Abstract
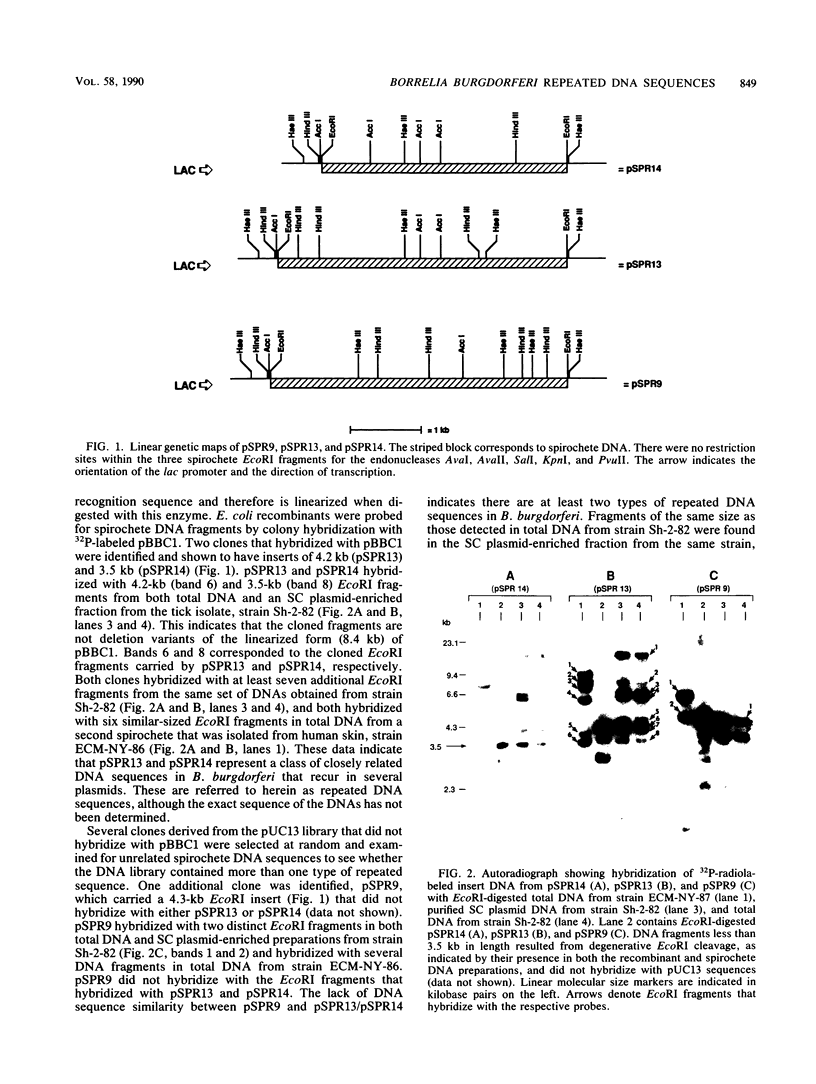
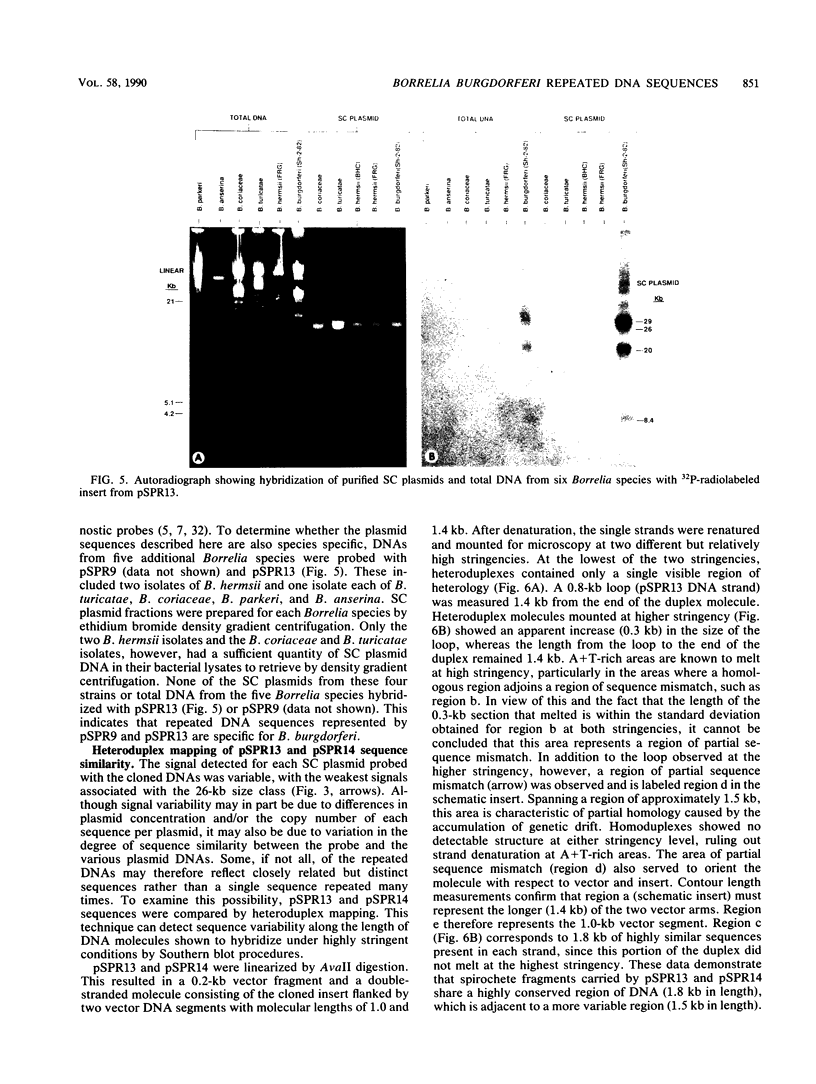
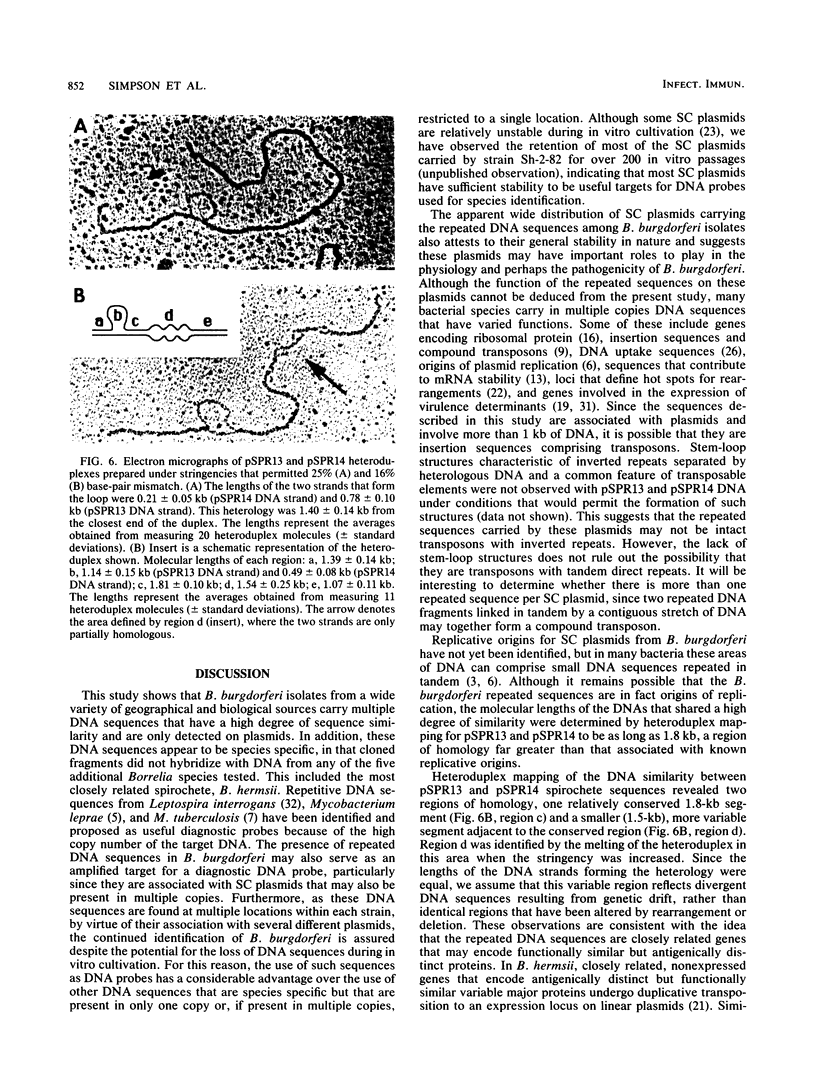
Borrelia burgdorferi, the causative agent of Lyme borreliosis, contains linear and supercoiled circular (SC) plasmids. Because SC plasmids are present in multiple copies, these plasmids were examined for species-specific sequences that could serve as high-copy-number target DNAs for a diagnostic probe. Three EcoRI fragments (4.3, 4.2, and 3.5 kilobase pairs [kb]) that hybridized with multiple DNA fragments from B. burgdorferi were identified and cloned from a SC plasmid-enriched fraction. The 4.2- and 3.5-kb fragments were similar in that they hybridized with each other and with similar-sized EcoRI fragments from two unrelated strains of B. burgdorferi. The 4.3-kb fragment did not hybridize with the other two cloned sequences. Both types of sequences hybridized with most of the SC plasmids in seven B. burgdorferi isolates, whereas only a single 49-kb linear plasmid, found in two of the seven strains tested, hybridized with the cloned sequences. None of the cloned sequences hybridized with chromosomal DNA from B. burgdorferi or with total DNA or SC plasmids from Borrelia hermsii, B. turicatae, B. coriaceae, B. parkeri, or B. anserina. These data indicate that the repeated DNA sequences described in this study appear to be plasmid associated and specific to B. burgdorferi. Heteroduplexes formed from the 4.2- and 3.5-kb fragments showed that hybridizing regions in each fragment comprise a 1.8-kb conserved region that is adjacent to a 1.5-kb region that exhibits greater sequence variability. The sequence divergence seen in the variable region is likely the result of genetic drift and may mean that these regions represent closely related genes that encode functionally similar but antigenically distinct proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Docherty M. A. A species-specific repetitive sequence in Mycobacterium leprae DNA. J Infect Dis. 1989 Jan;159(1):7–15. doi: 10.1093/infdis/159.1.7. [DOI] [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Crawford J. T., Bates J. H. Repetitive DNA sequences as probes for Mycobacterium tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2240–2245. doi: 10.1128/jcm.26.11.2240-2245.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Petersen L. L. An improved method for the isolation of supercoiled DNA molecules using ion-exchange column chromatography. Gene Anal Tech. 1987 Jan-Feb;4(1):5–8. doi: 10.1016/0735-0651(87)90006-9. [DOI] [PubMed] [Google Scholar]
- Habicht G. S., Beck G., Benach J. L. Lyme disease. Sci Am. 1987 Jul;257(1):78–83. doi: 10.1038/scientificamerican0787-78. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., McLaren R. S., Newbury S. F. Repetitive extragenic palindromic sequences, mRNA stability and gene expression: evolution by gene conversion? A review. Gene. 1988 Dec 10;72(1-2):3–14. doi: 10.1016/0378-1119(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Hyde F. W., Johnson R. C. Characterization of a circular plasmid from Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol. 1988 Oct;26(10):2203–2205. doi: 10.1128/jcm.26.10.2203-2205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl S., Yamamoto M., Nomura M. Mapping of a cluster of genes for components of the transcriptional and translational machineries of Escherichia coli. J Mol Biol. 1977 Jan 5;109(1):23–47. doi: 10.1016/s0022-2836(77)80044-2. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Steere A. C. Lyme disease: infectious in origin, rheumatic in expression. Adv Intern Med. 1986;31:147–166. [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Minges C. G., Titus J. A., Strohl W. R. Plasmid DNA in colorless filamentous gliding bacteria. Arch Microbiol. 1983 Jan;134(1):38–44. doi: 10.1007/BF00429404. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J., Schrumpf M. E., Karstens R. H. Identification of Borrelia burgdorferi and B. hermsii using DNA hybridization probes. J Clin Microbiol. 1989 Aug;27(8):1734–1738. doi: 10.1128/jcm.27.8.1734-1738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Cleary P. P. Expression of M type 12 protein by a group A streptococcus exhibits phaselike variation: evidence for coregulation of colony opacity determinants and M protein. Infect Immun. 1987 Oct;55(10):2448–2455. doi: 10.1128/iai.55.10.2448-2455.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Spanier J. G., Cleary P. P. A DNA substitution in the group A streptococcal bacteriophage SP24. Virology. 1983 Oct 30;130(2):514–522. doi: 10.1016/0042-6822(83)90104-6. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Comstock L. E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989 Apr;57(4):1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wokke J. H., van Gijn J., Elderson A., Stanek G. Chronic forms of Borrelia burgdorferi infection of the nervous system. Neurology. 1987 Jun;37(6):1031–1034. doi: 10.1212/wnl.37.6.1031. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Escherichia coli heat-labile enterotoxin genes are flanked by repeated deoxyribonucleic acid sequences. J Bacteriol. 1981 Feb;145(2):850–860. doi: 10.1128/jb.145.2.850-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner R. L., Bolin C. A. Repetitive sequence element cloned from Leptospira interrogans serovar hardjo type hardjo-bovis provides a sensitive diagnostic probe for bovine leptospirosis. J Clin Microbiol. 1988 Dec;26(12):2495–2500. doi: 10.1128/jcm.26.12.2495-2500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]