Abstract
Purpose
To investigate the variability of CT colonography (CTC) scan quality obtained within and between institutions by using previously validated automated quality assessment (QA) software that assesses colonic distention and surface area obscured by residual fluid.
Methods
The CTC scans of 120 patients were selected retrospectively, 30 from each of 4 institutions. The bowel preparation included oral contrast for fecal and fluid tagging. Patients at one institution (Institution 4) drank ½ the amount of oral contrast compared to the patients at the other 3 institutions. Fifteen of the CTC scans were from the beginning of the protocol studied at each institution and 15 scans were from the same protocol acquired approximately one year later in the study. We used previously validated QA software to automatically measure the mean distention and residual fluid of each of five colonic segments (ascending, transverse, descending, sigmoid, and rectum). Adequate distention was defined as a colonic diameter of at least 2 cm. Residual fluid was determined by the percentage of colonic surface area covered by fluid. We compared how the quality varied across multiple institutions and over time within the same institution.
Results
No significant difference in the amount of colonic distention amongst the 4 institutions was found (p = 0.19). However, the distention in the prone position was significantly greater than the distention in the supine position (p < 0.001). Patients at Institution 4 had about ½ the amount of residual colonic fluid compared to patients at Institutions 1–3 (p < 0.01). The sigmoid and descending colons were the least distended segments, and the transverse and descending colon contained the most fluid on the prone and supine scans, respectively. More recently acquired studies had greater distention and less residual fluid but the differences were not statistically significant (p=0.30 and p=0.96, respectively).
Conclusion
Across institutions, a significant difference can exist in bowel preparation quality for CTC. This study reaffirms the need for standardized bowel preparation and quality monitoring of CTC exams to reduce poor CTC performance.
Keywords: CT, colon, colonography, virtual colonoscopy, quality
INTRODUCTION
Colorectal cancer is the second leading cause of cancer related deaths among men and women in the United States. Fortunately, advances in screening procedures may be able to reduce the number of colon cancer deaths. Computed tomographic colonography (CTC) is an emerging minimally invasive technique that can detect polyps and cancers in the colon. The sensitivity and specificity of CTC for detecting polyps has varied widely in several recent large clinical trials [1–4]. One potential reason for poor CTC performance is suboptimal exam quality. Although quality control is a well recognized component of an effective colonoscopy program [5], currently there is no existing quality standard for CTC.
Residual colonic fluid and colonic distention are two important factors that have an effect on CTC quality. The minimization of residual fluid and adequate colonic distention produce higher exam quality and improve the ability of radiologists to accurately identify lesions in the colon [6–14].
Previously, we developed and validated automated quality assessment (QA) software in an effort to facilitate assessment of CTC exam quality [15]. The software automatically measures colonic distention and luminal surface area obscured by residual fluid. The purpose of this study was to use this QA software to compare the colonic distention and residual fluid present in CTC exams at various institutions and to determine how the quality of the exams changed over time.
METHODS
The acquisition of patient data for this study was HIPAA-compliant and approved by an Institutional Review Board. Informed consent was obtained for retrospective analysis of the data. Subsequently, the data from 3 institutions were declared exempt from further IRB review; for the 4th institution, data were subject to ongoing IRB approval.
Patient Population
Patients were chosen retrospectively from the patient cohorts at 4 medical centers (Institutions 1–4) to assess the quality of CTCs between the different centers and over time. Patients in Institutions 1–3 were scanned between May 2002 and June 2003 [1], while patients in Institution 4 were scanned between April 2004 and September 2005 [2]. To determine sample size, we used Altman’s nomogram for calculating sample size with p = 0.05, power of 0.80, target difference of 10%, standard deviation of 10% [16]. In chronological order, we used the first consecutive 15 (Group A) and last 15 (Group B) patients’ CTCs within each of the four institutions to make comparisons. Group B patients from Institution 4 were part of an ongoing study, whereas Group B patients from Institutions 1–3 were scanned at the conclusion of a study. 9/30 (30%), 8/30 (27%), and 8/30 (27%) of the patients at Institution 1, 2, and 3 had positive findings of a 6 mm or larger polyp at CTC, respectively. Patients from Institution 4 were the subset of all patients with positive findings of a 6 mm or larger polyp at CTC taken from a consecutive series of patients who underwent CTC. The time intervals between the last patient scanned in group A and the first patient scanned in group B were 308, 331, 363, and 328 days, respectively.
Fifteen patients were excluded from the study due to the inability of preprocessing software to accurately segment the colon or create a centerline for the colon. Of the excluded patients, 10 and 5 were from Groups A and B, respectively. The number of patients excluded by institution was 2, 4, 7, and 2, respectively.
The mean age of the patients kept in the study was 57.5 [range 42 – 80] years, while the mean age of the patients excluded from the study was 57.1 [range 50 – 67] years. The number of males and females kept in the study was 71 and 34, respectively, while the number of males and females excluded from the study was 10 and 5, respectively.
Bowel Preparation
At Institutions 1–3, patients underwent a 24-hour colonic preparation that consisted of a clear-liquid diet and oral administration of 90 mL sodium phosphate, 10 mg bisacodyl, 500 mL barium (2.1% by weight), and 120 mL diatrizoate meglumine and diatrizoate sodium given in divided doses [1]. While considered appropriate at the time of scanning of these patients, a ”double dose” of sodium phosphate was subsequently found to be associated with a rare but serious form of renal failure; consequently, in May, 2006, the FDA issued a recommendation to healthcare professionals to not exceed the single dose (45 mL) [http://www.fda.gov/cder/drug/infopage/OSP_solution/default.htm, last accessed April 2, 2008].
At Institution 4, patients underwent a standard 24-hour colonic preparation that consisted of oral administration of 45 mL sodium phosphate, 10 mg bisacodyl, 250 mL barium (2.1% by weight), and 60 mL diatrizoate sodium [9]. Patients were placed on a clear-liquid diet on the day prior to the colonic scan and did not eat or drink after midnight. This simplified preparation used half the amount of contrast agent and sodium phosphate and has been equally effective for polyp detection [17–19].
CT Colonography
At Institutions 1–3, a small, flexible rectal catheter was inserted and pneumocolon was achieved by patient-controlled insufflation of room air. Each patient was scanned in the supine and prone positions during a single breathhold using a 4-channel or 8-channel CT scanner (General Electric LightSpeed or Light Speed Ultra; GE Healthcare Technologies, Waukesha, WI). CT scanning parameters included 1.25- to 2.5-mm section collimation, 15 mm/s table speed, 1-mm reconstruction interval, 100 mAs, and 120 kVp [1].
At Institution 4, pneumocolon was achieved by either patient-controlled manual insufflation of room air (n = 10) or automated insufflation of CO2 with an equilibrium pressure setting of 20 – 25 mm Hg (n = 18) [9]. The patients were scanned in the supine and prone positions using 8-channel or 16-channel multi-detector scanners (General Electric LightSpeed Series; GE Medical Systems, Milwaukee, WI). In Group A, 10 patients’ colons were insufflated with room air and 3 with CO2. In Group B, all 15 patients’ colons were insufflated with CO2. CT scanning parameters included 1.25- to 2.5-mm section collimation, 1-mm reconstruction interval, 50–70 mAs, and 120 kVp [9].
Image Analysis by automated QA
A multi-stage procedure was performed which included computation of the colon centerline, manual correction of centerline connectivity errors, and determination of the colonic segments.
The colon centerline was computed using an investigational, previously published automatic procedure [20]. The version of the centerline program used in this study was an improved algorithm from the centerline method that was used in the previous QA study [15]. The new centerline algorithm was validated using the manual distention and fluid scoring results from the prior QA study.
Collapsed segments of the colon could have prevented accurate connectivity of the centerline between discrete colonic segments. A graphical user interface was used to manually correct these connectivity errors.
Since the analyses in this paper are reported in part on the basis of colonic segments, the segments of the colon needed to be determined. The locations of the colonic segments were pre-defined manually with the assistance of computer software that provided a graphical user interface. The graphical user interface enabled a user to manually place separators along the colon centerline to subdivide the colon into five segments (ascending colon, transverse colon, descending colon, sigmoid colon, and rectum). The cecum was considered part of the ascending colon for ease of analysis.
A medical student (blinded for review) and a premedical student (blinded for review), under the supervision of a board-certified diagnostic radiologist (blinded for review), placed the colonic segment separators. The segments were defined using a modified version of a procedure developed by Taylor et al.[10]. The separators were placed at the proximal and distal ends of the colon and the junctions between segments. The portion of the colon from the anorectal junction proximally to the level of the acetabular roof was defined as the rectum. The portion of the colon proximal to the rectum to the level of the iliac crest at which the colon does not reenter the pelvis was defined as the sigmoid. The portion of the colon proximal to the iliac crest to the midpoint of the splenic flexure was defined as the descending colon. The portion of the colon between the midpoints of the splenic and hepatic flexures was defined as the transverse colon. The ascending colon was defined as the midpoint of the hepatic flexure to the portion of the cecum distal to the ileocecal valve. For the purposes of this project, the standardization of the colonic segment positions was more important than the precise definitions of the beginning and end of each segment.
After the separators were placed, the QA software computed the distention in each colonic segment. Each segment was subdivided into narrow slices about 1 cm wide perpendicular to the centerline. The mean diameter of the colon in each slice was computed. Finally, based on a previous study showing that 2 cm colonic diameter indicated adequate distention [21], the percentage of centerline slices in each colonic segment that had a corresponding distention greater than or equal to 2 cm was calculated.
Next, the number of colon surface vertices abutting air or fluid was calculated by the QA software, using the CT attenuation values at each point, and converted into a percentage of surface area covered by fluid. The percentage of each segment obscured by fluid was the fluid surface area divided by the total colonic surface area.
Evaluation across Multiple Institutions
We performed inter- and intra-institution comparisons to evaluate trends in quality. For evaluations not comparing supine and prone quality, the supine and prone scores were first averaged per patient. For evaluations not comparing quality by colonic segment, an over-all colon score for each patient was obtained by averaging the scores for each segment; the segment scores were not weighted by their relative length.
Statistical Analysis
Paired t-tests were used to compare supine and prone quality. Unpaired t-tests were used to compare Group A patients with Group B patients and patients at different institutions. ANOVA was used to perform hypothesis testing across all four institutions. P values less than 0.05 were considered significant.
RESULTS
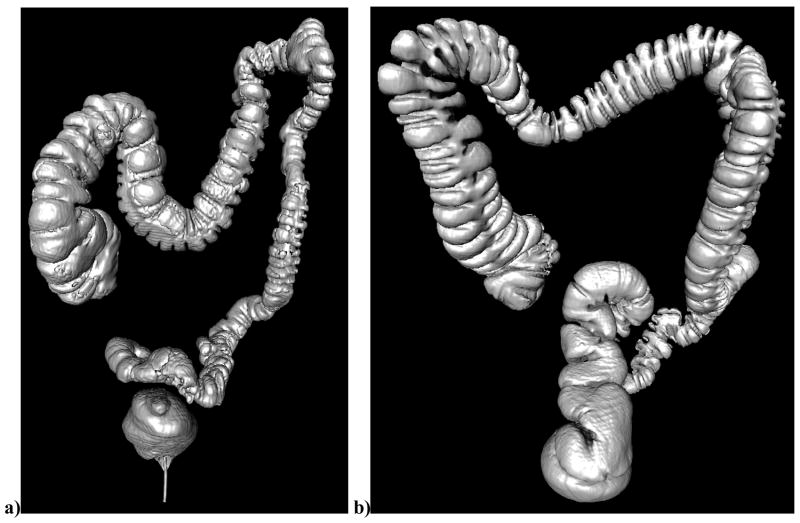
Examples of the automated assessments of distention and residual fluid are shown in Figure 1 and Figure 2, respectively.
Figure 1.
Anteroposterior surface reconstructed view of the colon from CTC showing representative distention scores. (a) The colon of a 69 year old female at supine CTCa poorly-distended descending colon with a distention score of 11.8% (b) The colon of a 52 year old male at prone CTC showing a well distended descending colon with a distention score of 95.7%.
Figure 2.
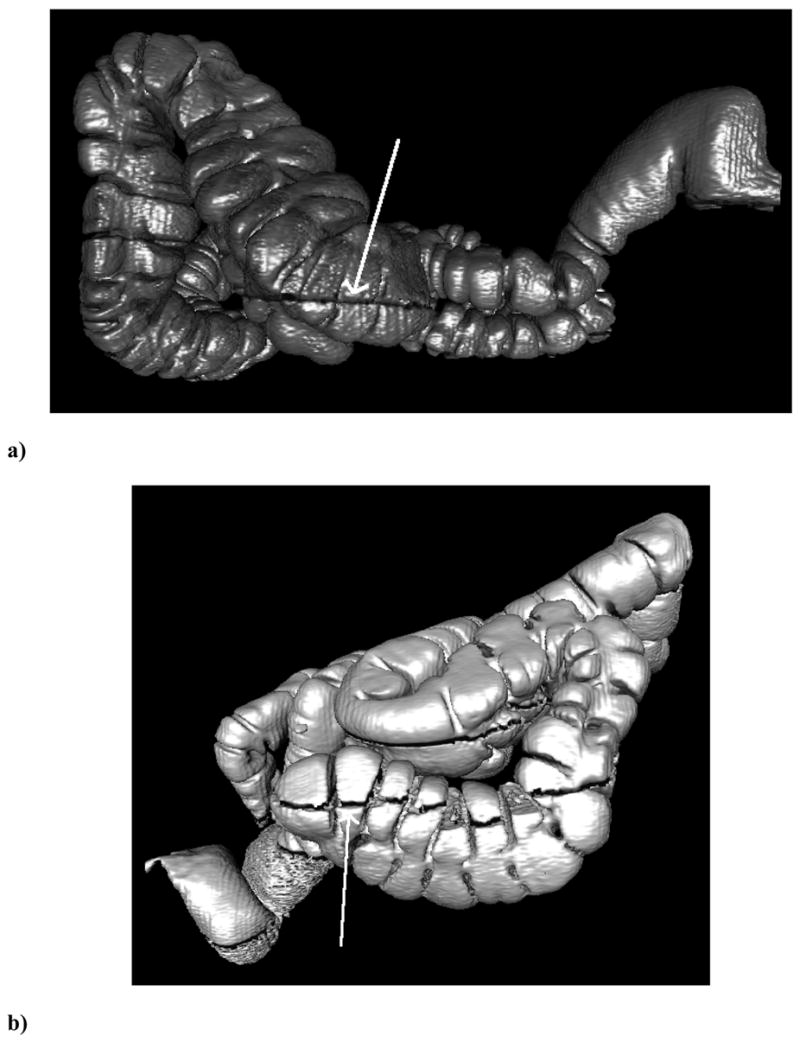
Lateral surface-rendered three-dimensional reconstructions of the colon from CTC showing representative fluid levels and their automated measurements. The arrows indicate the air/fluid boundary. The CT scanner table (not shown) is at the bottom of the image so that the fluid is dependent. (a) The ascending colon of a 69 year old male on prone CTCs showing low surface area obscured by fluid with a fluid score of 1.2%. (b) The ascending colon of a 54 year old male on supine CTC showing a high surface area obscured by fluid with a fluid score of 83.1%.
Table 1 shows the average percent colonic distention for each of the 4 institutions. The distention in the prone position, 88.0% ± 14.7%, is significantly larger than the distention in the supine position, 77.5% ± 19.5% (p < 0.001). There was no significant difference in the amount of colonic distention amongst the institutions (p = 0.19).
Table 1.
Distention in CTC examinations from four institutions as assessed by automated QA software. Numbers are percentage of colonic length (±standard deviation) having distention greater than or equal to 2 cm. Institution 3 had the greatest distention in the prone position and Institution 4 had the greatest distention in the supine position, although the differences for a particular position across institutions were not statistically significant.
| Institution | Number of Patients (n = 105) | Prone | Supine |
|---|---|---|---|
| 1 | 28 | 84.8 ± 16.2† | 72.1 ± 20.5† |
| 2 | 26 | 89.0 ± 15.5‡ | 81.1 ± 18.2‡ |
| 3 | 23 | 89.8 ± 10.4** | 73.2 ± 19.1** |
| 4 | 28 | 88.8 ± 15.5 | 83.1 ± 18.7 |
| Average | 26.3 | 88.0 ± 14.7* | 77.5 ± 19.5* |
p < .001
p < .01
p < .05 by paired t-test
Table 2 shows the average percent colonic wall surface area obscured by fluid for all four institutions. There was a significant difference between the amount of residual colonic fluid at each of the institutions (p < 0.001). The patients at Institution 4 had significantly less fluid obscuring the colonic surface in both prone and supine scans compared to the patients at Institutions 1–3 (p < 0.01). There was no significant difference in residual fluid on the prone, 15.8% ± 8.7%, versus the supine scans, 16.6% ± 10.2%, (p = 0.16).
Table 2.
Residual colonic fluid in CTC examinations from four institutions as assessed by automated QA software. Numbers are percent of colonic wall (±standard deviation) obscured by fluid. Institution 4 had significantly less residual fluid in both prone and supine positions compared to Institutions 1–3 (p < 0.01 and p < 0.001, respectively).
| Institution | Number of Patients (n = 105) | Prone | Supine |
|---|---|---|---|
| 1 | 28 | 14.9 ± 9.7‡ | 17.8 ± 12.1‡ |
| 2 | 26 | 19.0 ± 6.7 | 20.3 ± 9.5 |
| 3 | 23 | 21.6 ± 7.7 | 21.2 ± 8.0 |
| 4 | 28 | 9.1 ± 4.9 | 8.4 ± 3.7 |
| Average | 26.3 | 15.8 ± 8.7 | 16.6 ± 10.2 |
p < .05 by paired t-test
Over time, the distention increased and the residual fluid decreased, although the differences were not statistically significant (p=0.30, p=0.96) (Table 3). Regarding the different insufflation methods used at Institution 4, the average distention of the patients’ colons in Group A, 87.0% ± 10.6%, was not significantly different from the distention of the patients’ colons in Group B, 85.0% ± 18.6% (p = 0.73).
Table 3.
Distention and fluid quality over time as measured by QA software. Group A patients were the first consecutive 15 patients scanned and Group B patients were the last consecutive 15 patients scanned from each of four institutions, excluding a total of 15 patients whose CTC data could not be analyzed. Group B patients were scanned approximately 1 year after Group A patients. Although distention increased and fluid decreased slightly, the differences were not statistically significant (p=0.30 for distention, p=0.96 for fluid).
| Distention | Fluid | |
|---|---|---|
| Group A (n = 50) | 81.2 ± 14.1 | 16.3 ± 10.1 |
| Group B (n = 55) | 84.1 ± 14.4 | 16.2 ± 8.0 |
Quality varied by colonic segment and patient position. In the prone position, the least-distended segment was the sigmoid colon while in the supine position the least-distended segment was the descending colon (Table 4). Conversely, in the prone position, the best-distended segment was the rectum while in the supine position the best-distended segment was the ascending colon. In the prone position, the fraction of colonic surface obscured by residual fluid was greatest in the transverse colon while in the supine position it was greatest in the descending colon (Table 5).
Table 4.
Distention scores from the QA software by colonic segment. Numbers are percent of colonic segment length having a distention ≥ 2 cm in diameter. In the prone position, the sigmoid colon was the least distended with statistical significance compared to the rectum and ascending colon (p < 0.01). In the supine scans, the descending colon was least distended with statistical significance compared to the rectum, transverse, and ascending colon (p < 0.05).
| Segment | Prone (n=105) | Supine (n = 105) |
|---|---|---|
| Rectum | 92.3 ± 15.3* | 77.4 ± 26.1* |
| Sigmoid | 84.1 ± 27.0† | 69.0 ± 34.0† |
| Descending | 85.1 ± 26.9‡ | 66.2 ± 39.2‡ |
| Transverse | 87.1 ± 24.8 | 83.7 ± 26.6 |
| Ascending | 91.4 ± 14.2 | 91.3 ± 13.1 |
p < .001 by paired t-test.
Table 5.
Fluid scores from the QA software by colonic segment. Numbers are percent of colonic wall obscured by fluid in each colonic segment. In the prone scans, the transverse colon was most fluid-filled with statistical significance compared to the descending colon (p < 0.05). In the supine scans, the descending colon was most fluid-filled with statistical significance compared to the rectum, sigmoid, and transverse colon (p < 0.01).
| Segment | Prone (n=105) | Supine (n=105) |
|---|---|---|
| Rectum | 17.1 ± 17.1 | 15.6 ± 18.3 |
| Sigmoid | 15.4 ± 13.9† | 12.2 ± 14.0† |
| Descending | 13.4 ± 12.6* | 21.6 ± 19.2* |
| Transverse | 17.7 ± 13.3** | 14.8 ± 12.7** |
| Ascending | 15.5 ± 13.8‡ | 18.8 ± 13.3‡ |
p < .001
p < .05 by paired t-test.
DISCUSSION
Patients from Institution 4 had about 50% less residual fluid obscuring their colonic surfaces compared to those from Institutions 1–3 (Table 2). This finding was expected because patients at Institution 4 underwent a bowel prep consisting of 50% of the oral contrast volume given to patients at Institutions 1–3 [22]. Of interest, however, is that the bowel prep at Institution 4 also contained 50% of the amount of cathartic, yet this change did not lead to an increase in the amount of colonic fluid, perhaps due to the timing of the cathartic. The improved quality of the patient friendly bowel preparation (less oral contrast and cathartic) in the scans from Institution 4, along with the high polyp detection rates recently reported from that Institution [18], suggest that their bowel preparation is better than that used at Institutions 1–3.
Although a larger percentage of patients at Institution 4 used the automatic CO2 insufflator in Group B than in Group A, there was not a significant difference in the colonic distention. In a larger sample of patients at Institution 4, it has been reported that automatic CO2 insufflation may provide a small benefit over insufflation with room air in certain colonic segments on the supine view only [9].
In a busy clinical practice, quality can drift upward or downward over time and slow drifts can be difficult to detect. However, although a slight increase in quality (better distention and less fluid) was observed over about a one year interval, the change was not statistically significant (Table 3). This finding suggests that in the settings of these clinical research programs, the radiologists and support staff were able to maintain consistent quality without the use of quantitative assessments of quality. Whether this would be the case in a non-research clinical program is unknown. This result also suggests that the patients’ adherence to the bowel preparation was consistent over the one year period.
Colonic distention varied by segment and patient position. Overall, the prone scans were better distended than the supine scans. The distal colon was particularly poorly distended on the supine scans. In prone scans the sigmoid was the least distended. In the supine scans the sigmoid had lower distention than all segments but the descending colon. Radiologists must ensure adequate distention of the sigmoid and descending colons, as distention was relatively poor in these areas and it is known that polyps frequently occur there.
We found that residual colonic fluid shifted between the supine and prone positions. The descending colon and ascending colon had less residual fluid in the prone position and the rectum, sigmoid, and transverse colon had less fluid in the supine position. These fluid shifts are not unexpected since the patient must be turned 180° between the two positions and peristalsis is present. The fluid shifts can be helpful, particularly when fluid tagging is not used, by improving visibility of previously obscured colonic mucosa and are one of the rationales behind scanning patients in two positions [14, 23].
The amount of residual stool and the thickness of the colonic wall are two other important factors in the quality assessment of CTC. Residual stool is known to be a reason for missing polyps [24]. Developing automated software to determine the quantity of residual stool is a difficult task, particularly prospectively and if fecal tagging is not used, since stool can appear like polyps or masses. The thickness of the colonic wall is known to vary with differing degrees of colonic distention. In two studies, good or optimal colonic distention was defined as a pencil-thin bowel wall [10, 14]. In the future, QA software will need to assess the amount of residual stool and the thickness of the colonic wall in CTC scans.
Of the 12.5% (15/120) of patients who were excluded from analysis, 66.7% (10/15) were in Group A. These patients were excluded because the colon centerline did not pass through a segment or part of a segment in the patient scan. Possible reasons the centerline missed part of the segment were that leakage occurred (organs outside the colon were segmented, i.e., bone and liver), two segments were near each other and the centerline passed through the colon wall, or that a segment was so collapsed that the centerline passed through to the next closest portion of the colon that was not necessarily the adjacent segment. The large number of cases excluded from Institution 3 may be due to the increased amount of noise present in that CT scan data. The exclusion of 50% more patients from Group A than from Group B may also be an indication that the bowel preparation techniques improved over time so that the CTC scans could be processed and analyzed automatically.
Another limitation of the study is that our QA software requires the use of oral contrast for fluid tagging since it is much more difficult to assess residual fluid without tagging. However, the best reported CTC results have been in studies that used fluid tagging [1, 18, 25].
In conclusion, the QA software can be used to assess the quality of CTC examinations between institutions and over time. This software may help improve the consistency and quality of CTC examinations and lead to better polyp detection.
Acknowledgments
We thank William O. Schindler, DO, for supplying CT colonography data. The Intramural Research Program of the National Institutes of Health Clinical Center supported this work.
References
- 1.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Taylor AJ, Kim DH, Reichelderfer M, Gopal DV, Pfau PR. Screening for colorectal neoplasia with CT colonography: initial experience from the 1st year of coverage by third-party payers. Radiology. 2006;241:417–425. doi: 10.1148/radiol.2412052007. [DOI] [PubMed] [Google Scholar]
- 3.Cotton PB, Durkalski VL, Benoit PC, et al. Computed tomographic colonography (virtual colonoscopy) - A multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA-J Am Med Assoc. 2004;291:1713–1719. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635–650. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 6.Macari M, Lavelle M, Pedrosa I, et al. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218:274–277. doi: 10.1148/radiology.218.1.r01ja31274. [DOI] [PubMed] [Google Scholar]
- 7.Burling D, Taylor SA, Halligan S, et al. Automated insufflation of carbon dioxide for MDCT colonography: distension and patient experience compared with manual insufflation. AJR Am J Roentgenol. 2006;186:96–103. doi: 10.2214/AJR.04.1506. [DOI] [PubMed] [Google Scholar]
- 8.Yee J, Hung RK, Akerkar GA, Wall SD. The usefulness of glucagon hydrochloride for colonic distention in CT colonography. Am J Roentgenol. 1999;173:169–172. doi: 10.2214/ajr.173.1.10397121. [DOI] [PubMed] [Google Scholar]
- 9.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR Am J Roentgenol. 2006;186:1491–1496. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 10.Taylor SA, Halligan S, Goh V, et al. Optimizing colonic distention for multi-detector row CT colonography: effect of hyoscine butylbromide and rectal balloon catheter. Radiology. 2003;229:99–108. doi: 10.1148/radiol.2291021151. [DOI] [PubMed] [Google Scholar]
- 11.Dachman AH. Advice for optimizing colonic distention and minimizing risk of perforation during CT colonography. Radiology. 2006;239:317–321. doi: 10.1148/radiol.2392051374. [DOI] [PubMed] [Google Scholar]
- 12.Hung PW, Paik DS, Napel S, et al. Quantification of distention in CT colonography: development and validation of three computer algorithms. Radiology. 2002;222:543–554. doi: 10.1148/radiol.2222010600. [DOI] [PubMed] [Google Scholar]
- 13.Gollub MJ, Ginsberg MS, Cooper C, Thaler HT. Quality of virtual colonoscopy in patients who have undergone radiation therapy or surgery: How successful are we? Am J Roentgenol. 2002;178:1109–1116. doi: 10.2214/ajr.178.5.1781109. [DOI] [PubMed] [Google Scholar]
- 14.Yong AA, Harris JE, Shorvon PJ. The value of prone imaging in CT pneumocolon. Clin Radiol. 2000;55:959–963. doi: 10.1053/crad.2000.0568. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande KK, Summers RM, Van Uitert RL, et al. Quality assessment for CT colonography: validation of automated measurement of colonic distention and residual fluid. AJR Am J Roentgenol. 2007;189:1457–1463. doi: 10.2214/AJR.07.2327. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1991. [Google Scholar]
- 17.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Pickhardt PJ, Hinshaw JL, Taylor AJ, Mukherjee R, Pfau PR. Prospective blinded trial comparing 45-mL and 90-mL doses of oral sodium phosphate for bowel preparation before computed tomographic colonography. J Comput Assist Tomogr. 2007;31:53–58. doi: 10.1097/01.rct.0000230003.61392.2b. [DOI] [PubMed] [Google Scholar]
- 20.Van Uitert RL, Summers RM. Automatic correction of level set based subvoxel precise centerlines for virtual colonoscopy using the colon outer wall. IEEE Trans Med Imaging. 2007;26:1069–1078. doi: 10.1109/TMI.2007.896927. [DOI] [PubMed] [Google Scholar]
- 21.Van Uitert R, Bitter I, Summers RM, Choi JR, Pickhardt PJ. Quantitative assessment of colon distention for polyp detection in CT virtual colonoscopy. In: Manduca A, Amini AA, editors. Progress in Biomedical Optics and Imaging -Proceedings of SPIE Medical Imaging 2006: Physiology, Function, and Structure from Medical Images. San Diego: 2006. pp. 451–457. [Google Scholar]
- 22.Kim DH, Pickhardt PJ, Hinshaw JL, Taylor AJ, Mukherjee R, Pfau PR. Prospective Blinded Trial Comparing 45-mL and 90-mL Doses of Oral Sodium Phosphate for Bowel Preparation Before Computed Tomographic Colonography. J Comput Assist Tomogr. 2007;31:53–58. doi: 10.1097/01.rct.0000230003.61392.2b. [DOI] [PubMed] [Google Scholar]
- 23.Chen SC, Lu DS, Hecht JR, Kadell BM. CT colonography: value of scanning in both the supine and prone positions. AJR Am J Roentgenol. 1999;172:595–599. doi: 10.2214/ajr.172.3.10063842. [DOI] [PubMed] [Google Scholar]
- 24.Park SH, Ha HK, Kim MJ, et al. False-negative results at multi-detector row CT colonography: multivariate analysis of causes for missed lesions. Radiology. 2005;235:495–502. doi: 10.1148/radiol.2352040606. [DOI] [PubMed] [Google Scholar]
- 25.The National CT Colonography Trial. ACRIN Protocol 6664. http://www.acrin.org/6664_protocol.htm.