque from the outside but could actually be seen through. Infants this age do not follow the gaze of a blindfolded adult (Brooks & Meltzoff, 2002). Infants, through random assignment, received systematic training that they themselves could see through a trick blindfold. Results showed that infants given such experience followed the gaze of the adult who wore the blindfold. This result underscores the power and influence of infant self-experience. Infants were given a particular novel experience under experimental control, and as soon as they gained such experience, it changed their construal of the behavior of others. They treated the other as if she could also see through the blindfold, despite the fact that the adult’s eyes were covered, and it looked, from the outside, like she could not see.
The current studies strengthen the inferences that can be drawn from previous work reporting that infants’ first-person experience is connected to their understanding of others. For example, studies show that infants who produce a motor behavior (goal-directed reaching, pointing, crawling, walking) prefer, parse, or understand the same behavior when it is exhibited by others differently than nonproducers (e.g., Sommerville & Woodward, 2005b). However, one cannot infer a causal relation between first-person experience and understanding of others based on an association between the two in development: A third factor (e.g., information processing, inhibitory control, or general intelligence) could always underlie the association.
The way to resolve this is to conduct a training study with random assignment. Sommerville et al. (2005) conducted such a study with Velcro mittens and infant reaching. That training study and the current one have similarities, but there are three differences that are important for theory. First, the reaching study involved training of a manual act with concrete, physical contact between hand and object; in contrast, the current study involved a mental state (vision) with a spatial gap between self and object. Second, in the reaching study, the visual experience in the training phase (infant hand contacts or grasps target) is highly similar to what the infant sees in the test phase (adult hand grasps target); in contrast, in the current study the training experience bears no visual similarity to the test trials—a case of far transfer. Third, the effects reported here are run in two directions: The training experience of 12-month-olds led them to appreciate that blindfolds blocked the adult’s view, and the training experience of the 18-month-olds led them to appreciate that the trick blindfold did not block vision. The fact that we demonstrated training effects in both directions is an important lever for theory construction.
What Is Infant Training the Training of?
The fundamental question for theory is the basis of the training effect. How does first-person experience change infants’ interpretation of others’ behavior? The data support three inferences: (a) mentalism—self-experience changes infants’ understanding of the inner experience of the other; (b) affordances— experience changes infants’ interpretation of the psychological affordance of the object (the blindfold); and (c) understanding visual perception—infants are learning about the spatial requirements of seeing. These three points draw attention to, or highlight, different aspects of infant learning during training, and each provides a slightly different perspective. They will be illustrated below using the case of the opaque blindfold training, but they also apply to the trick-blindfold experience.
Infant Mentalism
According to the first perspective, training teaches infants that the blindfold shuts off their own vision—they learn that their own visual experience is affected. When they are confronted with the other person wearing the blindfold, they infer that the adult’s vision is likewise cut off. Infants project their own inner experience to others. If so, it illuminates the rudiments of “mentalizing” and understanding other minds. It shows that 12- to 18-month-olds attribute mental states (perceptual experience) to others.
Even if one accepts that the infants are using a mentalistic interpretation of the other person, it is useful to inquire further. The mentalism demonstrated here can be described as on–off, seeing versus not seeing—a kind of perception–ignorance distinction. The current results do not show qualitative perspective taking about how something appears to the other—only that it can be seen (or not) in the first place. It would not be surprising if infants understood the basic on–off experience of vision before more complex mental states such as false belief (see Flavell’s, 1977, 1992, Level 1 vs. Level 2 perspective taking).
There are other reports that children under approximately 2 years old attribute visual experiences to others (e.g., Lee, Eskritt, Symons, & Muir, 1998; Lempers, Flavell, & Flavell, 1977; Moll & Tomasello, 2004, 2006; O’Neill, 1996; Repacholi, Meltzoff, & Olsen, 2008; Tomasello & Haberl, 2003). The specific advances of the current work are that it uses young infants (12- to 18-month-olds) and a controlled intervention paradigm with random assignment to show that infants use first-person experiences as leverage for understanding the visual experiences of others (congruent with the “like me” hypothesis, Meltzoff, 2007a, 2007b).3
Psychological Affordances
The second view is more deflationary and focuses on the properties of the blindfold rather than on the inner experiences of the adult. On this view, the training period provides infants with information about affordances of the blindfold. The affordance of the opaque blindfold might be expressed as “unable to be seen through” or “opaque barrier.”
Although the affordance terminology may seem simple, it is worth unpacking. These proposed affordances (if they can be called that) differ from more common physical examples such as “squeakable” (a rubber toy) or “able to be traversed” (a rigid vs. flexible surface). Squeakable is an object function describing the consequences of a manual act. Traversable is a more complex affordance—what is perceived to be traversable depends on whether one is a crawler or a walker (Adolph, Eppler, & Gibson, 1993). The affordance involved in the current study concerns how the object affects one’s line of regard, which is inextricably bound with seeing and vision. At a minimum, then, and even under the most deflationary framing, the infants are learning a psychological affordance, not a simple physical or motor affordance—and moreover are learning how it affects the self and applying it to others.
Understanding Visual Perception
The third perspective is that infants are learning abstract relations from the training. We believe this perspective has special merit. The view is that infants are learning about the spatial–causal relations among three entities: viewer, barrier, and object. These three form a seeing triangle, with the spatial relations determining whether the object can be seen by the viewer. Infants might learn that if the blindfold is interposed between viewer and object, the viewer cannot see the object. This abstract description applies equally well to self and other. If infants can recognize that the spatial relation is similar—blindfold over eyes—they could generalize that the causal effect is similar. The emphasis here is not on the nature of the inner mental experience of others, or the psychological affordances of the blindfold, but on the spatial–causal demands of vision and visual access to distal objects.
Future research will help researchers elaborate these perspectives. It will be particularly useful to explore the scope of the generalization both with respect to the barrier and the perceiver. Do infants generalize only to the particular blindfold, or all blindfolds, or all opaque barriers? Does first-person experience influence infants’ interpretation of entities other than people: puppets, robots, or self-propelled entities lacking perceptual features of humanness?
First-Person Experience and Learning via Observation
We now come to a crucial issue for all theories of mind. Is self-experience the sole or privileged pathway to understanding other minds? Or can infants learn about other minds via observation of others’ behavior? The current work shows that self-experience can play a pivotal role in infants’ construal of the acts of others in psychological terms. Infants treated the blindfolded adult as seeing or not seeing depending on their own self-experience with the blindfold. It remains possible, however, that first-person experience could be bypassed and that observing the actions of others could inform infants about adult vision (and other mental states). A critical test would be to show infants a person with a cloth over her eyes and have the person systematically behave in one of two distinctive ways: (a) the adult fumbles for objects or (b) she performs goal-directed motor skills. The blindfolded adult then turns to face an external object. We think it is possible that infants could use the adult’s behavioral envelope to determine whether or not to gaze follow this blindfolded adult. This would entail abstracting information about whether the adult was (or was not) in visual contact with the world based on the cues, contingencies, and structural patterns in the person’s behavior while wearing the blindfold—in other words, based on the others’ behavior, not first-person experience. We are currently working on studies along these lines.
We favor the idea that infants can take multiple sources of information—both from first-person experience and from observation of third-person acts—and map them into the same abstract framework. This would support bidirectional learning—mapping from others to self and a mapping from self to others. In this view, first-person experience is a richly detailed source of knowledge that is constantly interwoven with third-person information gained from observing the acts of others. Infants surely are not limited to understanding what they have already experienced through self-production (Meltzoff, 1988). However, self-experience motivates infants to attribute properties, powers, and inner experiences to others and imbue observed acts with a felt meaning.
The current studies focused on the role of self-experience in understanding others’ visual perception. We found that first-person experience provides a framework for understanding like experiences in others. The broader theoretical claim is that self-experience provides a mechanism of change in social understanding: As children’s self-experience broadens, their appreciation of others’ minds and behavior is enriched and refined. This propels infants beyond what they see or know innately.
Acknowledgments
Portions of these data were presented at the International Conference on Infant Studies, Chicago, May 2004. We gratefully acknowledge support from the National Institutes of Health (HD-22514), the National Science Foundation (SBE-0354453), the Tamaki Foundation, and the James S. McDonnell Foundation. The thoughts expressed in the article are those of the authors and do not necessarily reflect the views of these agencies. We thank the following colleagues for helpful discussions and/or comments on drafts of this article: P. Kuhl, A. Gopnik, R. Williamson, B. Repacholi, J. Watson, P. Marshall, G. Gergely, G. Csibra, D. Liu, D. Keleman, J. Sommerville, S. Carlson, H. Moll, H. Wellman, and A. Woodward.
Footnotes
The “like me” hypothesis does not award sole priority to information flowing in one direction only (Meltzoff, 2007b; Meltzoff & Moore, 1997; Repacholi, Meltzoff, & Olsen, 2008). The framework encompasses mapping both from self to other and from other to self. Infants imitate novel acts, showing they learn from observing others’ acts even when these behaviors are not in the infant’s prior repertoire (Meltzoff, 1988)—this is mapping from other to self. They also recognize when others imitate their own behavior (Meltzoff, 2007a)—this is mapping from self to other.
As is standard in the gaze-following literature, the text reports analyses using looking scores (as defined in the Scoring section). Alternative analyses isolating only infants’ correct looks to target (ignoring incorrect looks) yield identical results in both Experiment 1 and 2.
Note that infants’ phenomenological self-experience with the blindfold is visually very different from their visual experience of the other person. When the opaque blindfold comes up in front of the infant’s eyes, they see the world go black. When they look at another person wearing the blindfold they see a partially obscured face. Infants use the former to make inferences about the latter. See Understanding Visual Perception for a larger spatial–causal framework that might aid such infant inferences.
References
- Adolph KE, Eppler MA, Gibson EJ. Development of perception of affordances. Advances in Infancy Research. 1993;8:51–98. [Google Scholar]
- Agnetta B, Rochat P. Imitative games by 9-, 14-, and 18-month-old infants. Infancy. 2004;6:1–36. [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Biro S, Leslie AM. Infants’ perception of goal-directed actions: Development through cue-based bootstrapping. Developmental Science. 2007;10:379–398. doi: 10.1111/j.1467-7687.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The importance of eyes: How infants interpret adult looking behavior. Developmental Psychology. 2002;38:958–966. doi: 10.1037//0012-1649.38.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Developmental Science. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SC, Caron AJ, Brooks R. Infant understanding of the referential nature of looking. Journal of Cognition and Development. 2000;1:359–377. [Google Scholar]
- Butterworth G, Cochran E. Towards a mechanism of joint visual attention in human infancy. International Journal of Behavioral Development. 1980;3:253–272. [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: Spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Caron AJ, Kiel EJ, Dayton M, Butler SC. Comprehension of the referential intent of looking and pointing between 12 and 15 months. Journal of Cognition and Development. 2002;3:445–464. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63 4, Serial No. 255. [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Corkum V, Moore C. Development of joint visual attention in infants. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 61–83. [Google Scholar]
- Csibra G, Gergely G. Social learning and social cognition: The case for pedagogy. In: Munakata Y, Johnson MH, editors. Processes of change in brain and cognitive development: Attention and performance XXI. Oxford, England: Oxford University Press; 2006. pp. 249–274. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains SMJ, Muir DW. A demonstration of gaze following in 3- to 6-month-olds. Infant Behavior & Development. 1997;20:569–572. [Google Scholar]
- D’Entremont B, Morgan R. Experience with visual barriers and its effects on subsequent gaze-following in 12- to 13-month-olds. British Journal of Developmental Psychology. 2006;24:465–475. [Google Scholar]
- Dunphy-Lelii S, Wellman HM. Infants’ understanding of occlusion of others’ line-of-sight: Implications for an emerging theory of mind. European Journal of Developmental Psychology. 2004;1:49–66. [Google Scholar]
- Flavell JH. The development of knowledge about visual perception. In: Keasey CB, editor. Nebraska symposium on motivation: Vol. 25. Social cognitive development. Lincoln: University of Nebraska Press; 1977. pp. 43–76. [PubMed] [Google Scholar]
- Flavell JH. Perspectives on perspective taking. In: Beilin H, Pufall P, editors. Piaget’s theory: Prospects and possibilities. Hillsdale, NJ: Erlbaum; 1992. pp. 107–139. [Google Scholar]
- Flavell JH. Cognitive development: Children’s knowledge about the mind. Annual Review of Psychology. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- Flom R, Lee K, Muir D, editors. Gaze-following: Its development and significance. Mahwah, NJ: Erlbaum; 2007. [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf P, Aschersleben G, Prinz W. Baby do—Baby see! How action production influences action perception in infants. Cognitive Development. 2007;22:16–32. [Google Scholar]
- Hobson RP. Autism and the development of mind. Hove, England: Erlbaum; 1993. [Google Scholar]
- Johnson SC. Detecting agents. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2003;358:549–559. doi: 10.1098/rstb.2002.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Slaughter V, Carey S. Whose gaze will infants follow? The elicitation of gaze-following in 12-month-olds. Developmental Science. 1998;1:233–238. [Google Scholar]
- Király I, Jovanovic B, Prinz W, Aschersleben G, Gergely G. The early origins of goal attribution in infancy. Consciousness and Cognition. 2003;12:752–769. doi: 10.1016/s1053-8100(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Lee K, Eskritt M, Symons LA, Muir D. Children’s use of triadic eye gaze information for “mind reading. Developmental Psychology. 1998;34:525–539. doi: 10.1037//0012-1649.34.3.525. [DOI] [PubMed] [Google Scholar]
- Lempers JD, Flavell ER, Flavell JH. The development in very young children of tacit knowledge concerning visual perception. Genetic Psychology Monographs. 1977;95:3–53. [PubMed] [Google Scholar]
- Leslie AM. ToMM, ToBY, and agency: Core architecture and domain specificity. In: Hirschfeld LA, Gelman SA, editors. Mapping the mind: Domain specificity in cognition and culture. New York: Cambridge University Press; 1994. pp. 119–148. [Google Scholar]
- Meltzoff AN. Infant imitation after a 1-week delay: Long-term memory for novel acts and multiple stimuli. Developmental Psychology. 1988;24:470–476. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Like me”: A foundation for social cognition. Developmental Science. 2007a;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. The “like me” framework for recognizing and becoming an intentional agent. Acta Psychologica. 2007b;124:26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Brooks R. Eyes wide shut: The importance of eyes in infant gaze following and understanding other minds. In: Flom R, Lee K, Muir D, editors. Gaze-following: Its development and significance. Mahwah, NJ: Erlbaum; 2007. pp. 217–241. [Google Scholar]
- Meltzoff AN, Brooks R. Social cognition and language: The role of gaze following in early word learning. In: Colombo J, McCardle P, Freund L, editors. Infant pathways to language: Methods, models, and research directions. New York: Psychology Press; 2009. pp. 169–194. [Google Scholar]
- Meltzoff AN, Gopnik A. The role of imitation in understanding persons and developing a theory of mind. In: Baron-Cohen S, Tager-Flusberg H, Cohen DJ, editors. Understanding other minds: Perspectives from autism. New York: Oxford University Press; 1993. pp. 335–366. [Google Scholar]
- Meltzoff AN, Moore MK. Explaining facial imitation: A theoretical model. Early Development and Parenting. 1997;6:179–192. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll H, Tomasello M. 12- and 18-month-old infants follow gaze to spaces behind barriers. Developmental Science. 2004;7:F1–F9. doi: 10.1111/j.1467-7687.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- Moll H, Tomasello M. Level 1 perspective-taking at 24 months of age. British Journal of Developmental Psychology. 2006;24:603–613. [Google Scholar]
- Moore C. Gaze following and the control of attention. In: Rochat P, editor. Early social cognition: Understanding others in the first months of life. Mahwah, NJ: Erlbaum; 1999. pp. 241–256. [Google Scholar]
- Moore C. The development of commonsense psychology. Mahwah, NJ: Erlbaum; 2006. [Google Scholar]
- Moore C, Corkum V. Infant gaze following based on eye direction. British Journal of Developmental Psychology. 1998;16:495–503. [Google Scholar]
- Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20:115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Nadel J. Imitation and imitation recognition: Functional use in preverbal infants and nonverbal children with autism. In: Meltzoff AN, Prinz W, editors. The imitative mind: Development, evolution, and brain bases. Cambridge, England: Cambridge University Press; 2002. pp. 42–62. [Google Scholar]
- Novey MS. The development of knowledge of others’ ability to see. Unpublished doctoral dissertation, Harvard University; 1975. [Google Scholar]
- O’Neill DK. Two-year-old children’s sensitivity to a parent’s knowledge state when making requests. Child Development. 1996;67:659–677. [Google Scholar]
- Rakison DH, Poulin-Dubois D. Developmental origin of the animate–inanimate distinction. Psychological Bulletin. 2001;127:209–228. doi: 10.1037/0033-2909.127.2.209. [DOI] [PubMed] [Google Scholar]
- Repacholi BM, Meltzoff AN, Olsen B. Infants’ understanding of the link between visual perception and emotion: “If she can’t see me doing it, she won’t get angry. Developmental Psychology. 2008;44:561–574. doi: 10.1037/0012-1649.44.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife M, Bruner JS. The capacity for joint visual attention in the infant. Nature. 1975 January 24;253:265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Searle JR. Intentionality: An essay in the philosophy of mind. New York: Cambridge University Press; 1983. [Google Scholar]
- Sommerville JA, Woodward AL. Infants’ sensitivity to the causal features of means-end support sequences in action and perception. Infancy. 2005a;8:119–145. [Google Scholar]
- Sommerville JA, Woodward AL. Pulling out the intentional structure of action: The relation between action processing and action production in infancy. Cognition. 2005b;95:1–30. doi: 10.1016/j.cognition.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences. 2005;28:675–691. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Haberl K. Understanding attention: 12- and 18-month-olds know what is new for other persons. Developmental Psychology. 2003;39:906–912. doi: 10.1037/0012-1649.39.5.906. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Lehmann H, Call J. Reliance on head versus eyes in the gaze following of great apes and human infants: The cooperative eye hypothesis. Journal of Human Evolution. 2007;52:314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders. 2006;36:993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA, Meltzoff AN, Markman EM. Prior experiences and perceived efficacy influence 3-year-olds’ imitation. Developmental Psychology. 2008;44:275–285. doi: 10.1037/0012-1649.44.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AL. Infants’ developing understanding of the link between looker and object. Developmental Science. 2003;6:297–311. [Google Scholar]
- Woodward AL. Infants’ understanding of actions involved in joint attention. In: Eilan N, Hoerl C, McCormack T, Roessler J, editors. Joint attention: Communication and other minds: Issues in philosophy and psychology. New York: Oxford University Press; 2005. pp. 110–128. [Google Scholar]
- Woodward AL, Guajardo JJ. Infants’ understanding of the point gesture as an object-directed action. Cognitive Development. 2002;17:1061–1084. [Google Scholar]