Abstract
Purpose
The lens grows continuously throughout life, but the factors that influence the size of the adult lens are not known. Lens thickness is a significant risk factor for age-related cataract. It has been postulated that the hypoxic environment in the eye protects the lens from nuclear cataracts. The authors sought to determine whether the PO2 in the eye regulates lens growth.
Methods
Lens cell proliferation was determined by counting BrdU-labeled and total nuclei in the germinative zone in flat-mounts of lens epithelia. Oxygen levels in the eye were altered by having rats breathe 11%, 21% (room air), or 60% oxygen. Oxygen levels in the vitreous were measured with a fiberoptic oxygen sensor.
Results
The BrdU-labeling index in the germinative zone declined from approximately 3.5% at 1 month to less than 0.7% at 8 months. Raising oxygen levels in the eyes of 1-month-old animals did not alter the rate of lens cell proliferation. Elevating intraocular oxygen in animals older than 1 month increased proliferation to the more rapid rate seen at 1 month. Decreasing oxygen levels below their normally low level did not affect the BrdU-labeling index at any age. Chronic exposure to increased oxygen led to the production of more lens fiber cells and larger lenses.
Conclusions
Normal age-related decline in lens growth requires the low oxygen level normally present in the eye. Increases in lens cell number and mass may account for some of the increase in cataract risk caused by chronic exposure of the lens to elevated oxygen levels.
It has been difficult to discover the aspects of aging that are responsible for the exponential increase in cataract risk after age 50. Environmental risk factors for cataract are often difficult to modify (smoking), or they account for only a small fraction of the total cataract burden (sunlight exposure).1 Family history is a major contributor to cataract risk.2–6 However, the genes that contribute to increased risk for age-related cataract have not been identified, and the pathways in which these genes act to increase or decrease cataract risk are unknown. Therefore, understanding the physiology of the normal lens is important for identifying the aspects of lens biology that are altered with age.
The lens is an epithelial tissue with cells that exist in three states of growth (Fig. 1A). The anterior surface of the lens is covered by a simple cuboidal epithelium. In adults, epithelial cells in the central region of the epithelium are mitotically quiescent. However, cells at the periphery of the epithelium, in the germinative zone, proliferate throughout life. When germinative zone cells divide, their daughter cells move posteriorly, withdraw from the cell cycle, and differentiate into fiber cells. Fiber cells, which make up the bulk of the lens, are greatly elongated and accumulate high levels of crystallins, which account for its transparency and refractive power. As the lens grows, fiber cells that were originally near the surface are buried deeper within the lens by the differentiation of more superficial fiber cells. At the completion of their elongation and maturation, fiber cells lose all their membrane-bound organelles.7 However, the remaining cellular components persist for the life of the lens. By these processes, the lens grows in cell number, mass, and size throughout life.8
Figure 1.
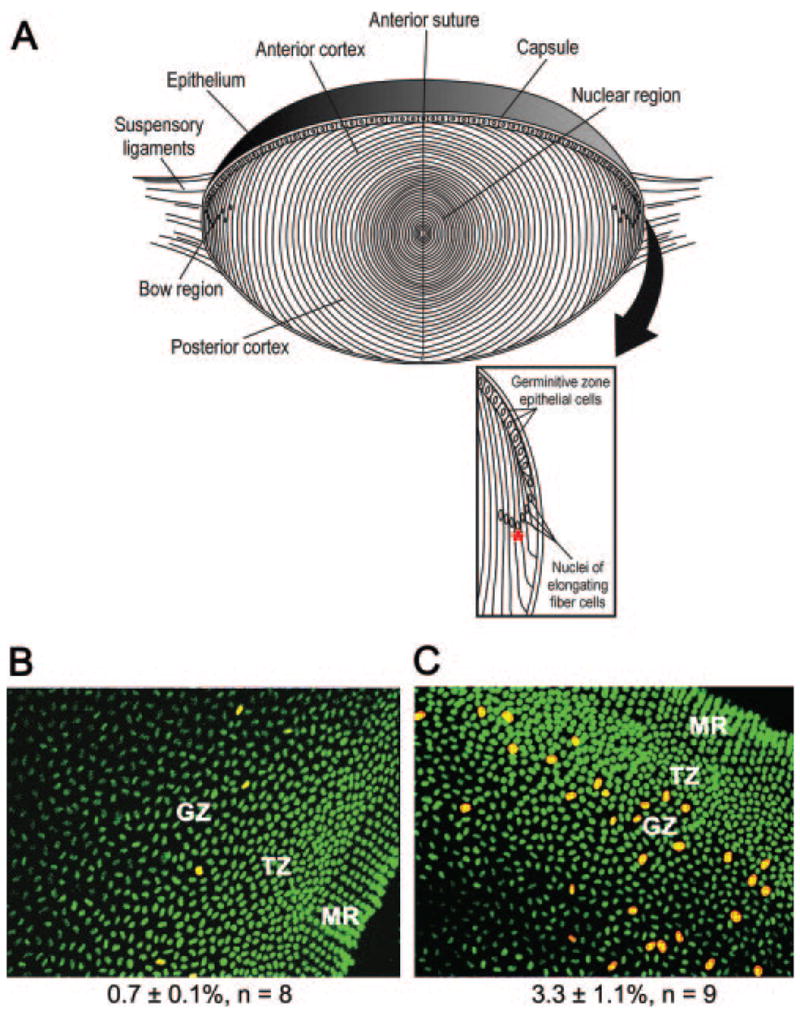
(A) Diagram of the adult lens. Inset: details of the equatorial region, including the location of the germinative zone at the periphery of the lens epithelium. Red asterisk: nucleus at the lowest point of the “lens bow.” In one experiment, this nucleus was used as a reference point to measure the extent of fiber cell differentiation. (B) Example of BrdU labeling in a flatmount preparation of the peripheral region of the lens epithelium from an untreated 8-month-old rat. BrdU was injected 1 hour before death. Cells labeled with an antibody to BrdU are red; nuclei are stained green with the fluorescent stain for nucleic acids, TOTO-1. (C) BrdU labeling of lens cells from an 8-month-old animal that breathed 60% oxygen for 3 days; staining as in (B). The mean BrdU-labeling index (±SEM) and number of samples counted are indicated below (B) and (C). GZ, germinative zone where epithelial cells proliferated; TZ, transition zone where postmitotic cells prepared to differentiate; MR, meridional rows containing the most peripheral fiber cells.
Lens growth is rapid during fetal and early postnatal life but slows thereafter. In humans, lens mass increases logarithmically until soon after birth, then transitions to linear growth for the remainder of life.9–11 Unlike the remainder of the mammalian eye, the lens continuously increases in cell number and size. The consequences of this sustained growth in an eye that is not growing are thought to contribute to presbyopia, the decreased ability to focus on near objects that develops in middle age.
Several growth factors promote the proliferation of lens epithelial cells in culture. These include platelet-derived growth factor,12–15 fibroblast growth factor,16–20 insulin and insulin-like growth factors,21,22 epidermal growth factor,23–28 and hepatocyte growth factor.29,30 These mitogens are present in ocular tissues near the lens or in the aqueous humor that bathes the lens epithelium. However, none has been demonstrated to regulate the normal growth of the lens in vivo. It has been assumed that the decline in the rate of lens growth that occurs during postnatal life results from decreased levels of lens mitogens or decreased responses of lens cells to these agents.
The avascular lens exists in a hypoxic environment.31–35 Increased exposure to oxygen has been shown to be a risk factor for the most common type of age-related cataract, nuclear cataract.36,37 During aging, degeneration of the vitreous body, the gel between the lens and the retina, may expose the lens to increased oxygen.38 In the present study, examination of the effects of oxygen on the lens in vivo revealed that the normal level of intraocular hypoxia is required to suppress lens cell proliferation and to maintain a smaller lens. Given that increased lens thickness is a risk factor for nuclear cataract formation,39,40 these observations provide a potential link between oxygen exposure and cataract formation.
Materials and Methods
Experiments with animals were approved by the Washington University Animal Studies Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
BrdU Labeling of Lens Epithelial Cells
Rats were injected intraperitoneally with a 10:1 mixture of 5′-bromode-oxyuridine (BrdU) and fluorodeoxyuridine (50 and 5 mg/kg, respectively; Sigma-Aldrich Chemical Co., St. Louis, MO). One hour after injection, lens epithelial wholemounts were dissected, fixed, and stained with a monoclonal antibody to BrdU (1:500; BD Biosciences, San Jose, CA) and the fluorescent nucleic acid stain, TOTO-1 (100 ng/mL; Invitrogen-Molecular Probes, Carlsbad, CA), and were examined using a confocal microscope. Labeled and total nuclei in the germinative zone of the lens epithelium were counted using the public domain NIH Image program (developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/).
Short-term Hypoxia or Hyperoxia
Cages were placed in a large, plastic enclosure, and rats were exposed to room air (21% oxygen) or 11% or 60% oxygen for up to 3 days. Oxygen levels were regulated using an oxygen controller (Pro-Ox 110; Biospherix, Redfield, NY). One hour before the end of the exposure, animals were injected with BrdU, and the BrdU-labeling index was determined as described. To follow changes in BrdU labeling after oxygen exposure, a group of 8-month-old rats was exposed to 60% oxygen and killed 3, 6, 12, 24, or 72 hours later. The remaining animals were returned to room air and killed 84, 96, 120, 144, and 168 hours after the beginning of the experiment. In each case, BrdU was injected 1 hour before death.
Long-term Intermittent Oxygen Exposure
One- and 8-month old (n = 24 at each age) rats were divided into two groups and exposed to room air or 60% oxygen for 3 days each week for 3 months. Body weights were checked weekly and did not vary significantly between groups. After 3 months, lens wet weights were measured, and the BrdU-labeling index was determined.
In another study, BrdU was injected at the initiation of treatment of 1- and 8-month-old rats. Among the 1-month-old animals, five were exposed to room air, five to 11% oxygen, and five to 60% oxygen 3 days each week for 4 weeks. Groups of five 8-month-old animals were exposed to room air or 60% oxygen. Animals were killed, and the lenses were isolated, fixed, and prepared for histologic analysis. Lens sections were stained with anti-BrdU antibody, and the migration of BrdU-labeled cells from the germinative zone into the fiber mass was quantified as a measure of lens growth. The extent of migration was determined by counting the number of nuclei between the deepest BrdU-labeled nucleus and the nucleus at the lowest point of the arc of the lens bow (the lens fiber cell nucleus closest to the posterior of the lens; see asterisk in Fig. 1).
Intraocular Oxygen Measurements
PO2 in the vitreous chamber was measured using a fiberoptic oxygen sensor (Oxylab pO2 optode; Oxford Optronix, Oxford, UK). Cages containing 1- or 8-month-old rats were placed in large plastic enclosures and exposed for 1 hour to 12%, 21%, or 60% oxygen. Before removal from the container, the animals were anesthetized by intra-peritoneal injection of ketamine (30 mg/kg) and medetomidine (1 mg/kg). Immediately after anesthetization, a sclerotomy was made with a 30-gauge needle 1 mm from the corneal limbus on the temporal side of eye, and the tip of the optode was inserted into the center of the vitreous chamber. Oxygen measurements were obtained by holding the probe in place until stable values were noted (approximately 2 minutes). Optode calibration was checked before each set of measurements.
TUNEL Labeling
Terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) was performed (Apoptag kit; Chemicon, Temecula, CA). Lenses were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA), washed in PBS, dehydrated, embedded in paraffin, and sectioned at 4 μm. Deparaffinized slides were treated with 3% H2O2 in methanol for 30 minutes, followed by proteinase K treatment (20 μg/mL) for 15 minutes Epitope retrieval was performed in 0.01 M citrate buffer (pH 6.0) either at 100°C for 20 minutes using a water bath or by placing slides in a decloaking chamber (Biocare Medical, Walnut Creek, CA) for 3 minutes. Slides were incubated with TdT enzyme in equilibration buffer for 1 hour at 37°C. The reaction was terminated with wash buffer provided by the manufacturer for 10 minutes at room temperature. Anti–digoxigenin-peroxidase conjugate was added for 30 minutes at room temperature, followed by color development with diaminobenzidine and hydrogen peroxide. Slides were counterstained with hematoxylin.
Statistical Analysis
Differences between samples were evaluated using Student’s t-test. Bonferroni adjustment was applied for multiple comparisons.
Results
Regulation of Lens Cell Proliferation by Hypoxia
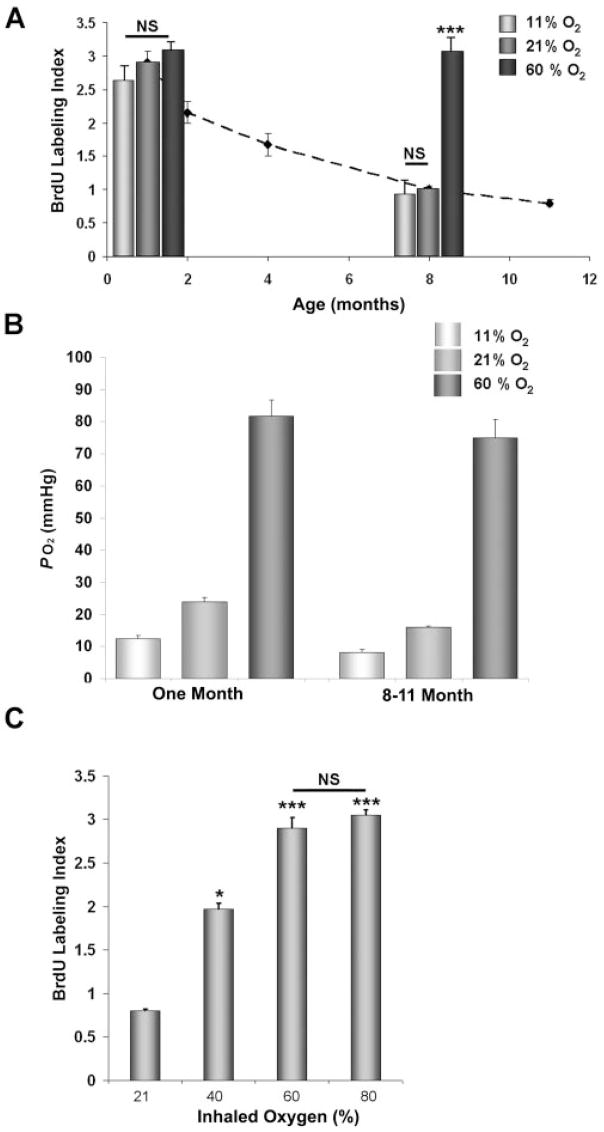
The lens of the eye is normally in a hypoxic environment.31–35,41–44 When humans or experimental animals breath higher than normal levels of oxygen, the PO2 around the lens increases.31–33 Pilot microarray studies performed on adult rat lenses exposed to higher or lower than normal levels of oxygen suggested that oxygen might alter the rate of lens cell proliferation (not shown). To test this possibility, 8-month-old Sprague–Dawley rats were kept in enclosures gassed with room air (approximately 21% oxygen) or 60% oxygen for 3 days. Animals were injected with BrdU 1 hour before death, and flat-mounts of lens epithelial cells were stained for BrdU. In adults, cell proliferation was restricted to the germinative zone, a band of epithelial cells near the lens equator (Fig. 1A). The germinative zone epithelial cells at the periphery of the lens epithelia of animals exposed to 60% oxygen had many more BrdU-labeled cells than animals maintained in room air, increasing from a BrdU-labeling index of 0.7% ± 0.1% in animals kept in room air to 3.3% ± 1.1% in animals breathing 60% oxygen (Figs. 1B, 1C). Oxygen exposure did not appreciably change the very low levels of BrdU labeling outside the germinative zone, in the more central regions of the lens epithelium (not shown).
Additional studies showed that the response of the lens epithelial cells to oxygen differed significantly in young and older rats. In an initial study, we measured proliferation in the germinative zone of rats at 1, 2, 4, 8, and 11 months of age (dotted line; Fig. 2A). The BrdU-labeling index of 1-month-old rat lenses was approximately 3%. The-labeling index gradually decreased with age, reaching less than 0.7% by 11 months of age.
Figure 2.
(A) BrdU-labeling index of germinative zone lens epithelial cells from 1- or 8-month-old rats breathing 11%, 21%, or 60% oxygen for 3 days. Dashed line: basal BrdU-labeling index at different ages. (B) Intraocular oxygen level, measured with an optical oxygen sensor, in young or older rats breathing 11%, 21%, or 60% oxygen. (C) BrdU-labeling index in the germinative zone of 8-month-old rat lens epithelia in animals breathing different levels of oxygen for 3 days. NS, not significantly different (P > 0.05). *P < 0.05 and ***P < 0.001 compared with 21% O2. All measurements are ± SEM.
Exposure of 1-month-old animals to 60% oxygen had no significant effect on the BrdU-labeling index of the epithelial cells in the germinative zone (Fig. 2A). To determine whether the higher BrdU-labeling index in younger rats was caused by higher intraocular oxygen levels, we measured oxygen in the eye with a fiberoptic oxygen sensor. Although approximately 50% higher than in 8-month-old rats, the oxygen levels around the lens in young rats kept in room air was still in the hypoxic range (approximately 3%; Fig. 2B). This level was similar to that previously reported for rats using a polarographic oxygen electrode.45 Exposing 1-month-old rats to hypoxic conditions decreased the intraocular PO2 to 12 mm Hg, well below the level found in older rats (16 mm Hg; Fig. 2B). However, this lower oxygen level did not significantly decrease the BrdU-labeling index in the germinative zone (Fig. 2A). Oxygen levels increased nearly fourfold when young rats breathed 60% oxygen (to more than 10%), but this did not significantly alter the BrdU-labeling index. Therefore, the proliferation of lens epithelial cells from young rats is not sensitive to intraocular oxygen levels.
As in our initial experiments, placing older rats in 60% oxygen increased the BrdU-labeling index approximately threefold (Fig. 2A). This was associated with a fourfold increase in the intraocular oxygen level (Fig. 2B). As in younger rats, maintaining older animals in lower than normal oxygen (11%) lowered the intraocular oxygen levels by 50% but did not significantly decrease the BrdU-labeling index below that seen in animals breathing room air (Figs. 2A, 2B). In 8-month-old rats, the BrdU-labeling index increased with increasing oxygen inhalation in a dose-dependent manner up to 60% oxygen, but exposure to more than 60% oxygen did not further increase the BrdU-labeling index (Fig. 2C).
Increased Cell Proliferation Results in Increased Fiber Cell Formation and Larger Lenses
After mitosis in the germinative zone, daughter cells normally cease proliferating and become terminally differentiated fiber cells.8 We sought to determine whether the excess cells formed in older lenses exposed to elevated oxygen differentiated into fiber cells. For this purpose, we used BrdU incorporated into DNA at the beginning of the study to track epithelial cells from the germinative zone into the fiber mass in animals breathing low, normal, or high levels of oxygen.
For logistic reasons, rats could only be exposed to altered oxygen levels for 3 days each week. Therefore, before beginning long-term studies, we examined the kinetics of BrdU-labeling when rats were exposed to 60% oxygen for 3 days, followed by 4 days in room air. The BrdU-labeling index of 8-month-old rats increased significantly within 3 hours after exposure to 60% oxygen (P < 0.05; n = 6) and approached maximum by 24 hours (Fig. 3A). Return to room air caused the BrdU-labeling index to decrease to near the baseline value within 24 hours. By estimating the area under the curve, we calculated that exposure to 60% oxygen for 3 days each week increased the average weekly BrdU-labeling index of 8-month-old lenses to 1.9 times the baseline in 21% oxygen. As shown in Figure 2, the already high BrdU-labeling index of young rat lens epithelial cells was not significantly altered by exposure to 60% oxygen.
Figure 3.
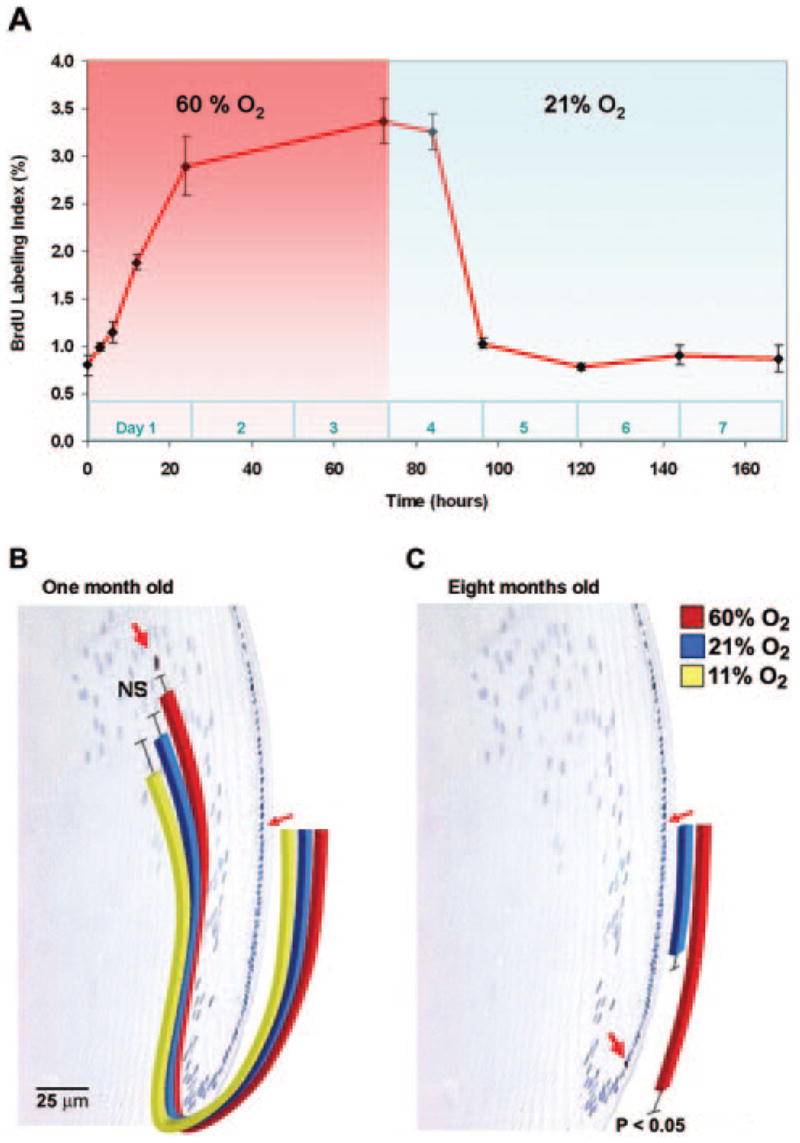
(A) BrdU-labeling index of germinative zone epithelial cells in rats breathing 60% oxygen for 3 days, followed by 4 days in room air. A significant increase in BrdU labeling was seen as early as 3 hours after breathing 60% oxygen. Calculation of the area under the curve showed that the weekly average BrdU-labeling index was approximately 1.9 times greater in mice intermittently breathing 60% oxygen than in those continuously breathing room air. (B) Measurement of the magnitude of new fiber cell formation in lenses of 1-month-old rats that were injected with BrdU, then intermittently exposed to 11% or 60% oxygen for 3 days each week for a total of 4 weeks. Rats maintained in room air (21% oxygen) served as controls. Bars show the routes and extents of migration of BrdU-labeled nuclei. (C) Extent of fiber cell formation in lenses of 8-month-old rats treated as in (B) with room air or 60% oxygen. NS, not significantly different. Smaller red arrows: germinative zone; larger red arrows: BrdU-labeled cells. The scale bar in (B) also applies to (C).
One- or 8-month-old rats were injected with BrdU. Beginning on the following day, they were exposed to 11%, 21%, or 60% oxygen for 3 days each week for 4 weeks. At the end of the treatment period, nuclei that had incorporated BrdU at the beginning of the experiment were located by antibody staining in central lens sections. In rats that were 1 month old at the beginning of treatment, labeled nuclei were located deep within the fiber mass. Exposure to low or high oxygen levels for 3 days each week did not significantly alter the depth within the fiber mass at which BrdU-labeled nuclei were detected (Fig. 3B). In the lenses of 8-month-old rats maintained in room air, BrdU-labeled cells barely moved out of the germinative zone and into the transitional zone, the region in which epithelial cells are postmitotic but have not yet begun to form fiber cells. However, in lenses treated intermittently with 60% oxygen, BrdU-labeled cells moved approximately twice as far, reaching the end of the transition zone, where cells are in the early stages of fiber cell differentiation (Fig. 3C). Because intermittent oxygen exposure increased the BrdU-labeling index in 8-month-old rats by a factor of 1.9 and the distance migrated by approximately 2, we concluded that most or all the excess germinative zone epithelial cells generated after breathing 60% oxygen differentiated into fiber cells.
If oxygen treatment decreased lens epithelial cell death, the additional cells that resulted might have contributed to the increase in epithelial cell migration in older rats. However, TUNEL-labeled nuclei were undetectable in the epithelial or superficial fiber cells of adult rat lenses (not shown). TUNEL-labeled cells were detected in adjacent tissues, confirming that the labeling reaction was successful. We concluded from this result that decreased apoptosis was unlikely to have contributed to the increased rate of fiber cell differentiation.
To test whether the increased rate of fiber cell differentiation resulted in larger lenses, 1- and 8-month-old rats were exposed to room air or 60% oxygen 3 days each week for 3 months. Lens wet weight was measured after the final treatment. In young animals, intermittent exposure to elevated oxygen had no significant effect on lens wet weight (Fig. 4A). By contrast, the lenses of rats that were intermittently exposed to 60% oxygen for 3 months, beginning at 8 months of age, had wet weights significantly greater than those of controls (Fig. 4B). Although this increase in wet weight might have resulted from the uptake of water, the increased rate of fiber formation observed in lenses exposed to elevated oxygen suggested that more rapid addition of lens fiber cells accounted for the increase in mass.
Figure 4.
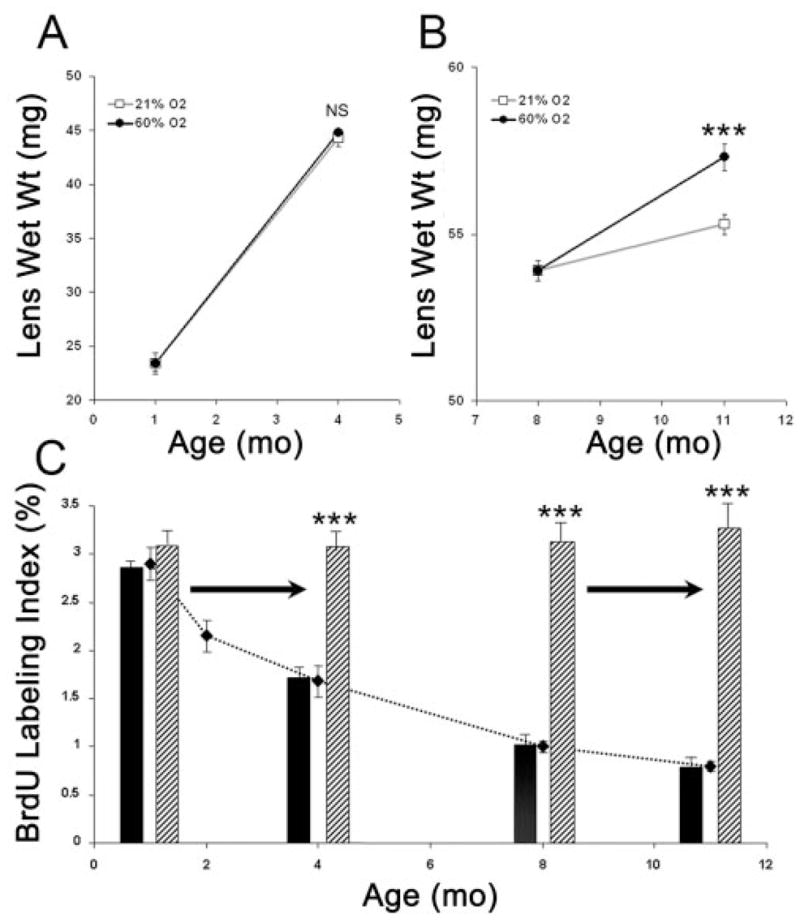
Long-term oxygen breathing alters the growth of older lenses. Shown are the wet weights and BrdU-labeling indexes in 4- and 11-month-old lenses from rats previously exposed to room air or intermittently to 60% oxygen for 12 weeks. (A) Lens wet weights in animals 1 month old at the initiation of oxygen treatment. (B) Lens wet weights in animals 8 months old at the initiation of oxygen treatment. Open squares: room air. Filled circles: sixty percent oxygen 3 days each week. n = 12 for each group. (C) Effect of oxygen treatment at different ages on BrdU incorporation. Dotted line: baseline BrdU-labeling index in untreated animals, taken from Figure 2. Data for 1-and 8-month-old rats are also from Figure 2. Labeling indexes at 4 and 11 months of age were obtained from rats treated intermittently with 60% oxygen 3 days each week for 12 weeks. Horizontal arrows: period during which animals were intermittently exposed to room air or 60% oxygen. Animals were injected with BrdU 1 hour before the end of the last 3-day oxygen treatment. Solid bars: room air. Striped bars: sixty percent oxygen. NS, not significantly different. ***P < 0.001.
Rat lens cells did not become refractory to the stimulatory effects of oxygen after repeated exposure. At the end of the twelfth week of intermittent oxygen exposure, the BrdU-labeling index was determined in the germinative zone. Figure 4C shows that the effect of oxygen on the percentage of cells incorporating BrdU was not diminished after 3 months of intermittent oxygen treatment. These measurements also revealed that, no matter at what age animals were exposed to 60% oxygen, the BrdU-labeling index reached a level indistinguishable from that at 1 month of age.
Discussion
Results of the present work suggest that the age-related decline in the rate of lens growth in rats depends on the normal level of intraocular hypoxia. These observations were possible because the levels of oxygen in the eye could be readily raised or lowered in vivo. In 1-month-old rats, the oxygen level around the lens was already in the hypoxic range. At this age, increasing the oxygen level in the eye or decreasing it to a level lower than that found in the eyes of older animals had no effect on the rate of epithelial cell proliferation. The rate of lens cell proliferation and, therefore, lens growth gradually declined after 1 month of age. As in the 1-month-old animals, making the lens more hypoxic did not decrease lens cell proliferation in older lenses. However, raising the oxygen level in the eyes of older rats returned the rate of lens growth to that seen at 1 month of age. These data suggest that if oxygen levels in the eye were not low, the lens would continue to grow at the same rate seen in 1-month-old animals. Because the rodent lens occupies a large proportion of the volume of the eye, the result would be a much larger lens and eye.
The mechanism by which intraocular hypoxia inhibits lens growth in older animals remains to be identified. Three general mechanisms seem possible, although they are not mutually exclusive.
First, with increasing age, low intraocular oxygen may increasingly suppress the synthesis or secretion of one or more critical growth factors normally present in the intraocular fluids. Exposing eyes to increased oxygen would relieve this inhibition, increasing growth factor levels in the aqueous humor and stimulating lens epithelial cell proliferation.
Second, growth factor levels in the aqueous humor may decrease with age, thereby reducing the rate of lens epithelial cell proliferation. However, oxygen treatment might increase lens epithelial cell proliferation, independent of growth factor levels. This might occur if, for example, oxygen treatment increases intracellular reactive oxygen species.13
Third, hypoxia may suppress the ability of lens epithelial cells to respond to growth factors normally present in aqueous humor. Increasing oxygen levels in the eye would relieve this inhibition and permit more rapid lens epithelial cell proliferation. Hypoxia is well known to inhibit the proliferation of cells cultured in growth stimulatory medium by increasing levels and activity of the hypoxia-dependent transcription factor HIF-1α. HIF-1 increases the levels of cyclin-dependent kinase inhibitors (CKIs).46–49 CKIs inhibit cell proliferation by inhibiting progression through the S-phase of the cell cycle. Removing this block would permit more cells to enter the S-phase, increasing the rate of proliferation. It is also possible that other mechanisms by which lens cells respond to growth factors are inhibited by hypoxia. For example, levels of FGF receptor-1 decrease as lens epithelial cells age, a change that correlates with the decreased ability of cells to respond to exogenously added FGF.50 It remains to be tested whether growth factor receptors, CKIs, or other aspects of growth factor signaling are regulated by intraocular oxygen.
The first two models predict that aqueous humor from older eyes would be less effective in promoting lens epithelial cell proliferation. Several studies have shown that aqueous humor can stimulate lens epithelial cell proliferation.15,23,51,52 We are aware of no evidence that aqueous humor from younger eyes is more effective in promoting proliferation than aqueous from older eyes. It would be useful to conduct such a test if a sufficient amount of aqueous humor could be obtained from young and old animals.
Results of the present study show that the cell number and mass of a lens can be increased by exposing animals to increased oxygen. Several studies have shown that exposure of the lens to increased oxygen is associated with increased opacification of the lens nucleus or frank nuclear cataracts.33,36,37,53–55 Epidemiologic studies revealed that lens thickness is a significant risk factor in age-related cataract formation.39,40 In these studies, individuals with smaller lenses at the time of examination were more likely to have cortical cataracts, whereas those with larger lenses more frequently had nuclear cataracts.39 Quantification of incident cataracts showed that individuals with smaller, initially clear lenses were more likely to develop cortical cataracts over a 5-year follow-up period, whereas those with thicker lenses were more likely to develop nuclear opacities.40
Studies of the physiology of the aging lens suggest an explanation for the association between larger lens size and nuclear cataract. Nuclear cataract involves the oxidative modification of crystallins and lens membrane proteins.56–59 Proteins in the nucleus are protected from oxidation by reducing agents, such as cysteine and glutathione.53,60–62 Reduced glutathione and cysteine are produced in cells near the lens surface, from which they can diffuse to cells deeper in the lens through gap junctions and, perhaps, fiber cell fusions.63–65 If a molecule of glutathione or cysteine is oxidized in the nucleus, it must diffuse back to the lens surface before it can be reduced. Older lenses have increased levels of oxidized glutathione in the nucleus, and even more oxidized glutathione is present in nuclear cataracts.53,66,67 As the lens ages, diffusion between the metabolically active cells close to the lens surface and the cells deeper in the lens is impeded.65,68–70 Supplying the lens nucleus with reducing agents, such as glutathione and cysteine, would be less efficient in larger lenses because the diffusion path from the metabolically active surface cells to the lens nucleus would increase as the size of the lens increases.
Exposure to increased oxygen would be expected to cause increased oxidative stress on the lens. At the same time, the present study suggests that increased oxygen would increase lens growth, which may decrease the ability of the lens to protect itself from oxidative damage. This suggests a vicious cycle in which oxygen promotes both oxidative damage and lens growth to accelerate the opacification of the lens nucleus. Better understanding of the mechanisms regulating lens growth, including the means by which intraocular hypoxia reduces lens cell proliferation, may lead to ways to protect the lens against nuclear cataract.
Acknowledgments
Supported by National Institutes of Health Grants EY04853 and EY015863 (DCB) and Core Grant P30 EY02687, a Research to Prevent Blindness Senior Scientist Award (DCB), and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences.
The authors thank Jean Jones and Belinda McMahan for assistance with histology.
Footnotes
Disclosure: Y.-B. Shui, None; D.C. Beebe, None
References
- 1.McCarty CA, Nanjan MB, Taylor HR. Attributable risk estimates for cataract to prioritize medical and public health action. Invest Ophthalmol Vis Sci. 2000;41:3720–3725. [PubMed] [Google Scholar]
- 2.Hammond CJ, Duncan DD, Snieder H, et al. The heritability of age-related cortical cataract: the Twin Eye Study. Invest Ophthalmol Vis Sci. 2001;42:601–605. [PubMed] [Google Scholar]
- 3.Hammond CJ, Snieder H, Spector TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 2000;342:1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 4.Heiba IM, Elston RC, Klein BE, Klein R. Genetic etiology of nuclear cataract: evidence for a major gene. Am J Med Genet. 1993;47:1208–1214. doi: 10.1002/ajmg.1320470816. [DOI] [PubMed] [Google Scholar]
- 5.Heiba IM, Elston RC, Klein BE, Klein R. Evidence for a major gene for cortical cataract. Invest Ophthalmol Vis Sci. 1995;36:227–235. [PubMed] [Google Scholar]
- 6.Iyengar SK, Klein BEK, Klein R, et al. Identification of a major locus for age-related cortical cataract on chromosome 6p12–q12 in the Beaver Dam Eye Study. Proc Natl Acad Sci USA. 2004;101:14485–14490. doi: 10.1073/pnas.0400778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 8.Beebe D. Lens. In: Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye. St Louis, MO: Mosby; 2003. pp. 117–158. [Google Scholar]
- 9.Augusteyn RC. Growth of the human eye lens. Mol Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- 10.Scammon RE, Hesdorfer MB. Growth in mass and volume of the human lens in postnatal life. Arch Ophthalmol. 1937;17:104–112. [Google Scholar]
- 11.Bours J, Fodisch H. Human fetal lens: wet and dry weight with increasing gestational age. Ophthalmic Res. 1986;18:363–368. doi: 10.1159/000265464. [DOI] [PubMed] [Google Scholar]
- 12.Brewitt B, Clark J. Growth and transparency in the lens, an epithelial tissue, stimulated by pulses of PDGF. Science. 1988;242:777–779. doi: 10.1126/science.3187521. [DOI] [PubMed] [Google Scholar]
- 13.Chen K, Zhou Y, Xing K, Krysan K, Lou MF. Platelet-derived growth factor (PDGF)-induced reactive oxygen species in the lens epithelial cells: the redox signaling. Exp Eye Res. 2004;78:1057–1067. doi: 10.1016/j.exer.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A alters lens growth and development. Dev Biol. 1996;180:554–565. doi: 10.1006/dbio.1996.0328. [DOI] [PubMed] [Google Scholar]
- 15.Ray S, Gao C, Wyatt K, et al. Platelet-derived growth factor D, tissue-specific expression in the eye, and a key role in control of lens epithelial cell proliferation. J Biol Chem. 2005;280:8494–8502. doi: 10.1074/jbc.M413570200. [DOI] [PubMed] [Google Scholar]
- 16.Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Chamberlain CG, McAvoy JW. IGF enhancement of FGF-induced fibre differentiation and DNA synthesis in lens explants. Exp Eye Res. 1996;63:621–629. doi: 10.1006/exer.1996.0156. [DOI] [PubMed] [Google Scholar]
- 18.Blanquet P, Patte C, Fayein N, Courtois Y. Identification and isolation from bovine epithelial lens cells of two basic fibroblast growth factor receptors that possess bFGF-enhanced phosphorylation activities. Biochem Biophys Res Commun. 1989;160:1124–1131. doi: 10.1016/s0006-291x(89)80120-2. [DOI] [PubMed] [Google Scholar]
- 19.Wormstone IM, Del Rio-Tsonis K, McMahon G, et al. FGF: an autocrine regulator of human lens cell growth independent of added stimuli. Invest Ophthalmol Vis Sci. 2001;42:1305–1311. [PubMed] [Google Scholar]
- 20.Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- 21.Reddan JR, Wilson-Dziedzic D. Insulin growth factor and epidermal growth factor trigger mitosis in lenses cultured in a serum-free medium. Invest Ophthalmol Vis Sci. 1983;24:409–416. [PubMed] [Google Scholar]
- 22.Reid T, Reid W. The labile nature of the insulin signal(s) for the stimulation of DNA synthesis in mouse lens epithelial and 3T3 cells. J Biol Chem. 1987;262:229–233. [PubMed] [Google Scholar]
- 23.Wickstrom K, Madsen K. The effect of transforming growth factor-alpha (TGF alpha) on rabbit and primate lens epithelial cells in vitro. Curr Eye Res. 1993;12:1123–1129. doi: 10.3109/02713689309033510. [DOI] [PubMed] [Google Scholar]
- 24.Ibaraki N, Lin LR, Reddy VN. Effects of growth factors on proliferation and differentiation in human lens epithelial cells in early subculture. Invest Ophthalmol Vis Sci. 1995;36:2304–2312. [PubMed] [Google Scholar]
- 25.Majima K. Human lens epithelial cells proliferate in response to exogenous EGF and have EGF and EGF receptor. Ophthalmic Res. 1995;27:356–365. doi: 10.1159/000267748. [DOI] [PubMed] [Google Scholar]
- 26.Ibaraki N, Lin LR, Reddy VN. A study of growth factor receptors in human lens epithelial cells and their relationship to fiber differentiation. Exp Eye Res. 1996;63:683–692. doi: 10.1006/exer.1996.0162. [DOI] [PubMed] [Google Scholar]
- 27.Ireland ME, Mrock LK. Expression and activation of the epidermal growth factor receptor in differentiating cells of the developing and post-hatching chicken lens. Exp Eye Res. 2004;79:305–312. doi: 10.1016/j.exer.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Ireland ME. Activated epidermal growth factor receptors in the adult human lens. Exp Eye Res. 2005;80:443–445. doi: 10.1016/j.exer.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Fleming TP, Song Z, Andley UP. Expression of growth control and differentiation genes in human lens epithelial cells with extended life span. Invest Ophthalmol Vis Sci. 1998;39:1387–1398. [PubMed] [Google Scholar]
- 30.Weng J, Liang Q, Mohan RR, Li Q, Wilson SE. Hepatocyte growth factor, keratinocyte growth factor, and other growth factor-receptor systems in the lens. Invest Ophthalmol Vis Sci. 1997;38:1543–1554. [PubMed] [Google Scholar]
- 31.Fitch CL, Swedberg SH, Livesey JC. Measurement and manipulation of the partial pressure of oxygen in the rat anterior chamber. Curr Eye Res. 2000;20:121–126. [PubMed] [Google Scholar]
- 32.Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–924. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 34.Shui Y-B, Fu J-J, Garcia C, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47:1571–1580. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- 35.Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2:161–164. [PubMed] [Google Scholar]
- 36.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giblin FJ, Padgaonkar VA, Leverenz VR, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995;60:219–235. doi: 10.1016/s0014-4835(05)80105-8. [DOI] [PubMed] [Google Scholar]
- 38.Harocopos GJ, Shui Y-B, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 39.Klein B, Klein R, Moss S. Correlates of lens thickness: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1998;39:1507–1510. [PubMed] [Google Scholar]
- 40.Klein BE, Klein R, Moss SE. Lens thickness and five-year cumulative incidence of cataracts: the Beaver Dam Eye Study. Ophthalmic Epidemiol. 2000;7:243–248. doi: 10.1076/opep.7.4.243.4176. [DOI] [PubMed] [Google Scholar]
- 41.Bassnett S, McNulty R. The effect of elevated intraocular oxygen on organelle degradation in the embryonic chicken lens. J Exp Biol. 2003;206:4353–4361. doi: 10.1242/jeb.00670. [DOI] [PubMed] [Google Scholar]
- 42.McLaren JW, Dinslage S, Dillon JP, Roberts JE, Brubaker RF. Measuring oxygen tension in the anterior chamber of rabbits. Invest Ophthalmol Vis Sci. 1998;39:1899–1909. [PubMed] [Google Scholar]
- 43.Kwan M, Niinikoski J, Hunt TK. In vivo measurements of oxygen tension in the cornea, aqueous humor, and anterior lens of the open eye. Invest Ophthalmol. 1972;11:108–114. [PubMed] [Google Scholar]
- 44.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu DY, Cringle SJ, Alder VA. The response of rat vitreal oxygen tension to stepwise increases in inspired percentage oxygen. Invest Ophthalmol Vis Sci. 1990;31:2493–2499. [PubMed] [Google Scholar]
- 46.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 47.Goda N, Dozier SJ, Johnson RS. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal. 2003;5:467–473. doi: 10.1089/152308603768295212. [DOI] [PubMed] [Google Scholar]
- 48.Galvin DJ, Watson RW, O’Neill A, et al. Hypoxia inhibits human bladder smooth muscle cell proliferation: a potential mechanism of bladder dysfunction. Neurourol Urodyn. 2004;23:342–348. doi: 10.1002/nau.20035. [DOI] [PubMed] [Google Scholar]
- 49.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 50.de Iongh RU, Lovicu FJ, Hanneken A, Baird A, McAvoy JW. FGF receptor-1 (flg) expression is correlated with fibre differentiation during rat lens morphogenesis and growth. Dev Dyn. 1996;206:412–426. doi: 10.1002/(SICI)1097-0177(199608)206:4<412::AID-AJA7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Kurosaka D, Nagamoto T. Inhibitory effect of TGF-β2 in human aqueous humor on bovine lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1994;35:3408–3412. [PubMed] [Google Scholar]
- 52.Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JEJ, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006;83:667–678. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2004;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Holekamp NM, Shui Y-B, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006;141:1027–1032. doi: 10.1016/j.ajo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Schaal S, Beiran I, Rubinstein I, Miller B, Dovrat A. Lenticular oxygen toxicity. Invest Ophthalmol Vis Sci. 2003;44:3476–3484. doi: 10.1167/iovs.03-0122. [DOI] [PubMed] [Google Scholar]
- 56.Dische Z, Zil HA. Studies on the oxidation of cysteine to cystine in lens proteins during cataract formation. Am J Ophthalmol. 1951;34:104–113. doi: 10.1016/0002-9394(51)90013-x. [DOI] [PubMed] [Google Scholar]
- 57.Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Bio-phys Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- 58.Spector A. The search for a solution to senile cataracts: Proctor lecture. Invest Ophthalmol Vis Sci. 1984;25:130–146. [PubMed] [Google Scholar]
- 59.Lohmann W, Schmehl W, Strobel J. Nuclear cataract: oxidative damage to the lens. Exp Eye Res. 1986;43:859–862. doi: 10.1016/s0014-4835(86)80015-x. [DOI] [PubMed] [Google Scholar]
- 60.Giblin FJ, Schrimscher L, Chakrapani B, Reddy VN. Exposure of rabbit lens to hyperbaric oxygen in vitro: regional effects on GSH level. Invest Ophthalmol Vis Sci. 1988;29:1312–1319. [PubMed] [Google Scholar]
- 61.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 62.Calvin H, Medvedovsky C, David J, et al. Rapid deterioration of lens fibers in GSH-depleted mouse pups. Invest Ophthalmol Vis Sci. 1991;32:1916–1924. [PubMed] [Google Scholar]
- 63.Shestopalov VI, Bassnett S. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J Cell Sci. 2000;113:1913–1921. doi: 10.1242/jcs.113.11.1913. [DOI] [PubMed] [Google Scholar]
- 64.Shestopalov VI, Bassnett S. Development of a macromolecular diffusion pathway in the lens. J Cell Sci. 2003;116:4191–4199. doi: 10.1242/jcs.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweeney MH, Truscott RJ. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- 66.Lou MF. Thiol regulation in the lens. J Ocul Pharmacol Ther. 2000;16:137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]
- 67.Reddy V. Glutathione and its function in the lens—an overview. Exp Eye Res. 1990;50:771–778. doi: 10.1016/0014-4835(90)90127-g. [DOI] [PubMed] [Google Scholar]
- 68.Moffat BA, Pope JM. Anisotropic water transport in the human eye lens studied by diffusion tensor NMR micro-imaging. Exp Eye Res. 2002;74:677–687. doi: 10.1006/exer.2001.1164. [DOI] [PubMed] [Google Scholar]
- 69.Moffat BA, Landman KA, Truscott RJ, Sweeney MH, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69:663–669. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- 70.Truscott RJ. Age-related nuclear cataract: a lens transport problem. Ophthalmic Res. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]