Abstract
Decreased hind limb pressure pain threshold (PPT) is an early indicator of insulinopenia and neuropathy developing in STZ-rat models of type 1 diabetes and pre-diabetes. To test if pain on pressure is also a hallmark of compensated insulin resistance and type 2 diabetes in this work we measured PPT of Zucker lean (ZL), fatty (ZF) and fatty diabetic rats (ZDF; 8 animals per group). Using clinically accepted cut-off values for diagnosis of human diabetes and pre-diabetes, at 6th week of age (the study entry), all animals maintained random blood glucose within a normal range (< 7.9 mM). Over the following 4 weeks, the random glucose remained normal in lean and ZF rats; it however crossed 11 mM cut-off for the diagnosis of diabetes in all ZDF rats. With no detectable relation to blood glucose levels or changes throughout the study, lean, ZF and ZDF rats maintained respectively highest, intermediate and lowest PPT levels (83 ± 1g, 70 ± 1g and 59 ± 1 g; mean values for all tests per group). Thus in Zucker rat model, type 2 diabetes-associated impairment of nerve function precedes the development of hyperglycemia. Furthermore, since normoglycemic, but displaying decreased PPT, ZF rats were strongly hyperinsulinemic (plasma insulin concentration 30 ± 4 ng/ml vs. 2.4 ± 0.3 ng/ml in lean rats) these data suggest that hyperinsulinemia compensating for glucose metabolism might not restore compromised nerve function.
Keywords: pre-diabetes, hyperglycemia, neuropathy, pressure pain, insulin resistance
Introduction
Diffuse peripheral neuropathy (DPN) is a frequent and severe complication of diabetes [1;2]. Hyperglycemia is considered as an important trigger of DPN; however whether it is a sole cause for the variety of symptoms of the disease is not known [2;3]. Pain on pressure is present in people with diabetes and DPN with 70% prevalence [4]. It is also one of most consistent and early sign of DPN observed in rat model of streptozotocin- (STZ) induced type 1 diabetes and Zucker and Sand rat models of spontaneous type 2 disease [5–7]. Recent studies in STZ- rats have shown that the onset of pain on pressure occurs during pre-diabetes, preceding fasting and postprandial hyperglycemia in this model [8;9]. In this work, to test if the early onset of exaggerated pain on pressure is a model- or type of diabetes- specific phenomenon, we have measured and compared characteristics of glucose metabolism and PPT in Zucker lean (ZL, control), Zucker fatty (ZF) and in Zucker diabetic fatty (ZDF) before and after spontaneous onset of overt hyperglycemia in the latter model.
Materials and Methods
All experiments were conducted in accord with National Institute of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by UAMS Animal Use Committee.
Five-weeks-old male ZL+/+, ZF/Gmi-fa/fa and ZDF/Gmi-fa/fa rats, 8 per group, were purchased from Charles River Genetic Models Inc. (Indianapolis, IN) and maintained at local animal facilities with free access to water and Purina 5008 rat chow. Experiments started after one week of animal acclimation to animal facilities and condition of behavioral setting and continued until the onset of diabetes (random glucose ≥ 11 mM) in the last of ZDF rats. On the same day the last PPT test was conducted and all animals were anesthetized for terminal blood sample collection.
During the experiment animal’s weight, random plasma glucose and PPT were determined on regular, 2–3 days intervals. In addition three determinations of fasting glucose and food tolerance tests were conducted and glycated hemoglobin A1c (HbA1c) was assayed in the beginning and at the end of experiments. In all these tests tail blood samples for glucose and HbA1c were collected using a pin-prick technique. Glucose was measured using the colorimetric Accu-Chek blood glucose monitoring system (Roche Diagnostics Corporation, Indianapolis) and HbA1c was determined using DCA 2000 Analyzer, (Bayer Corporation, Elkhart, IN). In food tolerance test, overnight fasted rats were placed in individual cages with the access to a standard (3 g/kg of rat weight) piece of Purina 5008 chaw, the amount that was completely consumed by a rat within 5–10 minutes. Glucose was measured at 30, 60, 90, and 120 minutes thereafter. Food instead of glucose tolerance test (FTT vs. GTT) was chosen because while GTT amplifies glucose intolerance, FTT is less stressful and it is more relevant with respect to measures of post-prandial hyperglycemia the state that is frequently implicated as an alternative to chronic hyperglycemia as a trigger of DPN [10–12]. Progression of diabetes-induced impairment of glucose metabolism in rats seems to closely mimic that in humans [2]. Therefore, the clinically-recommended random and fasting blood glucose cut-off values [13] are used in this work to define pre-diabetes and diabetes in rats.
In behavioral tests, dorsal hind-limb paw pressure pain withdrawal thresholds (PPT) were determined using Randal-Selitto analgesia-meter and a standard for our laboratory technique [8;9]. Briefly, 10 determinations of PPT per animal (5 per each hind limb with interval between sequential measurements greater than 10 minutes) were collected in each test session, filtered using mean +/− SD cut-off, averaged for both limbs and expressed in grams. Threshold force of linearly increasing pressure (16 g/s) was defined as a force that induces the first physical attempt of the animal to escape the stimulus. To avoid tissue injury the cut-off force was set to 250 g of pressure. In terminal experiments, blood was collected by a ventricular puncture technique, plasma separated by centrifugation (5000g, 5 min), and stored at −20C for insulin measurements. Insulin was determined using Ultra Sensitive Rat Insulin ELISA Kit (Crystal Chem Inc., Downers Grove, IL) following the manufactures protocol.
Unless stated otherwise, the results were analyzed using multi-variate repeated measures ANOVA (RM ANOVA) with rat’s age and group as independent and weight, glucose and PPT determinations as dependent variables, and Tukey HSD test for post-hoc comparisons. Effects were considered as statistically significant at p < 0.05.
Results
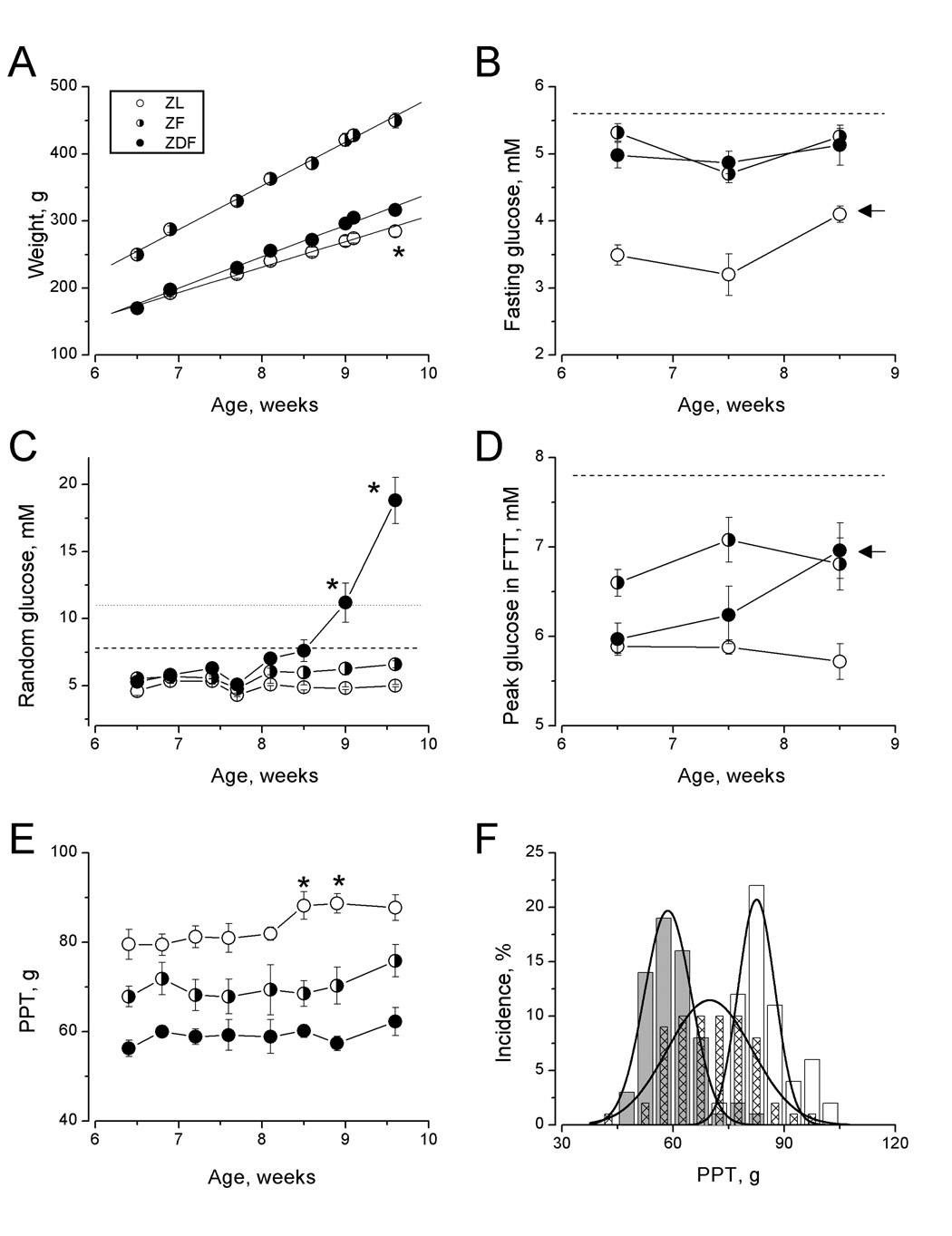
Throughout the study, ZF rats were heavier and gained weight faster than either ZDF or ZL rats (65 ± 2, 47 ± 1, and 38 ± 2 g/week, slopes of respective regression lines; Fig. 1A). Different weight gains by ZL and ZDF rats has translated into a significant difference in terminal weights of these animals (Fig.1A, asterisk, Tukey HSD test, p<0.05).
Figure 1.
Weight (A), fasting (B), random (C), and peak in FTT (D) plasma glucose and pressure pain thresholds (by age (E) and frequency distributions (F)) of Zucker lean, pre-diabetic fatty and diabetic fatty rats (ZL, ZF and ZDF, open circles/bars, half-shaded circles/hatched bars, filled circles/grey bars, respectively). Asterisks indicate statistical difference (Tukey test, p<0.05) for the given day of study between ZL vs. ZDF rats (A), ZDF vs. ZL and ZF rats (C) and ZL vs. ZDF rats (E). Arrows denote statistical difference of given measurement from baseline (first) measurement for the ZL (B) and ZDF (D) groups of rats. Lines in (A) are regression lines. Horizontal lines represent diagnostic cut-off limits: fasting glucose >5.6 mM (B, dashed line) or 2-h glucose in OGTT >7.9 mM (dashed lines in C and D) for pre-diabetes and 2-h glucose in OGTT for pre-diabetes and random glucose > 11mM for diabetes (dotted line in C). Curves in (F) are best-fit to the data Gaussian curves (Origin 7.0 Non-linear fit tool, OriginLab Corporation, Northampton, MA).
With regard to glucose metabolism, ZL rats maintained the lowest levels of all measured indexes, fasting and random glucose, and peak glucose in food tolerance test (Fig 1, B,C and D; open circles; for any given group of rats behavior of peak and 2-hr glucose in FTT was identical, therefore data on 2-hr glucose are not presented). Except for small but statistically significant increase in fasting glucose in 8.5-week-old animals (Fig 1B, arrow) no age-dependent changes in characteristics of glucose metabolism was detected in ZL rats (RM-ANOVA, p>0.05). Probably reflecting a raise in fasting glucose, the integral measure of glycemic history [14], the level of HbA1C has increased in ZL rats from 2.79 ±0.03 to 3.01 ± 0.01%, although the difference does not reach statistical significance (p=0.69, Tukey test).
When comparing with ZL rats, ZF rats displayed fasting hyperglycemia and glucose intolerance (manifested by heightened peak glucose in FTT; Fig.1 B,D and F; half-filled circles). These abnormalities were mild, always remaining below diagnostic of state of impaired fasting glucose or impaired glucose tolerance thresholds (5.6 mM or 7.9 mM, respectively, [13]; and dashed lines in Fig. 1B and D). Throughout the first half of experiment, ZL and ZF animals maintained the same levels of random glucose. Starting at the 8th week of age, random glucose of ZF rats slowly increased above the level in ZL rats. By Tukey HSD test however, even on the last day of experiment the difference between ZF and ZL rats random glucose did not approach the level of statistical significance (p>0.05; Fig 1C). Glycated hemoglobin increased from 2.8 ± 0.1% to 3.5 ± 0.2% (p<0.01, Tukey test).
Among studied animals, ZDF rats demonstrated the most prominent versatility in levels and changes of parameters of glucose metabolism (Fig. 1B–F; filled circles). Similarity to ZF, ZDF rats steadily maintained a mild degree of fasting hyperglycemia (Fig. 1B). Unlike that in ZF rats, peak and 2-hr glucose in FTT in ZDF rats were not different from those measured in ZL rats in the beginning of the study. However, both parameters of glucose intolerance started to increase in 7.5-week-old ZDF rats and by the 9.5-week-old ZDF rats they were statistically higher than those of ZL rats and not different from those of ZF animals (Fig. 1 D, arrow). Finally in the beginning of experiment, similarly to the other groups of animals, ZDF rats had low “normal” levels of random glucose and HbA1c (2.8 ± 0.1%), but starting in the 9th week of age all ZDF animals developed severe random hyperglycemia and diabetes, and by the end of the study the average glucose level in these animals was 19 ± 2 mM and mean HbA1c level was 4.3 ± 0.1% (the average age of onset of diabetes, defined as random plasma glucose equal or exceeding 11 mM, was 9.2 +/− 0.2 weeks, Fig. 1C).
Considering current views on the nature of the abnormalities of fasting glucose and the response to an oral glucose challenge [15], our data suggest that 6 – 10-week-old ZF rats maintain mild liver and skeletal muscle insulin resistance, while young, pre-diabetic ZDF rats constitute a model of maintained liver and progressive skeletal muscle insulin resistance. Furthermore by terminal tests, the ZF and ZDF rats maintained the same, 26 ± 3 ng/ml and 25 ± 5 ng/ml (p > 0.05, two-population t-test; 2.4 ± 0.2 ng/ml for ZL rats) levels of compensatory hyperinsulinemia. Therefore, it is likely not the failure of insulin production but the deterioration of muscle sensitivity to insulin constituted the major reason for the progression of ZDF rats to overt hyperglycemia in these experiments.
Hyperglycemia is undoubtedly an important factor of neuropathic complications of diabetes [3]. Therefore, perhaps the most novel and intriguing result of our work is that despite their very different glycemic state ZL, ZF and ZDF rats maintained throughout this study, respectively, highest, intermediate and lowest pressure pain thresholds (83 ± 1g, 70 ± 1g and 59 ± 1 g; mean values for all tests per group; Fig. 1E; p<0.01 for between-group comparisons by RM ANOVA; F = 129 (df = 2,168)). Furthermore, despite the progression of ZDF animals from the state of isolated mild fasting hyperglycemia to glucose intolerance and overt chronic hyperglycemia, the intensity of pressure pain in these animals remained without change (compare Fig.1 C and E). Post-hoc Tukey test has confirmed statistical significance (p<0.05) of differences of PPT measured in ZDF and ZL animals for each age point studied. By the same test however, ZF rats represent a transitional state with their PPT being not different from either ZL or ZDF rats at any but 8.5 and 9-weeks age points at which PPT 9 of ZL and ZF rats are different at p<0.05 (Fig. 1E). Such transitional state of ZF rats is also confirmed by the comparison of distributions of PPT measured in these, ZL and ZDF rats thought the study (Fig. 1F).
Discussion
In U.S., the number of cases of type 2 diabetes, the decrease of carbohydrate, lipid and protein metabolism associated with the impairment of peripheral sensitivity to the action of insulin (insulin resistance), has approached an epidemic scale [13;16]. Diffuse peripheral neuropathy is one of most severe and debilitating complications of diabetes; the natural causes of this complication are however not determined and treatment options are limited [1]. Chronic hyperglycemia affects endoneurial circulation and function of glial cells and neurons [3], and therefore control of blood glucose is a required step in treatment of diabetic complications [17]. However, intensive insulin replacement therapy fails to prevent new DPN in about 30% of treated type 1 diabetic patients and has even less efficacy toward neuropathy in type 2 diabetic patients [18;19]. Accumulating reports of increased prevalence of DPN in people with mild glucose intolerance and/or fasting hyperglycemia (see [20]), further suggest that some symptoms of this disease may be triggered by conditions other than chronic hyperglycemia. Identification of these non-glycemic triggers of DPN is of obvious importance [2].
Studied in this work, pain on pressure is the sign of abnormal sensitivity of group III and IV muscle nociceptors [21;22] that correlates with the presence of deep chronic aching pain symptom of DPN in people with diabetes [4]. It is also one of most consistent signs of DPN observed in rat models of overt hyperglycemia and type 1 and 2 diabetes [5–7;9]. Specifically after about 6 weeks of diabetes, PPT were decreased in Sand rat (Psammomys obesus) to about 80% and in ZDF rats to about 70% of respective age-matched controls [6;7]. Our study, in which PPT of ZDF rats was 71 ± 3% of that measured in ZL rats (Fig. 1E), confirms these observations. The novel finding of our work is this level of increased pressure pain is maintained in ZDF rats starting during pre-diabetes and without detectable changes during the progression of ZDF animals from glucose-tolerant to intolerant state and then to overt hyperglycemia and diabetes. Exaggerated sensitivity to pressure was also found in stably pre-diabetic, ZF rats displaying mild fasting hyperglycemia and glucose intolerance but without progression to diabetes (Fig 1E and F, mean PPT in ZF rats is 84 ± 4% of that in lean animals). Importantly, questioning the role of postprandial hyperglycemia as a factor (see [20]), while as compared to ZF animals, young ZDF rats had lower peak glucose in FTT they manifested stronger pain on pressure. Thus these data support the existence of non-glycemic triggers of certain aspects of diabetic impairment of function of PNS [2;23]. Finally, the point of mild degree of impairment of glucose metabolism in pre-diabetic Zucker rats was emphasized repeatedly throughout the paper. Indeed, clinical pre-diabetes is defined as a state of impaired fasting glucose and/or impaired glucose tolerance (fasting or 2-hr OGTT plasma glucose > 5.6 mM or 7.9 mM, respectively, [13]) and in none of studied ZF or young ZDF rats fasting glucose or peak post-meal glucose has reached these limits (dashed lines in Fig. 1B and D). The important implication of this observation is that in the natural history of diabetes, some pro-neuropathic conditions may exist as early as at the time of “pre-clinical” pre-diabetes.
Considering the putative reason for PPT differences detected in this study, it is important that Zucker lean, fatty and diabetic fatty rats are genetically uniform animals with exception of the presence or absence of two independent autosomal recessive genetic defects [24]. First defect is homozygous, fa/fa leptin receptor mutation leading to obesity, peripheral insulin resistance and compensatory hyperinsulinemia. Unlike Fa/Fa or Fa/fa (ZL rats), both ZF and ZDF animals have the fa/fa genotype and, starting at 3–4 weeks of age, insulin resistance. The second defect is a deficiency in β-cell gene transcription, which when occur on a background of fa/fa genotype results in failure of compensatory insulin production and progression of the disease to the stage of overt hyperglycemia (inbred from ZF-strain ZDF rat [24–27]). Thus, insulin resistance appears as a major common denominator distinguishing studied in this work 6 – 10 week-old ZF and ZDF rats from their lean counterparts. Pressure pain develops in STZ-rat models of type 1 pre-diabetes and diabetes, and in these models it was attributed to the direct effect of declining action of insulin in peripheral nervous system [2;9]. By analogy, it can be suggested that pressure hyperalgesia in type 2 pre-diabetes results from peripheral insulin resistance and the failure of targeting glucose homeostasis compensatory insulin production to meet the requirements of insulin-resistant PNS [2]. This suggestion however, requires verification by PPT measurements in other rat models of insulin resistance, such as female ZDF rats that progress from pre-diabetes to overt diabetes when maintained on high-fat diet only [28] or perhaps more importantly in an outbred (Sprague-Dawley, for example) rat with diet-induced insulin resistance [29]. Furthermore, the role of other non-glycemic factors, such as nerve effects of dyslipidemia, hyperinsulinemia and declining support by insulin-like growth factors (reviewed in [2;23]), has not been addressed in our work and awaits future studies in Zucker and other rat models of type 2 pre-diabetes and diabetes. Finally our work was focused on the measurements of PPT and pre-diabetic age of ZDF rats. Therefore, further studies in ZDF rats are needed to determine if prolonged period of overt hyperglycemia may augment pre-diabetic, non-glycemic pressure pain and to assess the role of glycemic and non-glycemic factors in the pathogenesis observed in ZDF rat manifestations of DPN other than mechanical hyperalgesia such as the slowing of nerve conduction velocity and heat hypoalgesia [30;31].
Acknowledgements
This work was supported by NIH NIDDK (grant DK067284) and by UAMS COM funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic Somatic Neuropathies. Diabetes Care. 2004;27:1458–1483. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 2.Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: Triggers and mechanisms. World J. Gastroenterol. 2007;13:175–191. doi: 10.3748/wjg.v13.i2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat. Rev. Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 4.Otto M, Bak S, Bach FW, Jensen TS, Sindrup SH. Pain phenomena and possible mechanisms in patients with painful polyneuropathy. Pain. 2003;101:187–192. doi: 10.1016/s0304-3959(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang HX, Snyder CK, Pu SF, Ishii DN. Insulin-like growth factors reverse or arrest diabetic neuropathy: effects on hyperalgesia and impaired nerve regeneration in rats. Exp. Neurol. 1996;140:198–205. doi: 10.1006/exnr.1996.0129. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang H-X, Wuarin L, Fei Z-J, Ishii DN. Insulin-like growth factor (IGF) gene expression is reduced in neural tissues and liver from rats with non-insulin-dependent diabates mellitus, and IGF treatment ameliorates diabetic neuropathy. J. Pharmacol. Exp. Ther. 1997;283:366–374. [PubMed] [Google Scholar]
- 7.Wuarin-Bierman L, Zahnd GR, Kaufmann F, Burcklen L, Adler J. Hyperalgesia in spontaneous and experimental animal models of diabetic neuropathy. Diabetologia. 1987;30:653–658. doi: 10.1007/BF00277324. [DOI] [PubMed] [Google Scholar]
- 8.Romanovsky D, Hastings SL, Stimers JR, Dobretsov M. Relevance of hyperglycemia to early mechanical hyperalgesia in streptozotocin-induced diabetes. J. Peripher. Nerv. Syst. 2004;9:62–69. doi: 10.1111/j.1085-9489.2004.009204.x. [DOI] [PubMed] [Google Scholar]
- 9.Romanovsky D, Cruz NF, Dienel GA, Dobretsov M. Mechanical hyperalgesia correlates with insulin deficiency in normoglycemic streptozotocin-treated rats. Neurobiol. Dis. 2006;24:384–394. doi: 10.1016/j.nbd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Owens DR, Wragg KG, Briggs PI, Luzio S, Kimber G, Davies C. Comparison of the metabolic response to a glucose tolerance test and a standardized test meal and the response to serial test meals in normal healthy subjects. Diabetes Care. 1979;2:409–413. doi: 10.2337/diacare.2.5.409. [DOI] [PubMed] [Google Scholar]
- 11.Russell JC, Graham SE, Dolphin PJ. Glucose tolerance and insulin resistance in the JCR:LA-corpulent rat: effect of miglitol (Bay m1099) Metabolism. 1999;48:701–706. doi: 10.1016/s0026-0495(99)90168-3. [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJ, Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve. 2007;36:536–541. doi: 10.1002/mus.20846. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2008;31:S55–S78. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 14.Ko GT, Chan JC, Yeung VT, Chow CC, Tsang LW, Li JK, So WY, Wai HP, Cockram CS. Combined use of a fasting plasma glucose concentration and HbA1c or fructosamine predicts the likelihood of having diabetes in high-risk subjects. Diabetes Care. 1998;21:1221–1225. doi: 10.2337/diacare.21.8.1221. [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 16.Steinbrook R. Facing the diabetes epidemic--mandatory reporting of glycosylated hemoglobin values in New York City. N. Engl. J. Med. 2006;354:545–548. doi: 10.1056/NEJMp068008. [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy : A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2006;49:1711–1721. doi: 10.1007/s00125-006-0316-2. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann. Intern. Med. 1995;122:561–568. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 20.Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. The Neurologist. 2008;14:23–29. doi: 10.1097/NRL.0b013e31815a3956. [DOI] [PubMed] [Google Scholar]
- 21.Simone DA, Marchettini P, Caputi G, Ochoa JL. Identification of muscle afferents subserving sensation of deep pain in humans. J. Neurophysiol. 1994;72:883–889. doi: 10.1152/jn.1994.72.2.883. [DOI] [PubMed] [Google Scholar]
- 22.Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. The effect of differential and complete nerve block on experimental muscle pain in humans. Muscle Nerve. 1999;22:1564–1570. doi: 10.1002/(sici)1097-4598(199911)22:11<1564::aid-mus12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Ishii DN. Implication of insulin-like growth factors in the pathogenesis of diabetic neuropathy. Brain Res. Brain Res. Rev. 1995;20:47–67. doi: 10.1016/0165-0173(94)00005-a. [DOI] [PubMed] [Google Scholar]
- 24.Griffen SC, Wang J, German MS. A genetic defect in beta-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes. 2001;50:63–68. doi: 10.2337/diabetes.50.1.63. [DOI] [PubMed] [Google Scholar]
- 25.Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, Sun X, Polonsky KS, Bell GI. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 26.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000;49:684–688. doi: 10.1016/s0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 27.Blonz ER, Stern JS, Curry DL. Dynamics of pancreatic insulin release in young Zucker rats: a heterozygote effect. Am. J. Physiol. 1985;248:E188–E193. doi: 10.1152/ajpendo.1985.248.2.E188. [DOI] [PubMed] [Google Scholar]
- 28.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis. 2000;148:231–241. doi: 10.1016/s0021-9150(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 29.Otukonyong EE, Dube MG, Torto R, Kalra PS, Kalra SP. High-fat diet-induced ultradian leptin and insulin hypersecretion are absent in obesity-resistant rats. Obes. Res. 2005;13:991–999. doi: 10.1038/oby.2005.116. [DOI] [PubMed] [Google Scholar]
- 30.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Adebara ET, Yorek MA. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition to reverse. Diabetes Obes. Metab. 2008;10:64–74. doi: 10.1111/j.1463-1326.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 31.Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am. J. Physiol Endocrinol. Metab. 2005;289:E113–E122. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]