Abstract
Background
Glycemic exposure activates 12-lipoxygenase (12LO) expression and formation of arachidonic acid-derived products. These products can induce cell hypertrophy, cell proliferation and extracellular matrix deposition, potentially leading to diabetic nephropathy (DN).
Study Design
cross-sectional study.
Settings & Participants
955 European American siblings from 369 Diabetes Heart Study families. Participants were categorized as: non-diabetic, diabetic with hemoglobin A1c < 6.5%, and diabetic with hemoglobin A1c > 6.5% (uncontrolled T2DM).
Predictor
Four haplotype-tagging variants in the arachidonate 12LO gene (ALOX12), glycemic control, and other covariates.
Outcomes & Measurements
Albuminuria measured by urinary albumin:creatinine ratio (ACR).
Results
The median ACR was 11.9 mg/g (interquartile range, 5.6–39.1). The overall test of the Arg261Gln genotypic association with ACR was significant (p=0.009). Compared to the 261Arg allele carriers, adjusted mean ACR was 42% higher among the 189 carriers of two 261Gln alleles (95% confidence interval, 10% to 83%; p = 0.007). This association was confined to the uncontrolled T2DM group (N=623) with the highest ACR values (P<0.001). Adjustments for additional determinants of ACR yielded similar results.
Limitations
Urine ACR was measured in duplicate on only a single occasion. This study was limited to European Americans.
Conclusions
Consistent with animal and cellular studies, these results provide further evidence of the importance of the 12LO pathway in the pathogenesis of human DN.
Keywords: lipoxygenase, ALOX12 gene, albuminuria, diabetic nephropathy, genetics, type 2 diabetes mellitus
INTRODUCTION
Albuminuria is associated with cardiovascular disease (CVD) and progressive chronic kidney disease. 1 Elevated urinary albumin excretion is an early and cardinal feature of diabetic nephropathy (DN), a microvascular complication observed in a minority of individuals with long-standing hyperglycemia. The association of suboptimal glycemic control and increased risk of albuminuria has been observed in several intervention studies.2;3 Two large clinical trials, the UK Prospective Diabetes Study and the Diabetes Control and Complications Trial (DCCT) reported that proportional reductions in the risk of microvascular and macrovascular complications accompanied reductions in glycated hemoglobin (HbA1c) over the entire spectrum of HbA1c values.4;5 A more intensive HbA1c goal of ≤ 6.5% has recently been recommended by the American College of Endocrinology6 and the International Diabetes Federation.7 Despite widespread promotion of the benefit of good glycemic control, most diabetes patients do not achieve recommended treatment goals. Current therapeutic options increase the risk of hypoglycemia and non-compliance, and numerous other barriers to intensive glycemic control are present in almost every aspect of diabetes care,8 A better understanding of the mechanisms underlying diabetes-related complications is needed to develop new treatment options to improve diabetes outcomes and target the subgroup at high risk of diabetes complications.
A matrix of factors, including hyperglycemia, advanced glycation end-product formation, oxidant stress, and growth factors (angiotensin II, platelet-derived growth factor, and transforming growth factor-beta), are thought to be associated with DN.9 However, renal complications develop in one-third of European American individuals with diabetes despite chronically elevated blood sugar, blood pressure and serum lipids. Genetic factors appear to play a role in the development of DN as individual responses to hyperglycemia vary. Our group has previously reported familial aggregation of urinary albumin excretion among diabetic families.10 While the precise molecular and signal transduction mechanisms responsible for DN are not fully understood, emerging research implies that the lipoxygenase (LO) pathway plays a major role in renal disease susceptibility.11;12
DN is histologically characterized by thickening of glomerular basement membranes, glomerular hypertrophy and deposition of extracellular matrix in the mesangium.9 In animals, several lines of evidence suggest that 12-lipoxygenase (12LO) is significantly associated with hyperglycemia-induced renal disease.11 In rat mesangial cells and mouse podocytes, 12LO mRNA and protein expression were increased more than 2-fold when cells were exposed to high extracellular glucose concentrations.13;14 In untreated diabetic rat kidneys, glomerular 12LO mRNA expression was more than 4-fold higher compared to control rats, with intermediate expression observed in treated diabetic rats.13 Mesangial cells cultured from 12/15-LO knockout mice (12/15LO is a single locus in the mouse) grow more slowly and synthesize less of the extracellular matrix protein fibronectin in the basal state and in response to angiotensin II, compared to wild-type mice.12 It appears that the stimulated release of 12LO arachidonic acid-derived products, 12(S)-hydroxyeicosatetraenoic acids (HETE), can directly induce cellular hypertophy13 and expression of extracellular fibronectin in mesangial cells.15 The association between urinary 12-HETE/creatinine and urinary albumin:creatinine ratio (ACR) in rats has been found to be highly significant (r = 0.79, p<10−5).9 These data suggest a potential role of 12LO in the pathogenesis of DN.
In humans, corresponding data are scarce. Compared to healthy controls, urinary 12-HETE is increased in diabetic patients with normal renal function, with the highest amounts present in diabetic individuals with albuminuria.16 These results suggest that hyperglycemia induces activity of arachidonate 12-lipoxygenase gene (ALOX12), the human homolog of 12LO, which may contribute to the development of DN. Therefore, we hypothesized that variation in ALOX12 could influence susceptibility to albuminuria, particularly in those exposed to hyperglycemia.
A non-synonymous polymorphism in the ALOX12 gene on chromosome 17p13.1, leading to an arginine to glutamine substitution at amino acid 261 (p.Arg261Gln), has been associated with bipolar disorder in Brazilians.17 Additional polymorphisms in the ALOX12 gene have recently been identified through re-sequencing. To our knowledge, none of these polymorphisms have been studied with respect to albuminuria or CVD related phenotypes. The Diabetes Heart Study (DHS) is composed of predominantly European American families containing multiple members with type 2 diabetes mellitus (T2DM). We examined the association of ALOX12 gene polymorphisms with albuminuria in DHS families, as well as their interaction with glycemic exposure.
METHODS
Study Design and Population
The DHS is conducted in Forsyth County, North Carolina, to study the genetic epidemiology of cardiovascular and renal disease in families containing multiple members with T2DM. Since January 1998, siblings concordant for T2DM have been recruited, along with one additional non-diabetic sibling, where possible.10 T2DM was defined as diabetes developing after age 34 and treated with insulin and/or oral agents, in the absence of historic evidence of ketoacidosis. Medications were reviewed to corroborate that the clinical diagnosis of T2DM was severe enough to require drug therapy. Non-diabetic siblings had fasting blood glucose levels <126 mg/dl (7.0 mmol/L). Socio-demographic data, age, sex, cigarette smoking, and duration of T2DM were attained by interviewer-administered questionnaire at baseline. Diabetic cases reporting known chronic renal failure or receiving renal replacement therapy were not recruited. Urinary ACR and ALOX12 polymorphisms were measured on 955 European American siblings from 369 DHS families. The study was approved by the Institutional Review Board at the Wake Forest University School of Medicine, and all participants provided written informed consent.
Laboratory Measurements
At enrollment, study participants presented fasting to the General Clinical Research Center at the Wake Forest University School of Medicine for measurement of urine ACR from a spot morning urine sample, blood pressure, serum chemistries (including serum creatinine), hemoglobin A1c, fasting glucose and lipids. HbA1c values were measured using high performance liquid chromatography.
Urine albumin was measured on a Model 1650 Advia (Bayer Diagnostics, Tarrytown, NY) using an automated immunoturbidity analysis. Urine creatinine was measured using the picric acid reaction on the Advia. In an alkaline medium, creatinine reacts with picric acid to form a yellow-orange complex. Rate of color formation is proportional to the concentration of creatinine present and is measured photo-metrically at 505 nm. The urine ACR was calculated using measurement of urinary albumin and creatinine.
Genomic DNA was extracted from peripheral lymphocytes. Based on DNA variations in the ALOX12 gene from the re-sequencing data of 24 African Americans and 23 Europeans,18 four ALOX12 haplotype-tagging single nucleotide polymorphisms (SNPs) (rs2292350:G>A, rs1126667:G>A [p.Arg261Gln], rs11078659:A>G, and rs2271316:G>C) were selected for evaluation in European Americans. These four polymorphisms were genotyped using a MassARRAY® Single Nucleotide Polymorphism (SNP) genotyping system (Sequenom, Inc., San Diego, CA).
Statistical Analysis
Each polymorphism was tested for departure from Hardy-Weinberg proportions using a chi-square goodness of fit test. The pairwise linkage disequilibrium (LD) coefficients, r2, were calculated.19 The distributions of urine ACR were highly skewed; thus, a natural logarithm transformation was applied to ACR in order to better approximate the distributional assumptions of conditional normality and homogeneity of variance.
In light of the absence of a glycemic threshold for the development of diabetic complications, we selected a HbA1c cutoff point of 6.5% in order to classify participants. Participants were categorized as: non-diabetic (non-T2DM), well controlled diabetic with hemoglobin A1c < 6.5% (controlled T2DM), and poorly controlled diabetic with hemoglobin A1c ≥ 6.5% (uncontrolled T2DM). Models were developed by these three strata.
To account for familial correlation in analyses of demographic and genetic data, generalized estimating equations (GEE1), assuming exchangeable correlations and using a robust estimator of the variance, were calculated for all analyses (SAS version 8.0, SAS Institute Inc., Cary, NC). The multivariate GEE1 models adjusted for potential non-genetic risk factors for ACR. P values < 0.05 were considered statistically significant. Haplotypes of ALOX12 were configured on the pedigree data (under the assumption that there were no recombination events between the polymorphisms) using the Zaplo program.20 Posterior probabilities of haplotypes for a subject, conditional on the observed marker data, were used as weights in the GEE1 models.
To minimize the type 1 error rate, an approach similar to Fisher’s protected least significant difference (LSD) multiple comparisons procedure was used.21 First, the genotype-based two degree-of-freedom general association test was computed. If this test of general association was significant, then the three a priori genetic models (i.e. dominant, additive and recessive) were tested without further adjustment for multiple comparisons. If the general test of association was not significant, then the three a priori genetic models were tested after making a sequential Bonferroni adjustment for the three comparisons.
To avoid the bias of population admixture by arising from population-based association study, the family-based association test (FBAT) was performed to assess association of transmitting a SNP allele to a child from a parent with the quantitative trait, ACR. Residuals of ACR adjusted for potential non-genetic risk factors for ACR, including age, sex, smoking, systolic and diastolic blood pressure, presence and duration of T2DM, in multivariate linear regression models was used as the phenotypes in FBAT.
RESULTS
Table 1 shows the demographic and clinical characteristics of the 955 participants according to their degree of glycemic control. There were 162 (17%) participants that were non-T2DM, 170 controlled T2DM, and 623 uncontrolled T2DM. Based on the cutoff HbA1c level of 6.5%, 79% of T2DM participants had poor glycemic control (less than the 87% that was reported by European Diabetes Policy Group8). The median ACR (interquartile range [IQR]) was 11.9 mg/g (5.6–39.1) overall. Compared to participants with controlled T2DM, uncontrolled T2DM participants tended to be slightly younger, more often had pre-existing CVD, and had longer diabetes durations and higher urine ACR.
Table 1.
Characteristics of 955 European American participants in Diabetes Heart Study, by glycemic environment.
| Characteristic | Non-T2DM (N=162) |
Controlled T2DM (N=170) |
Uncontrolled T2DM (N=623) |
p-value* for 3 group comparison |
p-value* uncontrolled Vs controlled |
|---|---|---|---|---|---|
| Women (%) | 62% | 52% | 51% | 0.05 | 0.9 |
| Mean (SD) Age, yr | 59.3 (10.1) | 63.3 (8.9) | 61.7 (9.3) | <0.001 | 0.05 |
| Cigarette Smoking | |||||
| Current Smokers (%) | 21% | 15% | 16% | ||
| Former Smokers (%) | 36% | 49% | 41% | 0.1 | 0.2 |
| Mean Body Mass Index (SD), kg/m2 | 29.1 (5.1) | 31.6(6.6) | 32.7 (6.9) | <0.001 | 0.06 |
| Mean duration of diabetes (SD), yr | 0 | 9.0 (7.1) | 10.7 (7.3) | <0.001 | 0.007 |
| % self-reported CVD | 15% | 28% | 37% | <0.001 | 0.04 |
| % Lipid lowering medication | 28% | 46% | 45% | <0.001 | 0.7 |
| % angiotensin-converting enzyme | 25% | 58% | 60% | <0.001 | 0.6 |
| inhibitor or angiotensin II receptor blocker | |||||
| Mean SBP (SD), mmHg | 135 (19) | 139 (18) | 140 (19) | 0.01 | 0.4 |
| Mean DBP (SD), mmHg | 74 (11) | 72 (11) | 73 (10) | 0.2 | 0.5 |
| Mean cholesterol (SD), mg/dL† | 194(34) | 183 (42) | 188 (43) | 0.04 | 0.1 |
| Mean HbA1c (SD), % | 5.6 (0.5) | 5.9 (0.4) | 8.2 (1.7) | <0.001 | <0.001 |
| Median (range) | 5.6 (3.5–7.4) | 6.0 (4.3–6.4) | 7.8 (6.5–18.3) | ||
| Mean serum creatinine (SD), mg/dL | 1.0 (0.2) | 1.1 (0.2) | 1.1 (0.3) | 0.01 | 0.7 |
| Median ACR (IQR), mg/g‡ | 7 (3–13) | 12 (5–28) | 16 (6–47) | <0.001 | <0.001 |
By chi-square, ANOVA, or Kruskal-Wallis test.
To convert values for cholesterol to millimoles per liter, multiply by 0.02586.
Albuminuria measured by urinary albumin:creatinine ratio.
Abbreviations: CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; ACR, albumin:creatinine ratio.
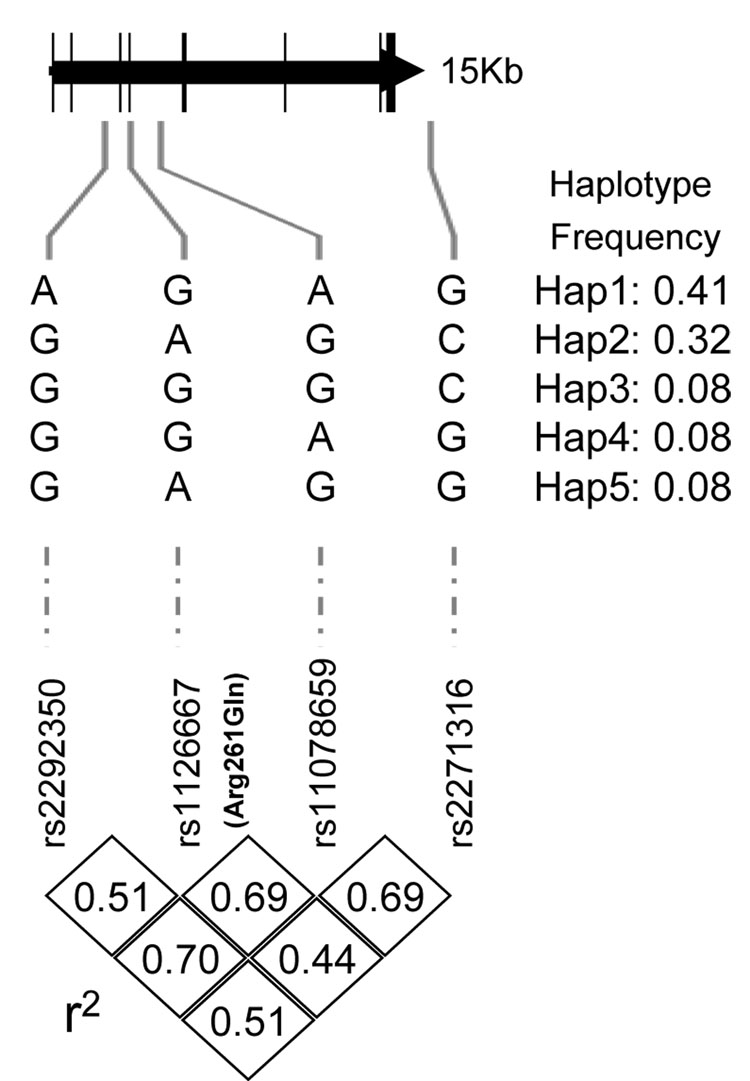
The four ALOX12 SNPs are in high linkage disequilibrium and belong to a single haplotype block, defined using the Confidence Interval Method22 (Figure 1). Five haplotypes were inferred using these four polymorphisms. Allele frequencies presented in the table 2 were assessed in the 369 unrelated probands and were consistent with Hardy-Weinberg expectations. The frequency of 261Gln allele (0.42 in European Americans) was similar to that in another recent report.17 The four SNPs were not statistically associated with presence of diabetes.
Figure 1.
Positions and linkage disequilibrium structure of 4 SNPs in ALOX12 gene. Exons are shaded gray.
Table 2.
Unadjusted mean log-transformed ACR, according to ALOX12 SNP genotype.
| dbSNP | PGA* | Alleles 1/2 |
Allele 2 freq. |
Mean lnACR (SE) |
p value† | ||
|---|---|---|---|---|---|---|---|
| 1/1 | 1/2 | 2/2 | |||||
| rs2292350 | 4216 | G/A‡ | 0.41 | 3.00 (0.08) | 2.92 (0.08) | 2.87 (0.11) | 0.4 |
| rs1126667 | 5304 | G/A | 0.42 | 2.97 (0.09) | 2.81 (0.06) | 3.16 (0.12) | 0.009 |
| rs11078659 | 6488 | G/A | 0.50 | 2.89 (0.10) | 2.88 (0.07) | 3.06 (0.10) | 0.5 |
| rs2271316 | 17947 | G/C | 0.43 | 3.03 (0.12) | 2.96 (0.07) | 2.89 (0.09) | 0.3 |
SeattleSNPs Programs for Genomic Applications (PGA) identifier
The Wald chi (2 d.f.) test for global effect with adjustment for age, sex, smoking, and history of T2DM.
Nucleotide substitutions: rs2292350:G>A, rs1126667:G>A [p.Arg261Gln], rs11078659:A>G, and rs2271316:G>C
Table 2 demonstrates that the mean log-transformed urine ACR significantly differed among the genotypes of the p.Arg261Gln polymorphism (Poverall [2 d.f]: 0.009), but not for the other three polymorphisms. Association analysis for the p.Arg261Gln polymorphism with ACR under the three genetic models (dominant, additive, recessive) suggested that the distribution of mean lnACR best fit a recessive mode of inheritance. GEE1 analysis adjusted for age, sex, and smoking in recessive association model revealed that the 261Gln allele was associated with increased ACR (an increase of 42% in ACR for homozygotes of the 261Gln allele compared to Arg carriers; P = 0.007). Further adjustment for diabetes duration, HbA1c, systolic and diastolic blood pressure, cholesterol concentration, prevalent CVD, and use of ACE inhibitors, angiotensin II receptor blockers, and lipid-lowering medications did not substantially alter the results (P = 0.02).
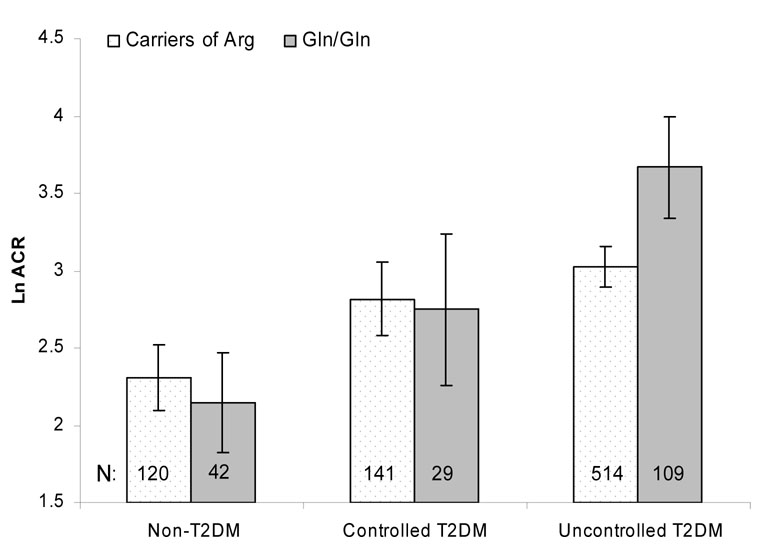
Figure 2 demonstrates the unadjusted log-transformed ACR by Arg261Gln genotype and glycemic exposure. Regardless of genotype, participants with uncontrolled T2DM had the highest ACR and non-T2DM participants had the lowest ACR. The association of the Arg261Gln polymorphism with albuminuria was significant in the uncontrolled T2DM group, but not in the other two subgroups.Table 3 reveals that adjustment for age, sex, and smoking indicated similar results. Homozygosity for the 261Gln allele (compared to individuals with at least one 261Arg allele) was associated with a 91% increase in ACR among participants with uncontrolled T2DM (p < 0.001). Urine ACR in uncontrolled T2DM carriers of two 261Gln alleles was 4-fold higher compared to that in non-T2DM carriers for 261Gln (p<0.001). The genetic association in the group with uncontrolled T2DM differed significantly from that in the non-T2DM group (p = 0.002) and the controlled T2DM group (p = 0.02).
Figure 2.
Crude lnACR means and their 95% confidence intervals according to the p.Arg261Gln genotype and glycemic control.
Table 3.
Association of ALOX12 SNP and haplotypes with urine ACR in a recessive model†.
| Genotype/ Haplotype |
Comparison group |
Non-T2DM (N=162) |
Controlled T2DM (N=170) |
Uncontrolled T2DM (N=623) |
Pinteraction Uncontrolled Vs Non-T2DM |
Pinteraction Uncontrolled Vs Controlled 2DM |
|||
|---|---|---|---|---|---|---|---|---|---|
| eβ (95% CI) | p | eβ (95% CI) | p | eβ (95% CI) | p | ||||
| Homozygous | Homozygous | 0.74 | 0.1 | 1.01 | 0.9 | 1.91 | <0.001 | 0.002 | 0.02 |
| A at | G or | (0.49, 1.11) | (0.62, 1.64) | (1.35, 2.66) | |||||
| rs1126667* | heterozygous | ||||||||
| at rs1126667 | |||||||||
| 2 copies | 0 or 1 copy | 1.02 | 0.9 | 1.04 | 0.9 | 0.79 | 0.2 | 0.7 | 0.5 |
| of Hap1 | of Hap1 | (0.17,6.14) | (0.56, 1.93) | (0.57, 1.09) | |||||
| (AGAG) | |||||||||
| 2 copies | 0 or 1 copy | 0.45 | 0.08 | 1.20 | 0.6 | 2.04 | 0.001 | 0.002 | 0.2 |
| of Hap2 | of Hap2 | (0.19, 1.10) | (0.61, 2.35) | (1.33, 3.13) | |||||
| (GAGC) | |||||||||
Adjusted for age, sex, and smoking.
rs1126667:G>A encodes p.Arg261Gln
The 4-SNP Haplotype (rs2292350, rs1126667 [p.Arg261Gln], rs11078659, and rs2271316) analyses revealed similar patterns as the single-locus analyses (Table 3). Of the five haplotypes, hap3, hap4, and hap5 were less frequent (frequency=0.08) and the number of carriers of two identical haplotypes was small; thus, their recessive effect could not be reliably estimated. Table 3 demonstrates that hap1, combining the most frequent alleles at each polymorphic site, was not related to urine ACR in any of the three subgroups. Hap2, containing the 261Gln allele, was positively associated with ACR in the uncontrolled T2DM group (p = 0.001), but not in the non-T2DM and controlled T2DM groups. The positive association of Hap2 with ACR was primarily driven by the single-locus association of the p.Arg261Gln polymorphism.
The distribution of urine ACR was different in males and females,23 thus, sex-specific associations were examined. The positive association of the 261Gln allele with urine ACR was observed in both males (an increase of 108% in ACR for homozygotes of the 261Gln allele; P = 0.006) and females (an increase of 75% in ACR for homozygotes of the 261Gln allele; P = 0.008) with uncontrolled T2DM. Because the uncontrolled T2DM group had slightly longer diabetes duration than the controlled T2DM group, we evaluated whether the genetic effect observed in uncontrolled T2DM was robust in the presence of presumed short-lived hyperglycemia. Among the 108 participants with uncontrolled T2DM who were diagnosed within the prior five years, urine ACR was 2-fold higher in 261Gln homozygotes (n=13), compared to carriers of 261Arg (p = 0.05).
The large group of individuals with poorly controlled hyperglycemia allowed us to stratify them further. There were 333 individuals with HbA1c level in between 6.5% and 8.0%, and 290 individuals with HbA1c level greater than 8.0%. Homozygosity for the 261Gln allele was associated with a 0.73 fold increase in ACR among participants with HbA1c level in between 6.5% and 8.0% (p = 0.003), and was associated with a 2.1 fold increase in ACR among participants with HbA1c level greater than 8.0%, (p = 0.006). The finding that association was even stronger in those with higher HbA1c further supports the modifying effect of hyperglycemia.
Finally, the findings of the population-based GEE1 analysis were examined using FBAT, the family-based association method which is less powerful but can avoid the potential confounding of population stratification. Under recessive genetic models, the positive LD between the 261Gln allele and ACR was not significant overall, but marginally significant in those with poorly controlled hyperglycemia (p = 0.07) and significant in those with HbA1c level greater than 8.0% (p = 0.02).
DISCUSSION
In this cohort of European American families enriched for the presence of T2DM, poorer glycemic control was associated with greater degrees of albuminuria. Although the presence of two copies of the 261Gln allele in ALOX12 appeared to be associated with an excess risk of albuminuria overall, we determined that this genetic predisposition to albuminuria was confined to the subset of T2DM study participants with poor glycemic control. The genetic association in the group with uncontrolled T2DM differed significantly from that in the non-T2DM group and the controlled T2DM group indicates an environmental-genetic interaction between prolonged hyperglycemia and p.Arg261Gln. These results suggest that the ALOX12 gene plays an important role in the pathogenesis of human DN.
In vivo and in vitro studies have demonstrated the 12/15LO activation induced hypertrophy and extracellular matrix synthesis in mesangial cell and podocytes, processes associated with the development and progression of albuminuria. The lipoxygenase pathway could potentially mediate the glomerular changes in DN through several mechanistic pathways.12 ALOX12 is expressed in multiple cell types involved in atherogenesis, including platelets, leukocytes, monocyte/macrophages, smooth muscle cells, and endothelial cells. Data from animal models demonstrate the key role of the lipoxygenase pathway in the pathogenesis of atherosclerosis, development of recurrent vascular stenosis and hypertension.12;24 Clinical data reveal that urinary 12-HETE excretion is markedly elevated in diabetic patients, particularly in those with albuminuria. The results of the current study further support the role of ALOX12 in development of albuminuria in response to hyperglycemia.
Two common coding variants have been identified in human ALOX12. The p.Arg261Gln (rs1126667:G>A in exon 6) and p.Ser322Asn (corresponding to rs434473:A>G in exon 8) polymorphisms are in almost complete linkage disequilibrium (r2, 1) and both reside in the lipoxygenase domain, one of the most important and conserved regions of this protein.17 Based on the 3-dimensional structure of a homologous protein,25 these two amino acids lie on the surface of the protein and may either alter the structure of ALOX12 or affect its ability to interact with other proteins. The potential functional significance of the two amino acids is supported by a recent Chinese study report that individuals with the 261Gln/Gln genotype had higher platelet 12-lipoxygenase activity (mean+/−SEM nmol/mg/min) than those with the 261Arg/Arg genotype (0.405+/− 0.047 versus 0.136+/−0.022; P = 0.001).26 Our study only reported the effect of the substitution of an arginine to a glutamine (p.Arg261Gln) on the risk of albuminuria. The p.Arg261Gln polymorphism and the haplotype carrying the 261Gln allele were associated with higher values of ACR, particularly in the group with uncontrolled T2DM. We also genotyped the p.Ser322Asn allele, and the association results were nearly identical to those for p.Arg261Gln. One or both of these polymorphisms may represent the functional alleles, as no other non-synonymous coding variants of this protein have been identified in European Americans.26a However, their effect may also be mediated through variants in regulatory regions as the observed LD is strong throughout the region. Based on sequence alone, p.Arg261Gln is more likely to have an overall effect on the protein since this is a non-conservative change of a positively charged side group (Arg) to a polar, uncharged side group (Gln). The presence of a glutamine at amino acid position 261 seems to be ancestral; as it is a consensus among the majority of mammals. This suggests that fixation of the allele coding for an arginine in this position in humans is a recent event.17 Future studies are needed to provide functional data directly supporting the p.Arg261Gln variant’s contribution to albuminuria.
Several limitations in this study need to be acknowledged. Urine ACR was measured in duplicate on only a single occasion. Variation in ACR is known to occur when frequent measurements are performed.27 However, unmeasured within-individual variation would be expected to reduce the magnitude of the association. Additionally, we classified glycemic control based upon a single measurement of HbA1c. HbA1c provides an average of serum glucose measurements over the preceding six to twelve weeks. Therefore, our classification of overall glycemic control was based upon recent data and provides a rough approximation of longer term control. Further, this study was limited to European Americans, so the ability to generalize these findings to other ethnic groups is uncertain. Lastly, the FBAT was marginally significant in those with poorly controlled hyperglycemia, even though it was significant in those with HbA1c level greater than 8.0%. The less significant association when analyzed using FBAT is likely due to the lack of power on the sibling data since inference of parental genotypes in an unknown mixture of populations is not straightforward and only families with at least two children with differing marker genotypes were included in the FBAT analyses.
In summary, the presence of homozygosity for the 261Gln allele in hyperglycemia independently predicts greater degrees of albuminuria in European Americans with poorly controlled type II diabetes. Our findings emphasize the importance of environmental factors, such as optimal glycemic control, on the risk for DN. The genetic risk of ALOX12 appeared to be mitigated in environments of well controlled blood sugar. However, more than 79% of the 18.2 million diabetic individuals in the U.S. have poorly controlled blood glucose (HbA1c > 7.0%).28;29 Therefore, interactive effects between ALOX12 gene polymorphisms and hyperglycemia on susceptibility to albuminuria (and ultimately DN) would appear to have a considerable impact on the public health. However, replication is essential for establishing the credibility of a genotype-phenotype association. The complex involvement of ALOX12 gene polymorphism in diabetic nephropathy warrants further investigation in other studies of type 2 diabetes. If confirmed, the findings would provide clear evidence of the role of 12LO pathway in DN. ALOX12 genotyping could assist in identification of diabetic patients who are at high risk for the subsequent development of DN and allow for targeted intensification of glycemic control. Identification of 12LO as an important constituent in the complex pathogenesis of DN could lead to the development of novel pharmacologic targets in nephropathy prevention.
ACKNOWLEDGEMENTS
We thank the patients, staff, and physicians who participated in the DHS.
Support: This study was supported in part by the General Clinical Research Center of the Wake Forest University School of Medicine grant M01 RR07122 and National Institutes of Health grants R01 HL67348 (Dr Bowden) and R01 HL071141 (Dr Hederick).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None.
REFERENCES
- 1.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Association of Clinical Endocrinologists. American College of Endocrinology Consensus Statement on Guidelines for Glycemic Control. Endocr Pract. 2002 Feb 2;8 suppl 1:5–11. [Google Scholar]
- 7.A desktop guide to Type 2 diabetes mellitus. European Diabetes Policy Group 1999. Diabet Med. 1999;16:716–730. [PubMed] [Google Scholar]
- 8.Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151 Suppl 2:T3–T7. doi: 10.1530/eje.0.151t003. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Natarajan R, LaPage J, et al. 12/15-lipoxygenase inhibitors in diabetic nephropathy in the rat. Prostaglandins Leukot Essent Fatty Acids. 2005;72:13–20. doi: 10.1016/j.plefa.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Langefeld CD, Beck SR, Bowden DW, et al. Heritability of GFR and albuminuria in Caucasians with type 2 diabetes mellitus. Am J Kidney Dis. 2004;43:796–800. doi: 10.1053/j.ajkd.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Kasinath BS. Lipoxygenases in renal injury--loading the matrix. Kidney Int. 2003;64:1918–1919. doi: 10.1046/j.1523-1755.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Reddy MA, Lanting L, et al. Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney Int. 2003;64:1702–1714. doi: 10.1046/j.1523-1755.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang SW, Adler SG, Nast CC, et al. 12-lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int. 2001;59:1354–1362. doi: 10.1046/j.1523-1755.2001.0590041354.x. [DOI] [PubMed] [Google Scholar]
- 14.Kang SW, Natarajan R, Shahed A, et al. Role of 12-lipoxygenase in the stimulation of p38 mitogen-activated protein kinase and collagen alpha5(IV) in experimental diabetic nephropathy and in glucose-stimulated podocytes. J Am Soc Nephrol. 2003;14:3178–3187. doi: 10.1097/01.asn.0000099702.16315.de. [DOI] [PubMed] [Google Scholar]
- 15.Reddy MA, Adler SG, Kim YS, et al. Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. Am J Physiol Renal Physiol. 2002;283:F985–F994. doi: 10.1152/ajprenal.00181.2002. [DOI] [PubMed] [Google Scholar]
- 16.Antonipillai I, Nadler J, Vu EJ, et al. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab. 1996;81:1940–1945. doi: 10.1210/jcem.81.5.8626861. [DOI] [PubMed] [Google Scholar]
- 17.Fridman C, Ojopi EP, Gregorio SP, et al. Association of a new polymorphism in ALOX12 gene with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2003;253:40–43. doi: 10.1007/s00406-003-0404-y. [DOI] [PubMed] [Google Scholar]
- 18.Carlson CS, Eberle MA, Rieder MJ, et al. Additional SNPs and linkage-disequilibrium analyses are necessary for whole-genome association studies in humans. Nat Genet. 2003;33:518–521. doi: 10.1038/ng1128. [DOI] [PubMed] [Google Scholar]
- 19.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 20.O'Connell JR. Zero-recombinant haplotyping: applications to fine mapping using SNPs. Genet Epidemiol. 2000;19 Suppl 1:S64–S70. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Milliken GA, Johnson DE. Van Nostrand Reinhold. New York: 1984. Analysis of Messy Data: Vol I: Designed Experiments. [Google Scholar]
- 22.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 23.Warram JH, Gearin G, Laffel L, et al. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 24.Reilly KB, Srinivasan S, Hatley ME, et al. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 25.Gillmor SA, Villasenor A, Fletterick R, et al. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol. 1997;4:1003–1009. doi: 10.1038/nsb1297-1003. [DOI] [PubMed] [Google Scholar]
- 26a.SeattleSNPs Programs for Genomic Applications. ALOX12:arachidonate 12-lipoxygenase. Available at http://pga.gs.washington.edu/data/alox12/. Accessed on: [Google Scholar]
- 26.Guo Y, Zhang X, Tan W, et al. Platelet 12-lipoxygenase Arg261Gln polymorphism: functional characterization and association with risk of esophageal squamous cell carcinoma in combination with COX-2 polymorphisms. Pharmacogenet Genomics. 2007;17:197–205. doi: 10.1097/FPC.0b013e328010bda1. [DOI] [PubMed] [Google Scholar]
- 27.Perkins BA, Ficociello LH, Silva KH, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 28.Davidson JA. Treatment of the patient with diabetes: importance of maintaining target HbA(1c) levels. Curr Med Res Opin. 2004;20:1919–1927. doi: 10.1185/030079904X6291. [DOI] [PubMed] [Google Scholar]
- 29.Supplement 1. American Diabetes Association: clinical practice recommendations 2000. Diabetes Care. 2000;23 Suppl 1:S1–S116. [PubMed] [Google Scholar]