Abstract
The GSH:GSSG ratio, which is the primary determinant of the cellular redox state, becomes progressively more pro-oxidizing during the aging process due to an elevation in the GSSG content and a decline in the ability for de novo GSH biosynthesis. The Km of glutamate-cysteine ligase (GCL), the rate-limiting enzyme in de novo GSH biosynthesis, significantly increases during aging, which would adversely affect the ability for rapid GSH biosynthesis, especially under stressful conditions. Experimental studies suggest that age-related accumulation of homocysteine, an intermediate in the tran-sulfuration pathway, may be responsible for causing the loss of affinity between GCL and its substrates. Over-expression of GCL has been shown to prolong the life span of Drosophila by up to 50 %, suggesting that perturbations in glutathione metabolism play a causal role in the aging process.
Keywords: glutathione, redox state, redox potential, glutathiolation, mitochondria, oxidative stress, aging
1. Introduction
An invariable feature of the life cycle of all multi-cellular organisms is that following a period of growth and reproduction there is a gradual decline in physiological fitness, and the ability to maintain homeostasis is progressively whittled, which inevitably culminates in the death of the organism. The nature of the mechanisms causing the various age-associated physiological decrements or those that determine the vast differences in the maximum life spans of various species are presently poorly understood. Among the many candidate causal hypotheses, one that has gained considerable interest postulates that senescent physiological changes emanate from the deleterious effects of reactive oxygen species (ROS), generated during cellular metabolism. This hypothesis, often referred to as the “oxidative stress hypothesis”, was originally advanced in 1956 [1], an era when there was relatively little understanding of the mechanisms of ROS production or the nature of their interactions with biomacromolecules. Nevertheless, considerable progress has since been made in understanding the role of ROS in the aging process, primarily due to the contemporaneous advances in the biochemistry of ROS. The main focus of current investigations is the elucidation of the mechanism by which ROS cause attenuations in specific cellular functions, which govern the progression of senescence and determine the species- specific maximum life spans.
2. Oxidative Stress, redox state and the aging process
Historically, ROS were generally regarded as potentially toxic and physiologically costly products of aerobic metabolism [2, 3]. Their formation was assigned to some intrinsic biochemical and/or structural flaws(s), requiring considerable expenditure of metabolic resources for their detoxification. In this view, ROS served no useful function, and if not totally eliminated by the anti-oxidant defenses, they would inflict random macromolecular damage, whose accumulation with age would cause functional losses. The mere existence of an elaborate array of anti-oxidant defenses was taken as evidence for the potential toxicity of ROS as well as for the physiological necessity of their elimination. Nevertheless, more recent evidence has suggested that the initial view of ROS being nothing but potentially toxic was overly simplistic[4–6]. There is mounting evidence that ROS, such as superoxide anion radical, hydrogen peroxide, carbon monoxide and nitric oxide, play an essential role in a variety of physiological processes, such as cellular proliferation and differentiation, gene regulation, and anti-bacterial defenses, among others. Because ROS can be simultaneously useful as well as hazardous, it has thus become quite problematic to experimentally establish a cause-and-effect relationship of their involvement in the aging process, by either globally enhancing the anti-oxidant defenses or by lowering the production of ROS. For instance, expression of ectopic catalase in mitochondrial matrix in combination with over-expression of Mn, superoxide dismutase, which results in a decrease in mitochondrial hydrogen peroxide production, was found to shorten rather than prolong the life span of Drosophila [7]. Such data strongly suggest that enhancements of anti-oxidant defenses do not necessarily retard the aging process. It thus seems that while basal levels of ROS generation are physiologically essential, excessive and/or unregulated production causes aging- and disease-associated alterations.
It is now widely recognized that in aerobic cells a dynamic equilibrium is maintained between oxidants and the anti-oxidant defenses. Because steady-state amounts of the molecular products of ROS attacks can be detected in young healthy animals, it has been inferred that ROS elimination by anti-oxidants does not achieve a level of 100% efficiency, thus cells exist under a certain level of oxidative stress (reviewed in[8]). A large body of evidence indicates that the magnitude of the imbalance between the antioxidant defenses and the oxidants, or the level of oxidative stress, widens during the aging process, progressing exponentially in the last trimester of life [9]. Studies on whether an elevation in the rate of ROS production, or a decline in the efficiency of anti-oxidant defenses, is responsible for the age-associated increase in oxidative stress, suggest that the anti-oxidant defenses do not keep pace with the age-related increases in ROS production [8]. Thus the balance between ROS and anti-oxidants shifts progressively towards a more pro-oxidant state. Furthermore, it is not only during the aging phase that there is an upsurge in the rate of ROS generation, there are other such increases when cells transition from a growth to a differentiated state [4, 5]. Thus during ontogeny there is a series of pro-oxidizing shifts in the cellular redox state [6]. Accordingly, aging process can be viewed as a manifestation of physiological inability to control both the production of ROS and infliction of damage by them. A progressive increase in the level of oxidative stress is postulated to exert a broad deteriorative effect on cellular physiology.
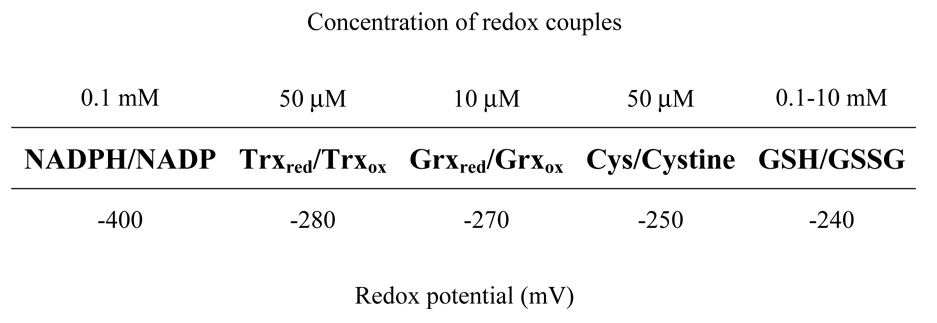
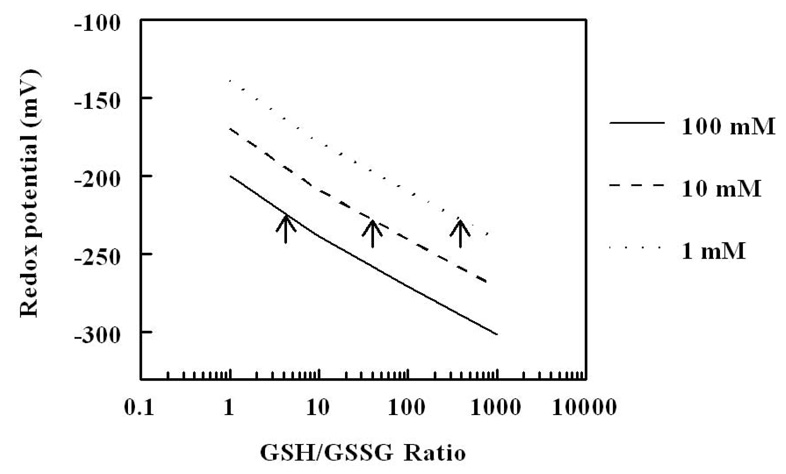
The oxidant/anti-oxidant balance is quantitatively reflected in the redox state, which, in turn, is dependent upon the ratios of the inter-convertible forms (reduced and oxidized) of redox couples, such as GSH/GSSG, NADH/NAD+, NADPH/NADP+, cysteine/cystine, thioredoxinred/thioredxinoxid, and glutaredoxinred/glutaredoxinoxid; however, GSH:GSSG couple is considered to be the primary intracellular determinant of the redox state, because it is 3 to 4 orders of magnitude more abundant than the other redox couples and because it has low standard redox potential (Eo=−240 mV) (Fig. 1). The critical functional component of GSH is the thiol moiety on the cysteinyl residue, which can act both as a reductant and a nucleophile. Furthermore, GSH is also tightly linked to other redox couples, either metabolically or because of its ability to form thiol-glutathione mixed disulfides with small molecules as well as with proteins [10–12]. The redox potential of GSH/GSSG couple is dependent upon two factors: the amounts of GSH and GSSG and the ratio between GSH and GSSH (Fig. 2). The reduction/oxidation reactions of glutathione involve two electron transfers:
This also underlies the main difference between glutathione redox couple and the others, all of which involve a one electron transfer reactions.
Figure 1.
A scale showing the relative redox potentials of glutathione (GSH/GSSG), cysteine (Cys/Cystine), thioredoxin (Trxred/Trxox), glutaredoxin (Grxred/Grxox) and NADPH/NADP+ redox couples and their intra-cellular concentrations.
Figure 2.
Redox potential of glutathione as a function of GSH/GSSG ratio at three different hypothetical concentrations of glutathione (1, 10 and 100 mM). Note that the same redox potential (for example E= −225 mV, indicated by arrow heads) can be achieved by variations in the concentration of glutathione and GSH/GSSG ratio.
3. Glutathione homeostasis
The intra-cellular concentration of GSH (γ-L-glutamylcysteinylglycine), a tripeptide, often approaches millimolar amounts in mammals; however, GSH concentration varies in different cellular compartments, implying that distinct intracellular redox microenvironments exist within different organelles. For instance, GSH levels are much lower in the nucleus than in the cytoplasm, but the nuclear GSH content increases during cell division [13]. There is increasing realization that intracellular redox compartmentalization is maintained dynamically in relation to the functional states of the organelles.
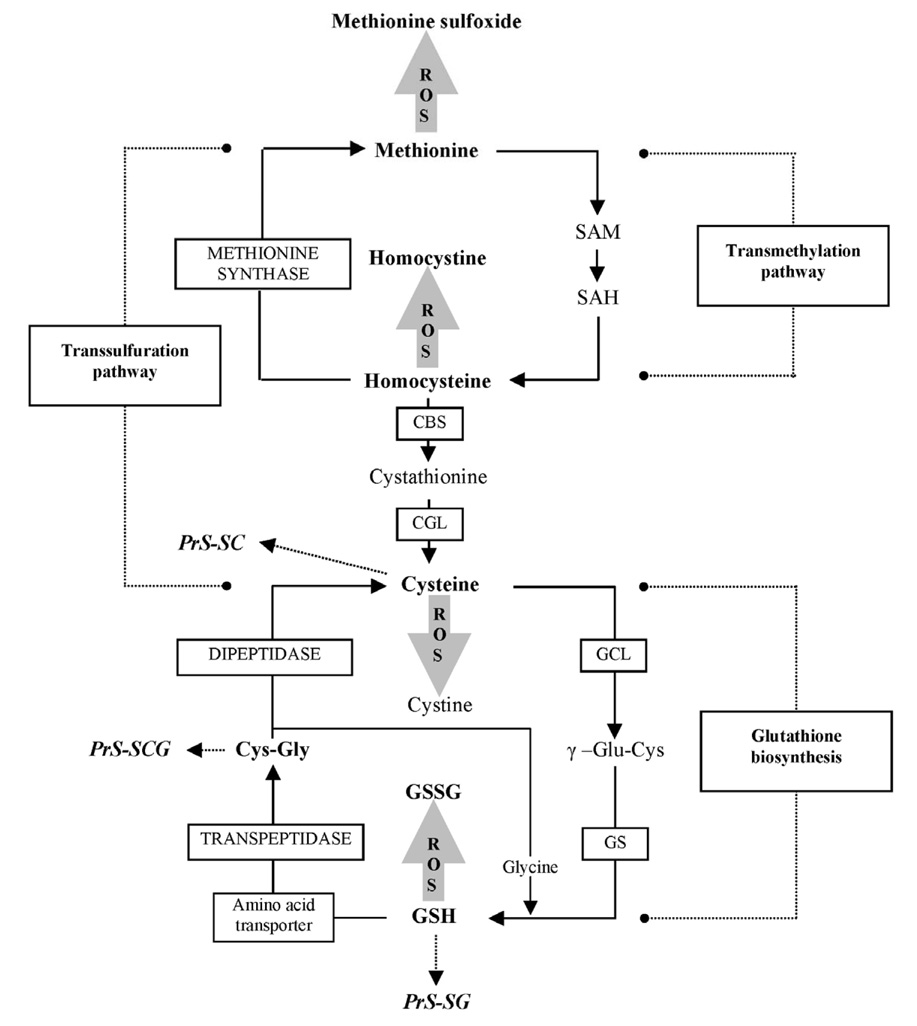
GSH synthesis occurs primarily in the cytoplasm, via two sequential ATP-dependent reactions: (1) ligation of L-glutamate and L-cysteine is catalyzed by the activity of γ-glutamylcysteine ligase (GCL), which is the rate-limiting step in the pathway, and (2) the addition of glycine to γ-glutamylcysteine by the activity of glutathione synthetase (reviewed in [14–16]). In higher eukaryotes, GCL is a heterodimer, consisting of a catalytic subunit (GCLc) and a modulatory subunit (GCLm). Degradation of GSH and GSSG involves cleavage of the γ-glutamyl-peptide bond by γ-glutamyltranspeptidase, followed by the cleavage of cysteinyl-glycine by a dipeptidase (Fig. 3).
Figure 3.
Synthesis and metabolism of glutathione including trans-sulfuration pathway.
As a versatile reductant, GSH serves several physiological functions, such as quenching of radicals by direct reaction, providing reducing equivalents for enzyme-mediated degradation of hydrogen peroxide and lipid peroxides, maintenance of protein thiol groups, conjugation and excretion of electrophilic xenobiotics, and acting as a coenzyme, among others [10, 12]. The main relevance of GSH to the aging process is that it provides important defense against oxidative stress, namely the reduction of hydrogen peroxide and lipid peroxides, which results in the oxidation of GSH to GSSG. The latter is either excreted out of the cell or is reconverted to GSH by the activity of NADPH-dependent glutathione reductase, or alternatively, GSSG may react with protein sulfhydryls to form mixed disulfides [15, 17, 18]. The GSH-NADPH system is the main provider of reducing power in cells and constitutes a major anti-oxidant axis for the elimination of ROS. In insects, GSSG is reduced back to GSH by the thioredoxin system [19]. The regeneration of the reduced form of thioredoxin from the oxidized state is dependent upon electrons supplied by NADPH. In addition, NADPH provides reducing equivalents for the thioredoxin peroxidase-mediated reduction of peroxides [20]. Because GSH has the dual ability to quench radicals directly as well as to donate electrons in enzyme-mediated reductions, it is regarded as the single most versatile antioxidant in cells, with broad effects on antioxidant potential [12]. Furthermore, GSH also maintains the cellular redox buffer, which is critical in processes such as cell proliferation, apoptosis, and maintenance of protein structure, among others [21–23].
In this context, an age-related loss of GSH can be predicted to have at least two potentially deleterious consequences: (1) steady-state levels of hydrogen peroxide would increase, leading to a rise in the formation of hydroxyl free radical and consequently greater macromolecular structural damage. Enhanced lipid peroxidation would result in corresponding increase in the level of products such as malondialdehyde and hydroxynonenals; both of which can form adducts with DNA or proteins, modifying their structure and function [24, 25]. (2) Formation of protein mixed disulfides can broadly interfere with the catalytic efficiency of enzymes, which may be reflected in their reduced ability to mount adaptive responses under stressful conditions [11, 21]. Both of these types of alterations do indeed occur during the aging process [26–29]. Thus, maintenance of an optimal GSH redox state is imperative for limiting the level of macromolecular oxidative damage.
Nevertheless, understanding the physiological consequences of variations in GSH: GSSG ratios during the aging process has been hampered by erroneous quantifications; specifically, flawed measurement of GSSG content can have a magnifying effect on the calculated redox potentials. Therefore, we provide below a brief critique of the analytical procedures for the measurement of GSH/GSSG.
4. Analytical procedures for measurement of glutathione and estimation of the redox potential
Measurements of GSH and GSSG in biological specimens can be erroneously affected during procedural handling due to the artificial oxidation of GSH. Analytical procedures in earlier studies, employing colorimetric, fluorimetric, and enzymatic assays were eventually found to have low sensitivity, inadequate specificity and/or low reproducibility [10, 12, 30]. Later procedures employing high-performance liquid chromatography, in combination with UV-absorbance or fluorescence detection, were found to be cumbersome, lack sufficient sensitivity for small samples, or unable to simultaneously measure GSH and GSSG. Moreover, multiple derivatization steps, involving thiol blocking, neutralization and acidification, can also lead to over- or underestimation of GSH/GSSG content. In contrast, ion-pairing HPLC in combination with coulometric electrochemical detection, used in the studies in our laboratory, offered significant advantages compared to the other procedures: our procedure is simple (no sample manipulation and or derivatization required), fast (analysis accomplished in 30 min) and specific (selective oxidation of GSH and GSSG at high electrode potential and elimination of interfering compounds at low electrode potential). Another unique advantage of this method is that GSH and GSSG along with other related aminothiols (GC, CG, cysteine, cystathionine and methionine) can be detected simultaneously [31, 32]. None of the other methods offer this capability. The multi-channel coulometric array detector also offers several other advantages over the commonly used amperometric electrochemical detectors. In the coulometric mode, almost 100% of the analyte is oxidized (or reduced) in flow-through porous graphite electrode, which contrasts with only 5–10% oxidation/reduction occurring on the surface of the amperometric detector. Other advantages include superior baseline stability and longer half-life of the electrode. Redox potentials of the glutathione redox couple (2GSH → GSSG + 2e− + 2H+ ) in mitochondria can be calculated by using experimentally determined concentrations of GSH and GSSG in the Nernst equation [30, 33]:
Eo is the standard potential of GSH (− 288 mV, at pH 7.8 in mitochondria), R is the gas constant (8.31 J/deg mol), T is the absolute temperature (310 K), n is the number of electrons transferred (2), and F is the Faraday constant (96,406 J/V).
5. Effect of age on glutathione redox state in mice
Comparisons of the amounts of GSH, GSSG and protein-glutathione (protein-SSG) disulfides and GSH:GSSG ratios in homogenates and mitochondria of different tissues (liver, kidney, heart, brain, eye, and testis) were made between 4-, 10-, 22- and 26- month-old C57BL/6 mice [26]. In 4-month-old mice, GSH content of tissue homogenates varied ~ 10-fold among different tissues, with the rank order: liver = testis > brain = heart > eye > kidney. GSSG content of tissue homogenates also varied ~ 10-fold. The GSH: GSSG ratios in different tissues were: liver, 61; kidney, 59; heart, 36; brain, 230; eye, 103 and testis, 165. There were up to 8.7-fold differences in protein-SSG content among different tissues. Such data clearly indicates that unique redox states exist in different tissues. During 4 to 26 months of age, GSH content of tissue homogenates decreased significantly in brain, eye, and testis; whereas GSSG level increased in all the tissues studied, ranging from 35 to 121 %. The GSH: GSSG ratios decreased significantly with age, ranging from 31 % in the heart to 121 % in the eye. Protein-SSG amount in homogenates increased with age in various tissues, except the brain.
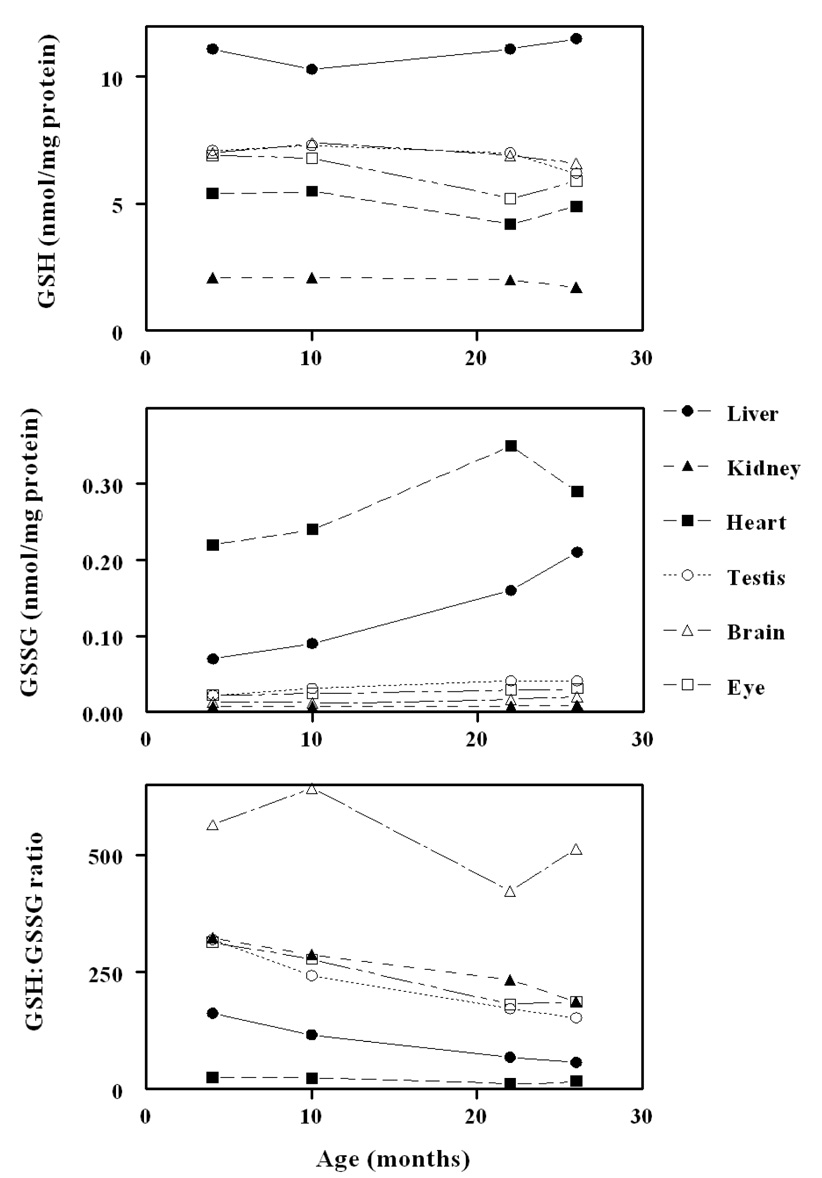
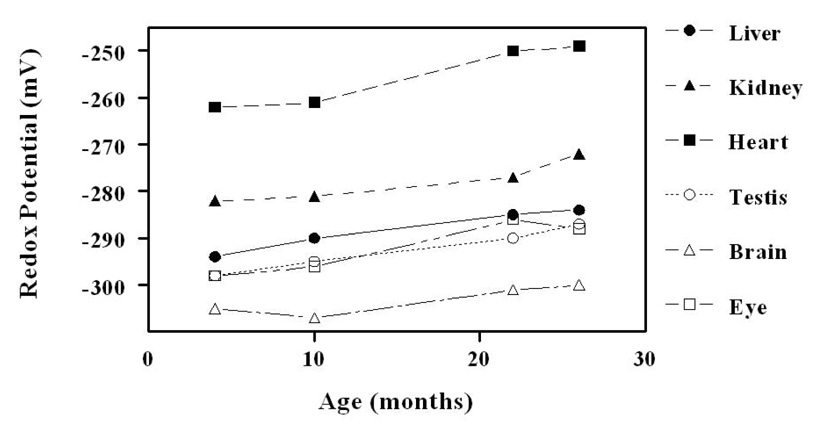
In 4-month-old mice, the mitochondrial GSH amount/mg protein was much lower than in the corresponding tissue homogenate, being 40 % in liver, 74 % in kidney, 31% in heart, 41 % in brain, 58 % in eye, and 26 % in testis. The GSSG content was also relatively lower in mitochondria than in the corresponding tissue homogenate; however, the GSH:GSSG ratios were much higher in mitochondria: liver,162; kidney, 323; heart, 25; brain, 564; eye, 314, and testis, 319. During 4 to 26 months of age (Fig. 4), mitochondrial GSH content decreased significantly (p < .05), ranging from 9 to 19 % in different tissues. Mitochondrial GSH: GSSG ratios decreased significantly with age in all tissues, except the brain. The protein-SSG content increased with age in mitochondria to a greater extent than in the corresponding tissue homogenates. Mitochondrial glutathione redox potentials declined (Fig. 5), that is, they became more oxidizing, or less negative, with age, ranging from 4.5 mV in the brain to 15 mV in the heart. Decreases in other tissues ranged between 9 and 12 mV. Such data unequivocally demonstrated that aging is associated with a significant pro-oxidizing redox shift in all the organs of the mouse examined. The proximate cause seemed to the increase in GSSG content and a decrease in GSH content in some tissues.
Figure 4.
Concentrations of GSH, GSSG and GSH;GSSG ratio in mitochondria of different tissues as function of age (adapted from [26]).
Figure 5.
Glutathione redox potentials in mitochondria from liver, kidney, heart, testis, brain and eye. Redox potentials become significantly (P< 0.01) more pro-oxidizing in the old (26 months) compared to young (4 months) mice in all the tissues, except brain (adapted from [26]). The standard deviation was less than 3–5% (error bars are omitted for the purpose of clarity).
To determine whether the glutathione redox state is correlated with life expectancy of mice, we compared GSH and GSSG contents in mitochondria and tissue homogenates between two different strains of mice, C57BL/6 and SAM (P8) (senescence-accelerated mouse); the latter has a 30% to 50% shorter life span than C57BL/6 mouse [27]. Although the mechanism underlying the short life span of the SAM mouse is not well understood, this strain exhibited a relatively higher level of oxidative stress, indicated by the GSH:GSSG ratios, than the C57BL/6 mouse.
6. Mechanisms of age-related oxidizing shift in glutathione redox state
The two main factors affecting the glutathione redox state during the aging process in the mouse seemed to be an increase in GSSG concentration and a decrease in the GSH pool size. In virtually all tissues, GSSG concentration is elevated during the last trimester of life. This is accompanied by an increase in the rates of mitochondrial superoxide and hydrogen peroxide production [34–36]. These two ROS are progenitors of a variety of other, some highly reactive, ROS. Age-related increases in ROS generation can be surmised to lead to enhanced utilization of GSH and formation of GSSG, due to either a direct interaction between ROS and GSH or increased utilization of GSH in enzyme-mediated elimination of peroxides. As a consequence, GSSG concentration would tend to increase if the capacity for its reduction lags behind the rate of its generation. The simultaneous increases in the rate of mitochondrial ROS production and the GSSG content, whose occurrence during the aging process has been well documented, are strongly suggestive of an overall enhancement of oxidative stress.
The other factor that may affect the cellular redox state during aging is the steady-state concentration of GSH, which remains relatively unchanged in most tissues, but as noted above, significant declines occur in some tissues. However, the ability for rapid de novo GSH synthesis together with its regeneration from disulfides are critical in GSH homeostasis, especially under conditions involving rapid upsurge in oxidant generation [12]. Our studies suggest that the capacity for de novo GSH biosynthesis may be compromised during the aging process, due to losses in the catalytic activity of GCL, the rate-limiting enzyme in GSH biosynthesis pathway [37]. For instance, comparison of Hanes-Woolf plots of GCL activity ([S/v] vs [S]) between relatively young and old houseflies indicated that GCL had significantly higher affinities for its substrates, glutamate and cysteine, in the young than in the old flies. The Km values increased in the old flies from 0.6 to 5.5 mM for glutamate and from 0.3 to 4.6 for cysteine. Another important potentially hazardous alteration was that the old flies did not exhibit substrate-dependent inhibition of GCL activity at >5 mM cysteine, indicating the loss of metabolic regulation during aging. Such loss of affinity by GCL for its substrates suggests that de novo GSH synthesis is less efficient in the old flies[38].
To understand the nature of the age-related perturbations in GSH biosynthesis in mammals, we compared the steady-state kinetics of hepatic GCL and levels of GSH precursors from the trans–sulfuration pathway, such as homocysteine, cystathionine and cysteine, between the young and old C57BL/6 mice (6- vs 24-month-old) [15]. There were no significant age-related differences in the Vmax, but the apparent Km for cysteine and glutamate were, respectively, 300 and 450 mM in the young and 800 and 710 mM in the old mice. Cysteine, cystathionine and Cys-Gly amounts increased with age by 91, 24, and 28%, respectively. The GSSG content increased 84% with age, while GSH amount remained unaltered, indicating a major oxidizing shift in GSH/GSSG ratio. The amount of homocysteine, a toxic trans- sulfuration/GSH biosynthesis pathway intermediate, was 154 % higher in the liver of the old than young mouse. The formation of cystathionine from homocysteine, which is the rate-limiting step in the trans-sulfuration pathway, was less efficient in the old compared to the young mouse, as indicated by the decrease in the efflux ratio (cystathionine/homocysteine). Incubation of liver homogenate with physiologically relevant concentrations of homocysteine was found to increase Km of GCL for glutamate 4.4-fold, however, there was no effect on Vmax of GCL. These experimentally-produced effects were similar to those observed ex vivo during aging, thereby suggesting that the age-related accumulation of homocysteine may underlie the loss of affinity of GCL for its substrates.
7. Effects of caloric restriction on glutathione redox state in mouse
A decrease in the amount of food consumption, relative to the ad libitum (AL) fed level, has been shown to extend life span of certain laboratory strains of mice and rats [39, 40]. There is also some evidence that caloric restriction (CR) retards the onset and progression of some age-associated changes linked to oxidative stress. A comparison between 22-month-old AL mice and those fed a diet containing 40% fewer calories than the AL group since the age of 4 months, indicated that CR had no effect on GSH content of tissue homogenates, whereas GSSG concentration was significantly lowered in the brain (42 %) and testis (37 %). In mitochondria, CR had no effect on GSH amount except in the heart and eye, where the increases were 24 % and 9 %, respectively [26]. The mitochondrial glutathione redox state was significantly more proreducing (−5 to −10 mV) in the heart, kidney, eye and testis of CR mice, compared to the AL mice. This proreducing shift was primarily due to a decrease in GSSG content. CR had no effect on glutathione redox potential in the brain or liver. In most tissues examined, CR caused a decrease in the amount of protein-SSG. In general, these studies indicated that CR attenuates the age-related oxidizing shift in the glutathione redox state [26].
8. Effects of dietary antioxidants on glutathione
The question whether intake of low molecular weight antioxidants causes a lowering of the level of oxidative stress has been intensely debated in the literature [36, 41, 42]. We addressed this issue by determining the effect of long term (8–10 months) dietary supplementation with two different mixtures of antioxidants on the glutathione redox state in senescence-accelerated mice (SAM-P8) [29]. Diet I was enriched with vitamin E, vitamin C, L-carnitine, and lipoic acid, while Diet II included vitamins E and C, and 13 additional ingredients containing micronutrients with bioflavnoids, polyphenols,and carotenoids. Both diets had a reductive effect in plasma, but only Diet II induced an increase in mitochondrial GSH and a decrease in GSSG content, and consequently a proreducing shift in GSH/GSSG redox couple. The effects on redox state in the tissue homogenates were insignificant.
9. Glutathione redox state in aging Drosophila melanogaster
The fruit fly (Drosophila melanogaster) provides several relative advantages as an experimental model for investigating the relationship between oxidative stress and aging, such as: i) relatively short life span (~8 weeks), ii) ability to manipulate the metabolic rate and life expectancy of flies by varying the ambient temperature, iii) availability of mutants and transformants (over-expressors). Glutathione redox state, amounts of the redox sensitive aminothiols (cysteine, Cys-Gly and methionine) and protein mixed disulfides were determined at different ages in a long-lived yw strain of D. melanogaster. The GSH:GSSG ratio decreased significantly with age due to an increase in GSSG content, while GSH concentration remained unaffected [43]. Amounts of Cys-Gly increased and of methionine decreased during aging. In contrast, amounts of protein mixed disulfides, measured as protein-Cys, protein-Cys-Gly and protein-SSG, increased with age. Together, the pro-oxidizing shift in glutathione redox state and the increase in protein mixed disulphide content indicated an enhancement of oxidative stress during aging.
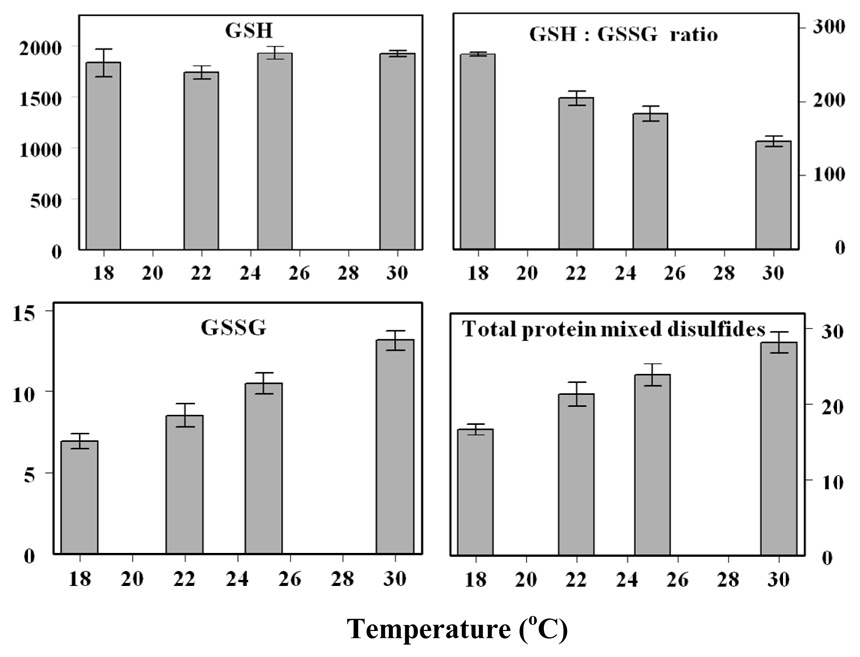
The effects of ambient temperature on life span, aminothiol content, glutathione redox state and protein thiolation were also examined in the aforementioned study [43]. Between 18° and 30° C, considered to be the physiological range, the life span of the flies is inversely related to the ambient temperature. The in vivo rate of oxygen consumption by flies increases with a rise in temperature, with a Q10 of 2. The amounts of Cys-Gly and GSSG increased, whereas the amount of free methionine and the GSH:GSSG ratio decreased with increasing ambient temperature (Fig. 6). The amounts of the three types of thiolated proteins measured increased linearly with ambient temperature. This study showed that shorter life span of flies at warmer temperatures was associated with a more rapid pro-oxidizing shift in the redox state.
Figure 6.
Effects of ambient temperature on amounts of GSH, GSSG and protein mixed disulfides, and GSH:GSSG ratio in y w flies (adapted from [43])
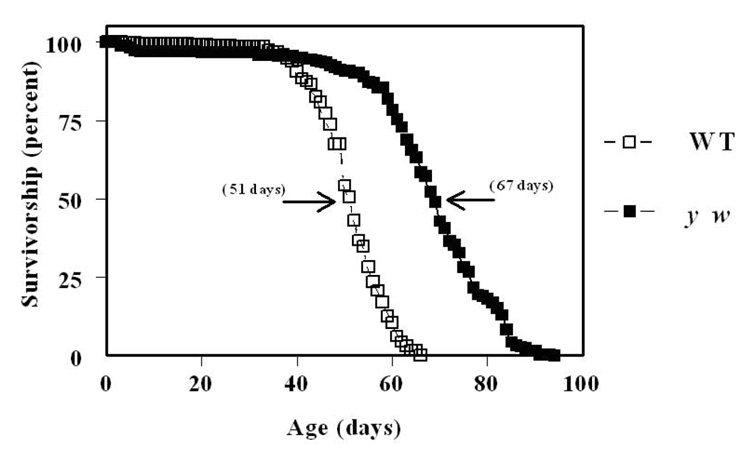
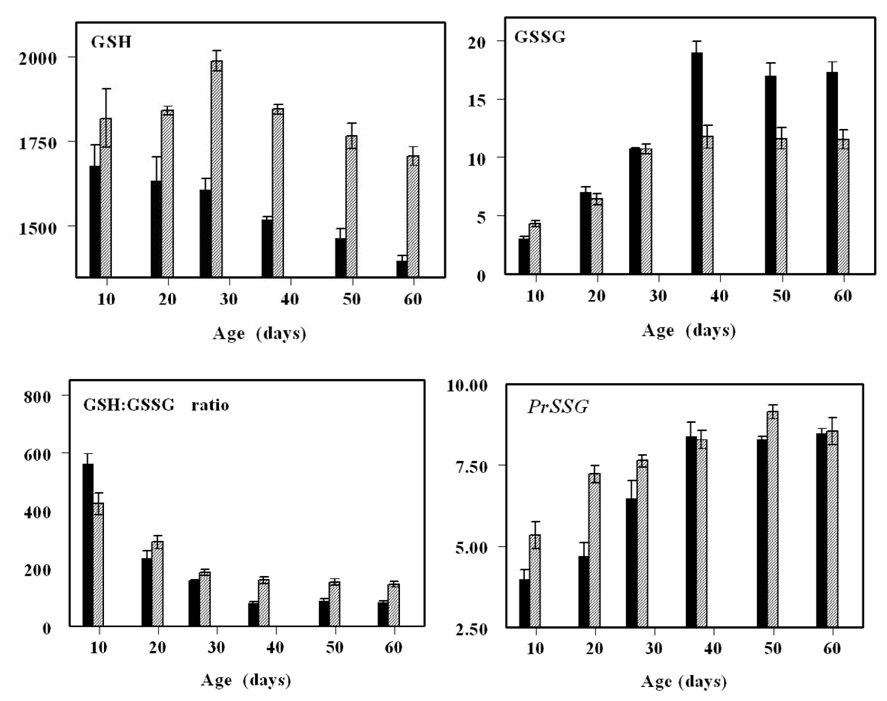
In another study, a comparison of glutathione redox state was made between two different strains of Drosophila melanogaster (Oregon R and y w), under normoxic and hyperoxic conditions (100% ambient oxygen) [28]. The reason for using the two strains was to address the question whether inter-strain differences in longevity (Fig. 7) were associated with resistance to oxidative stress. The shorter-lived Oregon R flies exhibited a relatively greater age-related decline in GSH:GSSG ratios, due to a more rapid increase in GSSG content, and a decrease in GSH amount (Fig. 8). Under hyperoxia, neither strain reproduced or exhibited accelerated trends of alterations in glutathione redox state, detected during normal aging. Thus, hyperoxia was inferred not to be an appropriate model for accelerated aging.
Figure 7.
Survivorship curves of WT and y w Drosophila melanogaster (adapted from [28]).
Figure 8.
Amounts of GSH, GSSG and protein mixed disulfides (PrSSG), and GSH:GSSG ratios in the shorter-lived WT (black bars) and longer-lived y w (grey bars) Drosophila melanogaster as function of age (adapted from [28]).
The existence of a possible causal link between extension of Drosophila life span and the enhancement of de novo glutathione biosynthesis was investigated by over-expressing glutamate-cycteine ligase (GCL). Using the GAL4-UAS binary transgenic system, flies over-expressing catalytic (GCLc) or modulatory (GCLm) subunits of the enzyme were generated with global or neuronal targeting [44]. As a consequence of the increased GCL activity and protein content, GSH amounts in fly homogenate was found to increased up to two-fold. Increased GSH content in the neuronal-targeted GCLc-expressing flies was correlated with extension of mean and maximum life spans of up to 50%. These results provided further evidence supporting the validity of the oxidative stress hypothesis of aging, by demonstrating that enhancing GSH biosynthesis can cause an extension of the life span of flies.
10. Role of glutathione in disease and toxicity
Depletion of glutathione or a disturbance in its redox state can lead not only to the disruption of a variety of cellular activities, but also to sensitization of cells to toxicants, thereby causing drug-induced cell toxicity. Indeed, some drugs have been shown to affect glutathione redox couple and mitochondrial function; e.g., usnic acid, a key constituent of LipoKinetix (a dietary supplement marketed as a weight-loss agent), can induce hepatic necrosis, triggered by GSH depletion and disruption of mitochondrial respiration[45]. Metabolites of acetaminophen can conjugate GSH and deplete mitochondrial GSH pool, with severe hepatotoxic consequences[46]. Chronic ethanol intake enhances susceptibility to acetaminophen toxicity, due to selective depletion of mitochondrial GSH[47–50]. Obviously, the toxicity of GSH-depleting drugs would tend to be relatively greater in the aged, due to a decline in the efficiency of GSH de novo synthesis.
Apparently, because of the presence of elevated concentrations of molecular products of ROS attacks on cellular macromolecules, oxidative stress has been postulated as a causal factor in the pathogenesis of a lengthy list of clinical disorders. But, whether enhanced oxidative damage is the primary cause of any particular abnormality, or it is only one among a set of coordinated deleterious alterations, still remains to be established. Similarly, the effect of GSH depletion in mitochondria and/or cytosol on cellular signaling pathways and the pathogenesis of specific diseases is presently unclear.
Acknowledgements
Our research has been supported by grants RO1 AG 13563 and RO1 AG 7657 from National Institutes of Health- National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Fridovich I. Superoxide dismutases. Annu. Rev. Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 3.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 4.Sohal RS, Allen RG, Nations C. Oxygen free radicals play a role in cellular differentiation: an hypothesis. J. Free Radic. Biol. Med. 1986;2:175–181. doi: 10.1016/s0748-5514(86)80067-8. [DOI] [PubMed] [Google Scholar]
- 5.Sohal RS, Allen RG. Oxidative stress as a causal factor in differentiation and aging: a unifying hypothesis. Exp. Gerontol. 1990;25:499–522. doi: 10.1016/0531-5565(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 6.Hitchler MJ, Domann FE. An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 2007;43:1023–1036. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayne AC, Mockett RJ, Orr WC, Sohal RS. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem. J. 2005;391:277–284. doi: 10.1042/BJ20041872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal S, Sohal RS. DNA oxidative damage and life expectancy in houseflies. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu. Rev. Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 11.Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry-glutathione-protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- 12.Meister A, Anderson ME. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 13.Markovic J, Borras C, Ortega A, Sastre J, Vina J, Pallardo FV. Glutathione is recruited into the nucleus in early phases of cell proliferation. J. Biol. Chem. 2007;282:20416–20424. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 14.Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch. Biochem. Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Toroser D, Yarian CS, Orr WC, Sohal RS. Mechanisms of gamma-glutamylcysteine ligase regulation. Biochim. Biophys. Acta. 2006;1760:233–244. doi: 10.1016/j.bbagen.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soltaninassab SR, Sekhar KR, Meredith MJ, Freeman ML. Multi-faceted regulation of gamma-glutamylcysteine synthetase. J. Cell. Physiol. 2000;182:163–170. doi: 10.1002/(SICI)1097-4652(200002)182:2<163::AID-JCP4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Forman HJ, Shi MM, Iwamoto T, Liu RM, Robison TW. Measurement of gamma-glutamyl transpeptidase and gamma-glutamylcysteine synthetase activities in cells. Methods Enzymol. 1995;252:66–71. doi: 10.1016/0076-6879(95)52009-0. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 19.Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Muller HM, Botella-Munoz J, Schneuwly S, Schirmer R, Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 21.Droge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp. Gerontol. 2002;37:1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 22.Ghezzi P. Oxidoreduction of protein thiols in redox regulation. Biochem. Soc. Trans. 2005;33:1378–1381. doi: 10.1042/BST0331378. [DOI] [PubMed] [Google Scholar]
- 23.Humphries KM, Szweda PA, Szweda LI. Aging: a shift from redox regulation to oxidative damage. Free Radic. Res. 2006;40:1239–1243. doi: 10.1080/10715760600913184. [DOI] [PubMed] [Google Scholar]
- 24.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Stadtman ER. Quantitation of 4-hydroxynonenal protein adducts. Methods Mol. Biol. 2000;99:25–34. doi: 10.1385/1-59259-054-3:25. [DOI] [PubMed] [Google Scholar]
- 26.Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebrin I, Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp. Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebrin I, Sohal RS. Comparison between the effects of aging and hyperoxia on glutathione redox state and protein mixed disulfides in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:869–874. doi: 10.1016/j.mad.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebrin I, Zicker S, Wedekind KJ, Paetau-Robinson I, Packer L, Sohal RS. Effect of antioxidant-enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free Radic. Biol. Med. 2005;39:549–557. doi: 10.1016/j.freeradbiomed.2005.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 31.Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 32.Harvey PR, Ilson RG, Strasberg SM. The simultaneous determination of oxidized and reduced glutathiones in liver tissue by ion pairing reverse phase high performance liquid chromatography with a coulometric electrochemical detector. Clin. Chim. Acta. 1989;180:203–212. doi: 10.1016/0009-8981(89)90001-6. [DOI] [PubMed] [Google Scholar]
- 33.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 34.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 35.Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech. Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- 36.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 37.Toroser D, Sohal RS. Age-associated perturbations in glutathione synthesis in mouse liver. Biochem. J. 2007;405:583–589. doi: 10.1042/BJ20061868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toroser D, Sohal RS. Kinetic characteristics of native gamma-glutamylcysteine ligase in the aging housefly, Musca domestica L. Biochem. Biophys. Res. Commun. 2005;326:586–593. doi: 10.1016/j.bbrc.2004.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 40.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 42.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Rebrin I, Bayne AC, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orr WC, Radyuk SN, Prabhudesai L, Toroser D, Benes JJ, Luchak JM, Mockett RJ, Rebrin I, Hubbard JG, Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J. Biol. Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 45.Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid-induced necrosis of cultured mouse hepatocytes: inhibition of mitochondrial function and oxidative stress. Biochem. Pharmacol. 2004;67:439–451. doi: 10.1016/j.bcp.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 46.Vendemiale G, Grattagliano I, Altomare E, Turturro N, Guerrieri F. Effect of acetaminophen administration on hepatic glutathione compartmentation and mitochondrial energy metabolism in the rat. Biochem. Pharmacol. 1996;52:1147–1154. doi: 10.1016/0006-2952(96)00414-5. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol. Appl. Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M, Ardite E, Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 49.Zhao P, Kalhorn TF, Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–335. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 50.Zhao P, Slattery JT. Effects of ethanol dose and ethanol withdrawal on rat liver mitochondrial glutathione: implication of potentiated acetaminophen toxicity in alcoholics. Drug Metab. Dispos. 2002;30:1413–1417. doi: 10.1124/dmd.30.12.1413. [DOI] [PubMed] [Google Scholar]