Abstract
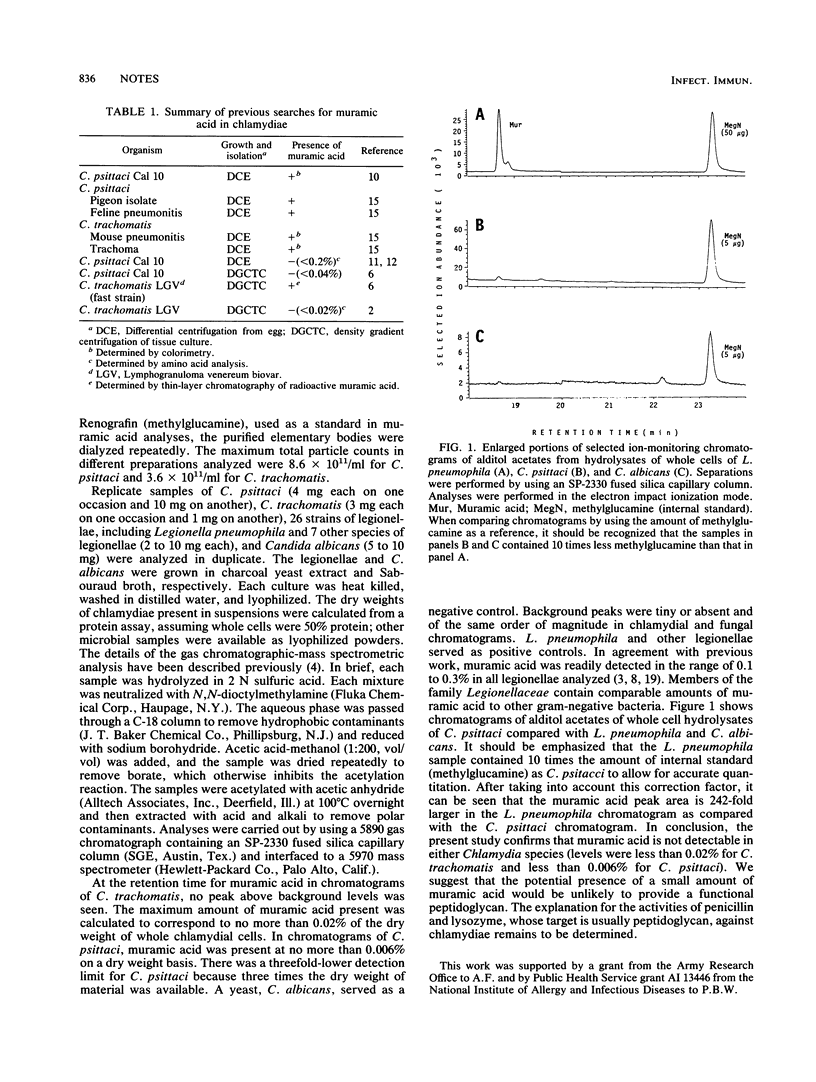
By using the powerful separation technique of capillary gas chromatography combined with the selectivity of mass spectrometric detection, muramic acid was not detectable in purified elementary bodies of Chlamydia psittaci Cal 10 (less than or equal to 0.006%) or C. trachomatis serovar E (less than or equal to 0.02%). This confirms previous reports which suggested the absence of a typical peptidoglycan in Chlamydia spp.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURKE D. C. The nucleic acid contents of viruses. J Gen Microbiol. 1962 Feb;27:181–194. doi: 10.1099/00221287-27-2-181. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Amano K., Hackstadt T., Perry L., Caldwell H. D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982 Jul;151(1):420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Lau P. Y., Brown A., Morgan S. L., Zhu Z. T., Lema M., Walla M. D. Capillary gas chromatographic analysis of carbohydrates of Legionella pneumophila and other members of the family Legionellaceae. J Clin Microbiol. 1984 Mar;19(3):326–332. doi: 10.1128/jcm.19.3.326-332.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Schwab J. H., Cochran T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect Immun. 1980 Aug;29(2):526–531. doi: 10.1128/iai.29.2.526-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Gilbart J., Fox A. Elimination of group A streptococcal cell walls from mammalian tissues. Infect Immun. 1987 Jun;55(6):1526–1528. doi: 10.1128/iai.55.6.1526-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbart J., Fox A., Morgan S. L. Carbohydrate profiling of bacteria by gas chromatography-mass spectrometry: chemical derivatization and analytical pyrolysis. Eur J Clin Microbiol. 1987 Dec;6(6):715–723. doi: 10.1007/BF02013084. [DOI] [PubMed] [Google Scholar]
- HURST E. W. Chemotherapy of virus diseases. Br Med Bull. 1953;9(3):180–185. doi: 10.1093/oxfordjournals.bmb.a074352. [DOI] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N., Morrison D. C. Lipopolysaccharide interaction with lysozyme. Binding of lipopolysaccharide to lysozyme and inhibition of lysozyme enzymatic activity. J Biol Chem. 1989 Mar 15;264(8):4434–4441. [PubMed] [Google Scholar]
- PERKINS H. R., ALLISON A. C. Cell-wall constituents of rickettsiae and psittacosis-lymphogranuloma organisms. J Gen Microbiol. 1963 Mar;30:469–480. doi: 10.1099/00221287-30-3-469. [DOI] [PubMed] [Google Scholar]
- Register K. B., Davis C. H., Wyrick P. B., Shafer W. M., Spitznagel J. K. Nonoxidative antimicrobial effects of human polymorphonuclear leukocyte granule proteins on Chlamydia spp. in vitro. Infect Immun. 1987 Oct;55(10):2420–2427. doi: 10.1128/iai.55.10.2420-2427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Preparation and chemical composition of the cell membranes of developmental reticulate forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1184–1188. doi: 10.1128/jb.94.4.1184-1188.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walla M. D., Lau P. Y., Morgan S. L., Fox A., Brown A. Capillary gas chromatography-mass spectrometry of carbohydrate components of legionellae and other bacteria. J Chromatogr. 1984 Apr 24;288(2):399–413. doi: 10.1016/s0021-9673(01)93716-1. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


