Abstract
Background
Neoadjuvant cisplatin-based chemotherapy improves survival in muscle-invasive urothelial cancer, with MVAC (methotrexate, vinblastine, doxorubicin and cisplatin) considered the standard regimen. Gemcitabine plus cisplatin (GC) has similar efficacy and less toxicity than MVAC in metastatic disease, but is untested as neoadjuvant treatment.
Methods
We retrospectively evaluated patients with muscle-invasive urothelial carcinoma who received neoadjuvant GC prior to radical cystectomy between November 2000 and December 2006 at Memorial Sloan-Kettering Cancer Center. Post-therapy pathological downstaging to either residual disease at cystectomy (pT0) or no residual muscle-invasion (< pT2, i.e. pT0, pTIS, pT1), chemotherapy delivery, and disease-free survival were the endpoints of interest. For comparison, similar endpoints were assessed in an historical cohort treated with neoadjuvant MVAC.
Results
Four cycles of neoadjuvant GC were given over 12 weeks (N=42). Thirty-nine of 42 patients (93%) received 4 cycles, with a median 91% drug delivery for cisplatin and 90% for gemcitabine. The pT0 proportion was 26% (95% confidence interval [CI], 14–42) and no residual muscle-invasive disease proportion (< pT2) was 36% (95% CI, 21–52); while pT0 was achieved in 28% (95% CI, 16–42) and < pT2 in 35% (95% CI, 23–49) of 54 MVAC-treated patients. All 15 GC patients achieving < pT2 pathologic stage remained disease-free at a median follow-up of 30 months.
Conclusion
Neoadjuvant GC is feasible and allows for timely drug delivery. The proportion of GC-treated patients whose primary tumors were downstaged, with prolonged disease-free survival and minimal or no residual disease, was similar to MVAC-treated patients.
Keywords: urothelial carcinoma, neoadjuvant, chemotherapy, cisplatin, radical cystectomy
INTRODUCTION
Despite surgery with curative intent (radical cystectomy [RC] and a bilateral pelvic lymph node dissection [BPLND]), approximately 50% of patients with muscle-invasive transitional cell carcinoma of the bladder will develop distant metastases and succumb to the disease.1 Attempts to improve survival have focused on perioperative chemotherapy in an attempt to eradicate micrometastases. A meta-analysis of over 3000 patients from neoadjuvant studies demonstrated a statistically superior survival benefit from neoadjuvant cisplatin-based combination chemotherapy compared with surgery alone.2.
The most successful neoadjuvant regimen reported in the literature is MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), a standard of care for treatment of patients with metastatic cancer.3 This consensus is supported by the Southwest Oncology Group (SWOG)-8710 trial that randomized patients with stage T2–T4a bladder cancer to receive either radical cystectomy alone or 3 cycles of MVAC followed by cystectomy.4 Disease-specific survival was superior for the patients receiving neoadjuvant MVAC (hazard ratio of 1.66, 95% confidence interval [CI], 1.22–2.45, P=.002). There was a trend toward superior overall survival (1.33 overall survival, 95% CI, 1.00–1.76) for MVAC-treated patients, with a 5-year survival of 57% compared with 43% for patients treated with surgery alone (P=.06). Pathologic response to neoadjuvant therapy also yields useful prognostic information. In the SWOG study, patients without residual disease (pT0) at cystectomy had an improved 5-year survival (85%) over those who had residual disease, and patients in the MVAC arm more frequently achieved pT0 status (38% vs 15%, P<.001).4
Despite these data, neoadjuvant chemotherapy has met resistance in the oncology community.5 Commonly cited reasons for lack of neoadjuvant therapy is that MVAC toxicity is too severe for routine clinical use,3 or delaying cystectomy leads to progression.6 Better-tolerated neoadjuvant chemotherapy may encourage greater utilization and potentially better outcomes. Cisplatin and gemcitabine therapy (GC) demonstrates efficacy similar to MVAC in terms of response and long-term survival for metastatic disease, but with far less toxicity.7,8 It therefore appears to be an excellent candidate for neoadjuvant therapy, but little information is available regarding optimum drug delivery, pathological response, and disease-free survival.9
Here, we report our experience with neoadjuvant GC prior to cystectomy in patients with clinical stage pT2–pT4a, N0 bladder cancer. Our main outcome of interest was the rate of pathologic downstaging to either pT0 or < pT2 because these endpoints are directly related to 5-year survival. In order to provide side-by-side assessments of similar therapy, we describe this experience in the context of a historical cohort of patients treated with neoadjuvant MVAC at our institution
PATIENTS AND METHODS
Patient Characteristics
Approval was obtained from the Institutional Review Board Approval of Memorial Sloan-Kettering Cancer Center (MSKCC) to review patients who had been treated at MSKCC with neoadjuvant chemotherapy, either MVAC or GC. For GC neoadjuvant therapy, both the pharmacy database and the institutional surgical database were queried for all patients who received this chemotherapy at MSKCC’s Sidney Kimmel Center for Prostate and Urologic Cancers. Then, medical records of bladder cancer patients treated with GC chemotherapy between November 2000 and December 2006 were reviewed. These records were cross-validated with a surgical database of MSKCC patients undergoing radical cystectomy. The IRB approval allowed us to capture patients with muscle-invasive disease seen at our center who met two criteria: 1) they were referred to a MSKCC medical oncologist for neoadjuvant therapy from an outside physician or a MSKCC urologist; and, 2) they received their chemotherapy at our center with GC. During the time period of this study and continuing to the present, it has been our practice in medical oncology to discuss neoadjuvant cisplatin-based regimens such as MVAC and GC with patients who have muscle-invasive disease and who are eligible for neoadjuvant therapy. Despite this discussion, no patients were treated with MVAC during the actual study period.
Patients referred to the Center for muscle-invasive urothelial cancer identified at transurethral resection of the bladder tumor (TURBT) had an independent MSKCC pathology review by dedicated genitourinary pathologists. Patients were then clinically staged with either computed tomography (CT) or magnetic resonance imaging (MRI), and a repeat examination under anesthesia (EUA) by a MSKCC urologist. A repeat TURBT was performed at the time of EUA at the surgeon’s discretion. All patients underwent post-chemotherapy radical cystectomy with pelvic lymphadenectomy. Pathologic staging was retrieved from the postoperative pathology reports. The study included patients with locally advanced disease clinically suspicious for tumor involving the anterior vagina or prostate (clinical stage T4a seen in 3 patients), but excluded patients with cT4b disease. Patients were excluded if there was clinical indication of metastatic disease, eg, any adenopathy >2 cm on pretreatment imaging, or if they had non-transitional cell carcinoma. These ineligible patients with more advanced disease were recommended to have 6 cycles of systemic chemotherapy, the choice of which was dependent on renal function and medical co-morbidities, interest in conventional or investigational therapy, and perceived tolerance of chemotherapy.
Patients were evaluated for chemotherapy by an MSKCC medical oncologist, typically recommending GC chemotherapy consisting of 4 cycles at 21-day intervals over 12 weeks. The 21-day schedule was based on the evidence of greater drug exposure of both cisplatin and gemcitabine compared with the original 28-day schedule, with completion of 4 rather than 3 cycles in 12 weeks.10,11 Two 21-day schedules predominated: standard single-dose and “split-dose” cisplatin. The former consisted of 4 cycles of GC with cisplatin 70 mg/m2 and gemcitabine 1000 mg/m2 on day 1, and gemcitabine 1000 mg/m2 on day 8.12 Split-dose GC consisted of 4 cycles of cisplatin 35 mg/m2 and gemcitabine 1000 mg/m2 on days 1 and 8 of each cycle.13 Two patients received all chemotherapy at local institutions, and 2 received the initial cycle at our institution but completed therapy locally. Specific drug doses were not retrievable in 1 patient who was excluded from the dose-intensity analysis.
For the comparator cohort, our institutional database was accessed for a previously reported cohort of 111 patients treated with 4 cycles of MVAC chemotherapy at 28-day intervals before cystectomy. These patients had heterogeneous surgical management.14 In that study, some patients refused cystectomy or deferred surgery until progression. We were able to identify 54/111 who underwent either a partial or a radical cystectomy after MVAC. Our institutional database did not include complete data regarding the delivered chemotherapy doses and the toxicity related to the MVAC chemotherapy regimen thus precluding a toxicity comparison with GC. However, it did contain the dates of initiating MVAC chemotherapy, the number of cycles, the date and extent of cystectomy, and pathological endpoints.
Statistical Analysis
Descriptive statistics were used for all endpoints of interest, including pathological outcome and survival. Disease-free survival was chosen as the endpoint of interest because of the relatively short follow-up in the GC cohort. Kaplan-Meier analysis was used to describe disease-free survival, but the GC and MVAC groups were not compared statistically. Disease-free survival was defined as the time from first chemotherapy to the appearance of local or regional disease, metastases, or death. Superficial urothelial cancer recurrences were not considered events. Drug intensity over time was analyzed for 4 planned cycles of therapy in 41 patients as previously reported by our group15 and others.11 The last 2 cycles in the 2 patients treated with 6 cycles prior to cystectomy were not included in the calculations.
RESULTS
Forty-two patients received neoadjuvant GC and 54 received MVAC. Both cohorts had similar clinical characteristics at presentation (Table 1). The GC cohort of 42 patients was selected from a surgical database of over 700 patients undergoing cystectomy for all stages of bladder cancer during the study period. Forty-one patients received GC on the 21-day schedule: 26 patients received standard-dose cisplatin, 15 patients received the split-dose cisplatin schedule. One patient was treated with the original 28-day GC schedule (days 1, 8, 15).7 Two patients received 6 rather than the usual 4 cycles of GC. Three patients received only 3 of 4 planned cycles. Reasons for early discontinuation were worsening renal function, dehydration with hypotension, and progressive urinary symptoms without tumor response (1 patient). No patient who received neoadjuvant GC as a bi-modal approach to treating muscle-invasive bladder cancer refused cystectomy. Nine patients were hospitalized during treatment. The most common causes were thromboembolic disease (3 patients) followed by emesis (2 patients).
Table 1.
Clinical Characteristics of Patients Receiving Neoadjuvant Chemotherapy
| Characteristics | GC (N=42) | MVAC (N=54) |
|---|---|---|
| Age (median, yrs) at first chemo | 64 (56–70) | 63 (58–67) |
| Age (median, yrs) at RC | 64 (57–70) | 63 (58–68) |
| Gender (male), No. (%) | 32 (76) | 43 (80) |
| Clinical Stage at Presentation, No. (%) | ||
| T2 | 19 (45) | 32 (59) |
| T3 | 19 (45) | 15 (28) |
| T4 | 4 (10) | 7 (13) |
| Days to cystectomy from first chemotherapy | ||
| dose, median (range) | 138 (123–155) | 125 (89–175) |
| RC | 138 (123–155) | 96 (80–163) (n=33) |
| PC | NA | 155 (118–182) (n=21) |
Abbreviations: GC, gemcitabine + cisplatin; MVAC, methotrexate, vinblastine, doxorubicin, cisplatin; RC, radical cystectomy; PC, partial cystectomy; NA, Not applicable
Pathologic outcomes at cystectomy were contrasted to prechemotherapy clinical stage for GC patients. (Table 2a). Twenty-six percent (95% CI, 14–42) of patients achieved pT0 stage and 36% (95% CI, 21–52) had < pT2 disease at cystectomy. Achievement of < pT2 status was achieved in 13/27 patients treated with standard-dose cisplatin and 2/15 receiving split-dose cisplatin; the small numbers of patients precluded statistical analysis (Table 2b). All patients underwent pelvic lymphadenectomy with a median lymph node count of 15. Node positivity (despite normal post-chemotherapy scans) was seen in two pT2 patients and nine pT3 patients. Although all patients had transitional cell carcinoma histology at diagnosis, the cystectomy specimens showed 2 patients with residual squamous differentiation and 1 with residual small cell carcinoma.
Table 2.
| Table 2a. Prechemotherapy clinical versus pathologic stage of the primary tumor at cystectomy in gemcitabine-cisplatin cohort for all patients irrespective of the dosing schedule (N=42) | |||||||
|---|---|---|---|---|---|---|---|
| Pre-Chemotherapy Clinical Stage | Pathologic Stage at Cystectomy |
||||||
| pT0 | pTIS | pT1 | pT2 | pT3 | pT4 | Total | |
| cT2 | 5 | 1 | 1 | 4 | 7 | 1 | 19 |
| cT3 | 5 | 1 | 1 | 5 | 7 | 0 | 19 |
| cT4 | 1 | 0 | 0 | 0 | 3 | 0 | 4 |
| Total | 11 | 2 | 2 | 9 | 17 | 1 | 42 |
| Table 2b. Clinical versus pathologic stage of the primary tumor at cystectomy in the gemcitabine-cisplatin cohort according to dosing schedule | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosing Regimen | Pathologic Stage at Cystectomy |
||||||||||
| ≤ pT1 | pT2 | pT3 | pT4 | Total | |||||||
| Split dose | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
 |
cT2 | 0 | 7 | 2 | 2 | 5 | 2 | 0 | 0 | 7 | 12 |
| cT3 | 2 | 5 | 1 | 4 | 2 | 5 | 0 | 0 | 5 | 14 | |
| cT4 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 3 | 1 | |
| Total | 2 | 13 | 3 | 6 | 10 | 7 | 0 | 1 | 15 | 27 | |
Of the 54 patients treated with neoadjuvant MVAC and surgery, 33 underwent radical cystectomy, and 21 underwent partial cystectomy after chemotherapy. The rate at which pT0 stage and < pT2 was achieved was 28% (95% CI, 16–42) and 35% (95% CI, 23–49), respectively. Because these patients underwent either radical or partial cystectomy based on response to chemotherapy, 15% of 33 patients (95% CI, 5–32) who underwent radical cystectomy were pT0, but 48% of 21 patients (95% CI, 26–70) were stage pT0 after partial cystectomy.
Disease-free survival was assessed in relation to pathological outcome. At last follow-up in the GC cohort, 15 patients had developed recurrent disease, of whom 10 died of disease and 5 were alive with disease. Twenty-six patients were without evidence of disease. One patient died of unknown causes 3½ years after initial chemotherapy. All 15 patients who were < pT2 stage at cystectomy remained disease-free at last follow-up, with a median follow-up of 30 months (interquartile range [IQR] 14–37). All 11 patients with positive lymph nodes at cystectomy recurred; 7 subsequently died of disease. Disease-free survival curves for the GC and MVAC cohorts are presented in Figures 1a and 1b. The median follow-up for survivors was 24.2 months in the GC cohort and 48.1 months in the MVAC cohort.
Figure 1.
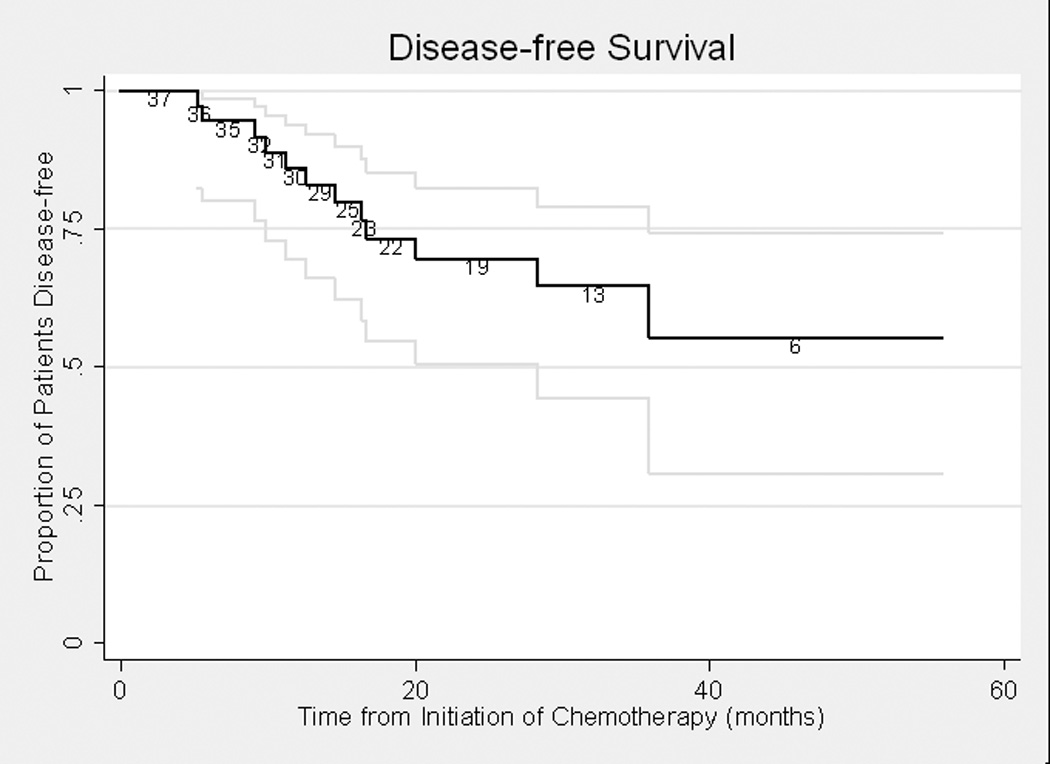
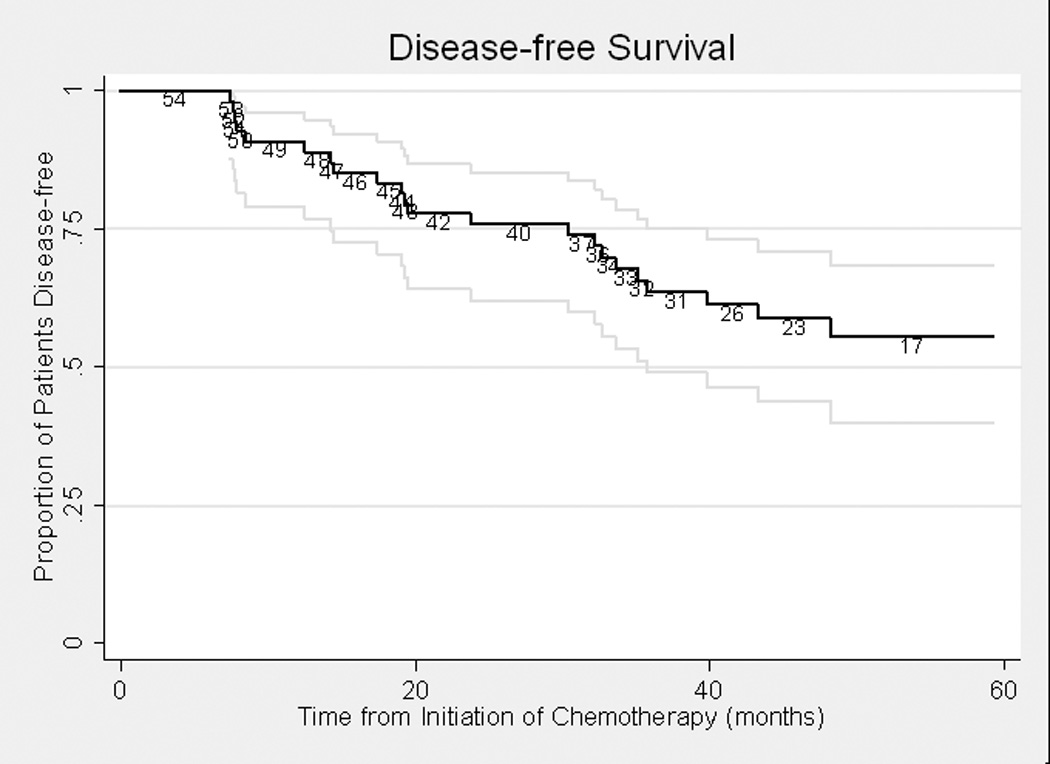
Figure 1a. Disease-free Survival in Gemcitabine-Cisplatin Cohort (n=42)
Figure 1b. Disease-free Survival in MVAC Cohort (n=54)
In evaluating the ability to deliver the planned GC chemotherapy, we found that the median time from the first day of GC chemotherapy to the last date of therapy was 77 days (IQR 70–89). The median time from the date of the last drug administration to the date of radical cystectomy was 57 days (IQR 46–76). The median interval from first chemotherapy treatment to radical cystectomy was 138 days (IQR 123–155). No significant differences were observed in the duration of therapy for patients treated with conventional cisplatin dosing (n=27) versus a split-dose schedule (n=15) (Table 3).
Table 3.
Duration of treatment for the gemcitabine-cisplatin cohort (N=42)
| Therapy Span | Conventional Dose Schedule, Median, days (range) (n=27) |
Split Dose Cisplatin, Median, days (range) (n=15) |
|---|---|---|
| Start of chemotherapy to cystectomy | 147 (123–169) | 129 (112–145) |
| Start to end of chemotherapy | 77 (70–97) | 77 (70–97) |
| End of chemotherapy to cystectomy | 62 (47–82) | 49 (40–66) |
The median time from first MVAC chemotherapy to radical cystectomy was 126 days (IQR 89–175). This interval was 96 days (IQR 80–163) among the 33 patients who underwent radical cystectomy versus 155 days (IQR 118–182) among the 21 patients who underwent partial cystectomy.
Dose-intensity of GC chemotherapy was reviewed for a planned 4 cycles to be given over 12 weeks. All 42 study patients received at least 3 cycles of neoadjuvant GC, 39 (93%) received 4 cycles, and 2 received 6 cycles. The achieved dose intensity for cisplatin was 90% (IQR 75%–100%), translating into a median weekly cisplatin dose of 21 mg/m2/week (IQR 18–23). The achieved dose intensity for gemcitabine was 90% (IQR 68%–100%) with a median dose of 615 mg/m2/week (IQR 542–667). Cisplatin dose intensity was slightly greater in patients receiving split-dose cisplatin (93%) compared with conventional single-dose cisplatin (88%).
DISCUSSION
This is the first comprehensive study demonstrating that neoadjuvant GC chemotherapy can downstage muscle-invasive bladder tumors to a degree observed with MVAC.14 The proportion of patients with complete eradication of tumor (pT0) was 26% for GC in this study and 28% for patients treated with MVAC in our prior study. Elimination of muscle-invasive disease (< pT2) was also similar--36% for GC and 35% for MVAC. Although these results do not come from a direct random comparison, the similarity of responses in primary bladder tumors across one institution’s studies is not surprising, given the comparable activity of these two regimens in metastatic cancer.7,8
Pathologic response to neoadjuvant therapy yields useful prognostic information. In the SWOG study, patients without residual disease (pT0) at cystectomy had an improved 5-year survival (85%) over those who had residual disease, and patients in the MVAC arm more frequently achieved pT0 status (38% vs 15%, P<.001).4 However, even less complete downstaging to non-muscle-invasive disease (<T2) is still prognostic. One analysis reported that patients with only residual “superficial” disease (including pT0, pTcis, pTa, pT1) after neoadjuvant chemotherapy experienced a 75% survival at 5 years, compared with 20% for those with residual muscle-invasive or node-positive disease.16 Thus, chemotherapy downstaging defines chemotherapy efficacy and is a surrogate for long-term survival.
At first glance, the pathologic complete response rate (pT0) of 26% achieved with GC in this study appears inferior to other studies in which 38% of patients were reported to have achieved pT0 with MVAC and 32% with CMV (cisplatin, methotrexate and vinblastine).4,17 A closer look at these trials suggests that the pT0 rates across studies are actually similar. In the neoadjuvant CMV trial, only 206 of 246 patients receiving chemotherapy had surgery; 35 had tumor progression precluding cystectomy.18 Thus, the reported pT0 rate of 32% drops to 27% (95% CI, 21–33) if the results are evaluated using an intent-to-treat analysis. SWOG 8710 reported a pT0 rate of 38% (48/126) in patients undergoing surgery,4 but that drops to 32% (48/150; 95 CI, 25%–40%) using an intent-to-treat analysis when all 150 patients receiving MVAC are included. Thus, these data suggest that GC chemotherapy has activity within the range of other neoadjuvant regimens known to improve survival.
Similar to prior observations with MVAC,4,14 GC patients with an excellent pathological response to therapy did well. All 15 GC patients achieving <pT2 disease in this study remain disease-free. Although the median follow-up is shorter than the previously reported MVAC series, these results support the concept that sustained benefit in disease-free survival follows downstaging to < pT2, and is similar across active cisplatin-based regimens.16 Moreover, no patient failed to undergo cystectomy due to progression during 12 weeks of neoadjuvant GC chemotherapy.
Drug delivery with the 21-day schedule of GC was excellent. Forty-two patients received at least 3 cycles, and 39 (93%) received 4 cycles. This delivery appears better than MVAC and CMV reported in randomized trials. SWOG-8710 reported that 87% of patients randomized to MVAC received 1 full cycle of chemotherapy.4 The CMV trial reported that 20% of patients received less than the 3 intended cycles, and approximately half the patients received the planned dose of cisplatin.18 These observations suggest that the GC regimen, better tolerated than MVAC in advanced disease, is superior to CMV in the delivered number of cycles and possibly better than MVAC.
In this study, dose intensity was higher using a 21-day schedule for GC than the original 28-day schedule. The achieved dose intensity for cisplatin was 90% (IQR 75%– 100%), translating into a median weekly cisplatin dose of 21 mg/m2/week (IQR 18–23), higher than cisplatin delivery seen in a prior MVAC study from our center.15 Our results for GC are similar to other studies following the 21-day GC regimen of 70 mg/m2/cycle and a delivered cisplatin dose of 21 mg/m2/week in bladder cancer and 23 mg/m2/week in non-small lung cancer.10,12,19 The achieved dose intensity for gemcitabine was 90% (IQR 68%–100%), with a median dose of 615 mg/m2/week (542–667), exceeding that observed for the 28-day GC schedule, with observed medians of 593–600 mg/m2/week.7,11 We also report on 15 patients treated with split-dose cisplatin who achieved a 93% dose intensity. Although less responses in the primary tumors were seen after split-dose cisplatin than single-dose cisplatin (2/15 versus 13/27), the numbers of patients are too small to compare statistically or draw inferences. More study of the regimens is indicated to address whether or not there is a schedule impact on tumor response.
The neoadjuvant GC regimen was generally well tolerated, but there were 9 hospitalizations among the 42 patients. Thromboembolic events accounted for one-third of the complications, 2 were incidental emboli detected on routine post-chemotherapy CTs, and no complication was life-threatening. Thromboembolic complications have been reported to occur in 13% of urothelial cancer patients on cisplatin-based chemotherapy so the rate of 7% seen in our study is consistent with that observed in the literature.20
There are limitations to our study. This is a retrospective study with selection biases, and a comparison between the GC and MVAC cohorts is limited because of historic rather than randomized comparisons. As with all retrospective analyses, this study may have missed patients receiving bi-modality therapy or patients who did not undergo cystectomy despite cross validation of the pharmacy and surgical datasets. Outside of hospitalization rates, we did not have complete chemotherapy toxicity data. However, this regimen has previously been studied in urothelial and non-small lung cancer, and the toxicity profile has been well characterized.
In conclusion, this study demonstrates that GC for muscle-invasive bladder cancer produces definitive clinical activity in the neoadjuvant setting, with a pathological complete response rate similar to MVAC and an excellent disease-free survival in responding patients. Using the 21-day schedule, GC could be given for 4 cycles in 12 weeks, with a higher dose intensity than the typical 28-day schedule. This well-tolerated regimen is worthy of more extended use and evaluation in the neoadjuvant setting.
Acknowledgments
AD was supported by a gift from the Tina and Richard V. Carolan Foundation. AD and JAP were supported by Training Grant T32–82088 from the National Institutes of Health.
Footnotes
This work was previously presented in part at the American Society of Clinical Oncology 2007 Annual Meeting, Chicago, IL
REFERENCES
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–206. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Loehrer P, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a Cooperative Group Study. J Clin Oncol. 1992;10:1066–1073. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. New Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 5.David K, Milowsky M, J R, et al. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998–2003: a report from the national cancer data base. J Urol. 2007;178:451–454. doi: 10.1016/j.juro.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 6.Mamhmud S, Fong B, Fahmy N, et al. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol. 2006;175:78–83. doi: 10.1016/S0022-5347(05)00070-4. [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large randomized multicenter, multinational multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 8.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 9.Dienstmann R, Herchenhorn D, Peixoto F, et al. Phase II trial of gemcitabine and cisplatin as neoadjuvant chemotherapy for invasive bladder cancer: Preliminary results [Abstract 14590] J Clin Oncol. 2006:24. (2006 ASCO Annual Meeting Proceedings, June 20 Supplement) [Google Scholar]
- 10.Dogliotti L, Cartenì G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase II trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Soto Parra H, Cavina R, Latteri F, et al. Three-week versus four-week schedule of cisplatin and gemcitabine: results of a randomized phase II study. Ann Oncol. 2002;13:1080–1086. doi: 10.1093/annonc/mdf186. [DOI] [PubMed] [Google Scholar]
- 12.Rinaldi M, Crino L, Scagliotti G, et al. A three-week schedule of gemcitabine-cisplatin in advanced non-small-cell lung cancer with two different cisplatin dose levels: A phase II randomized trial. Ann Oncol. 2000;11:1295–1300. doi: 10.1023/a:1008334610955. [DOI] [PubMed] [Google Scholar]
- 13.Hussain S, Stocken D, Riley P, et al. A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer. Br J Cancer. 2004;91:844–849. doi: 10.1038/sj.bjc.6602112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz PK, Herr HW, Zhang ZF, et al. Neoadjuvant chemotherapy for invasive bladder cancer: prognostic factors for survival of patients treated with M-VAC with 5-year follow- up. J Clin Oncol. 1994;12:1394–1401. doi: 10.1200/JCO.1994.12.7.1394. [DOI] [PubMed] [Google Scholar]
- 15.Dodd PM, McCaffrey JA, Mazumdar M, et al. Evaluation of drug delivery and survival impact of dose-intense relative to conventional-dose methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy in urothelial cancer. Cancer Invest. 2000;18:626–634. doi: 10.3109/07357900009032829. [DOI] [PubMed] [Google Scholar]
- 16.Splinter TA, Scher HI, Denis L, et al. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer- - Genitourinary Group. J Urol. 1992;147:606–608. doi: 10.1016/s0022-5347(17)37318-4. [DOI] [PubMed] [Google Scholar]
- 17.on behalf of the Intl Collaboration of Trialists of the MRC Advanced Bladder Cancer Group, MRC Clinical Trials Unit, London, UK. Hall RR. Updated results of a randomised controlled trial of neoadjuvant cisplatin (C), methotrexate (M) and vinblastine (V) chemotherapy for muscle-invasive bladder cancer [abstract 710] Proc Am Soc Clin Oncol. 2002:21. [Google Scholar]
- 18.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle- invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533–540. [PubMed] [Google Scholar]
- 19.Winquist E, Wilson J, Dorreen M, et al. A 3-week gemcitabine-cisplatin regimen for metastatic urothelial cancer. Can J Urol. 2004;11:2445–2449. [PubMed] [Google Scholar]
- 20.Czaykowski P, Moore M, Tannock I. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998;160:2021–2024. doi: 10.1097/00005392-199812010-00022. [DOI] [PubMed] [Google Scholar]


