Abstract
Background
Hemoglobin A1c (HbA1c) is a marker of cumulative glycemic exposure over the preceding 2–3 month period. Whether mild elevations of this biomarker provide prognostic information for development of clinically evident type 2 diabetes and cardiovascular disease among individuals at usual risk for these disorders is uncertain.
Methods
We examined baseline HbA1c levels as a predictor of incident clinical diabetes and cardiovascular disease (non-fatal myocardial infarction, coronary revascularization procedure, ischemic stroke, or death from cardiovascular causes) in a prospective cohort study beginning in 1992 of 26,563 US female health professionals aged ≥ 45 years without diagnosed diabetes or vascular disease (median follow-up 10.1 years).
Results
During follow-up, 1238 cases of diabetes and 684 cardiovascular events occurred. In age-adjusted analyses using quintiles of HbA1c, a risk gradient was observed for both incident diabetes and cardiovascular disease. In multivariable-adjusted quintile analyses, HbA1c remained a strong predictor of diabetes but was no longer significantly associated with incident cardiovascular disease. In analyses of threshold effects, adjusted relative risks for incident diabetes in HbA1c categories of <5.0%, 5.0–5.4%, 5.5–5.9%, 6.0–6.4%, 6.5–6.9%, and ≥7.0% were 1.0, 2.9, 12.1, 29.3, 28.2, and 81.2. Risk associations persisted after additional adjustment for C-reactive protein and after excluding individuals developing diabetes within 2 and 5 years of follow-up.
Conclusions
These prospective findings suggest that HbA1c levels are elevated well in advance of the clinical development of type 2 diabetes supporting recent recommendations for lowering of diagnostic thresholds for glucose metabolic disorders. In contrast, the association of HbA1c with incident cardiovascular events is modest and largely attributable to coexistent traditional risk factors.
Hemoglobin glycation, estimated by percentage hemoglobin A1c (HbA1c), was first used clinically 30 years ago to assess degree of chronic hyperglycemia among diabetic patients 1 in whom values reflect weighted mean glucose levels over the preceding 3-month period.2 Over the past three decades, elevated HbA1c has been firmly linked with long-term risk of microvascular complications and HbA1c assessment is now used ubiquitously for monitoring effective glycemic control as a cornerstone of diabetes care. With the introduction of reference method standardization, issues pertaining to high inter-laboratory and inter-assay analytic variability have been largely overcome such that in 2002, 98% of US laboratories surveyed used standardized methods.3
Given these favorable performance characteristics, recent investigative efforts have attempted to broaden the role of HbA1c as an index of cumulative glycemic exposure in diabetes and cardiovascular risk assessment among non-diabetic patients. Several studies have evaluated the ability of HbA1c levels to predict future type 2 diabetes in high-risk pre-diabetic individuals4–7 and more recent data suggest that HbA1c may also be useful in detecting risk for incident cardiovascular events.8–12 Importantly, whether a single HbA1c measurement can be used in this application remains uncertain and prospective population-based studies of individuals at low to average risk are rare.
In a prior nested case-control analysis,13 we found that an elevated HbA1c level was a univariate predictor of incident cardiovascular events but this effect was not significant after adjustment for other cardiovascular risk factors. However, we did not examine non-linear threshold effects which may have prognostic significance as has been demonstrated in several prospective studies of plasma glucose and incident cardiovascular events 14–19 and at least one study of HbA1c and cardiovascular mortality.8
We therefore evaluated whether baseline HbA1c levels predict clinical diabetes and first cardiovascular events among otherwise healthy middle-aged and older American women, a population in which diabetes is a potent vascular risk factor and among whom data pertaining to this issue are sparse. We utilized both traditional quantile analysis and examined potential threshold effects with a focus on HbA1c levels currently considered to be well within the normal range.
METHODS
Study Population
The Women’s Health Study (WHS)20 is a recently completed randomized clinical trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. Between November 1992 and July 1995 a total of 39,876 US female health professionals aged 45 years and older without prior cardiovascular disease or cancer (except non-melanoma skin cancer) were enrolled and randomized into the study.
Before randomization, 28,345 participants provided blood specimens which were stored in liquid nitrogen until laboratory analysis. Of samples received, 27,882 were usable for HbA1c determination. We restricted the population to subjects without diagnosed diabetes and excluded women with missing baseline BMI (1.9%, n=517). All other major known diabetes and cardiovascular risk factors assessed had less than 1% missing data. The final study population comprised 26,563 women followed for a median of 10.1 years (range, 0.07–10.8 years).
Outcome Ascertainment
The status of type 2 diabetes was indicated at baseline by self-report, and women with a history of diagnosed diabetes were excluded. Thereafter, all participants were asked annually whether and when (month and year) they had been diagnosed with diabetes since completing the previous questionnaire. Two complementary methods for diabetes confirmation have been used.21 First, as part of a nested case-control study22 406 consecutive cases of self-reported diabetes occurring between years 2 through 5 of follow-up were confirmed by telephone interview using American Diabetes Association (ADA) criteria.23 Second, a random sample of 147 women with self-reported diabetes was mailed a supplemental diabetes questionnaire. Among 136 respondents, 124 (91%) met ADA diagnostic criteria. Additionally, 113 of the 124 women gave permission to contact their primary care physician. Of 113 physicians approached, 97 responded and 90 provided adequate information to apply the ADA criteria. Among these 90, 89 (99%) were confirmed to have type 2 diabetes. Thus, we believe that self-reported type 2 diabetes is valid in the WHS.
Women with a self-reported history of diagnosed cardiovascular disease (myocardial infarction, coronary revascularization, angina, stroke, transient ischemic attack, and peripheral arterial surgery) were ineligible for randomization into the WHS. After randomization, all women were followed through annual mailed questionnaires for incident myocardial infarction, coronary revascularization, stroke, or death from cardiovascular causes. Medical records were obtained for all women reporting a cardiovascular endpoint. Records were reviewed in a blinded fashion by an endpoints committee of physicians. Myocardial infarction was confirmed if symptoms met World Health Organization criteria and if the event was associated with abnormal levels of cardiac enzymes or diagnostic electrocardiograms. Coronary revascularization was confirmed through review of procedural reports. A confirmed stroke was defined as a new neurologic deficit of sudden onset that persisted for at least 24 hours. Clinical information and radiographic reports were used to distinguish hemorrhagic from ischemic events. Death from cardiovascular was determined by autopsy or death certificates, medical records, and information obtained from family members.
Laboratory Analysis
HbA1c was estimated using the Tina-Quant turbidimetric inhibition immunoassay (Roche Diagnostics, Indianapolis, IN) on a Hitachi 911 autoanalyzer using packed red blood cells. The assay is specific for HbA1c, is standardized against the approved International Federation of Clinical Chemists (IFCC) reference method, and is traceable to the Diabetes Control and Complications Trial (DCCT) by use of a conversion factor. Values of HbA1c presented in this study are DCCT aligned. The reference range for healthy non-diabetic subjects is 4.8 to 5.9%. The coefficient of variation for HbA1c computed from blinded simultaneously analyzed quality controls was 7.2%.
EDTA specimens were analyzed for LDL- and HDL-cholesterol using direct measurement assays (Roche Diagnostics, Indianapolis, IN). C-reactive protein (CRP) was measured using a validated high-sensitivity assay (Denka Seiken, Niigata, Japan).
Statistical Analysis
Histograms of HbA1c levels were constructed according to 4 main groups: individuals remaining disease-free (N=24,725), developing cardiovascular disease only (N=600), diabetes only (N=1,154), or both cardiovascular disease and diabetes (N=84). The median, interquartile range (IQR), mean, and standard deviation (SD) were calculated. Differences in median HbA1c were tested using the Wilcoxon Rank Sum test. Cox proportional hazards models predicting incident diabetes and cardiovascular disease were constructed using HbA1c quintiles with the lowest quintile as referent. Tests of linear trends were computed using median values within each quintile. Models were first age-adjusted (5-year categories). Multivariable models further adjusted for ethnicity, smoking, history of hypertension, baseline anti-hypertensive therapy, BMI, diabetes in a first-degree relative (diabetes models) or parental history of myocardial infarction before age 60 (cardiovascular disease models), exercise frequency, alcohol consumption, use of menopausal hormone therapy (MHT), and measured LDL and HDL-cholesterol levels (see Table 1 footnote). Sensitivity analyses excluded diabetes cases diagnosed within 2 and 5 years of follow-up. We repeated our analysis of incident diabetes using only confirmed events.
Table 1.
Relative risks (RRs) of diabetes and cardiovascular disease by HbA1c quintiles
| Hemoglobin A1c Quintiles |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-Trend | |
| < 4.8 | 4.80 – 4.93 | 4.94 – 5.06 | 5.07 – 5.22 | > 5.22 | ||
| N | 5313 | 5313 | 5313 | 5311 | 5313 | |
| Incident diabetes mellitus | ||||||
| Events | 53 | 65 | 109 | 194 | 817 | |
| Events/1000 person-years | 1.0 | 1.2 | 2.1 | 3.8 | 17.0 | |
| Age-adjusted RR (95% CI) | 1.0 | 1.2 (0.9 –1.8) | 2.1 (1.5 – 2.9) | 3.7 (2.8 – 5.1) | 17.2 (13.0 – 22.8) | <0.001 |
| Multivariable-adjusted RR† (95% CI) | 1.0 | 1.1 (0.8 – 1.6) | 1.7 (1.2 – 2.3) | 2.6 (1.9 – 3.5) | 8.6 (6.5 – 11.6) | <0.001 |
| Multivariable-adjusted RR‡ (95% CI) | 1.0 | 1.3 (0.9 – 1.9) | 1.8 (1.3 – 2.6) | 2.8 (2.0 – 3.9) | 8.2 (6.0 – 11.1) | <0.001 |
| Incident cardiovascular events* | ||||||
| Events | 105 | 113 | 135 | 141 | 191 | |
| Events/1000 person-years | 2.0 | 2.2 | 2.6 | 2.7 | 3.7 | |
| Age-adjusted RR (95% CI) | 1.0 | 0.9 (0.7 – 1.2) | 1.1 (0.8 – 1.4) | 1.0 (0.8 – 1.3) | 1.2 (1.0 – 1.6) | 0.046 |
| Multivariable-adjusted RR† (95% CI) | 1.0 | 0.8 (0.6 – 1.1) | 0.9 (0.7 – 1.2) | 0.8 (0.6 – 1.0) | 0.9 (0.7 – 1.2) | 0.5 |
Cardiovascular disease: MI, CABG/PTCA, ischemic stroke, and cardiovascular death
Adjusted for age (5-year categories), ethnicity (Caucasian, African-American, Hispanic, Asian, American Indian, other, unknown), smoking (never, past, current), history of hypertension (no/yes self-report ≥ 140/90), baseline anti-hypertensive therapy (no/yes), BMI category (WHO category), family history of MI/DM (parental MI <60y, 1st degree relative DM), exercise (never, <1 time per week, 1–3 times per week, 4+ times per week), alcohol consumption (non-drinker, 1–3 per month, 1–6 per week, 1+ per day), MHT use, LDL (linear continuous), and HDL (linear continuous).
Adjusted as above after excluding women diagnosed with diabetes during the first two years of follow-up (N=175).
In analyses examining alternate cutpoints of HbA1c, individuals were categorized into groups beginning at values below 5.0%, the population mean, in 0.5% increments up to a value ≥ 7.0%, the cutpoint corresponding to the optimal treatment target24 and a level proposed as diagnostic of drug-requiring diabetes.25 Kaplan-Meier survival curves were plotted and differences in event-free survival assessed using the log-rank test for multiple group comparisons.
All confidence intervals (CIs) are 2-tailed and calculated at the 0.05 level. Analyses were conducted using SAS statistical software version 8.01 (SAS Institute, Cary, NC).
RESULTS
The study population was predominantly non-Hispanic white (94.8%) having a mean age of 54.6 years (SD 7.1) and mean BMI of 25.8 kg/m2 (SD 4.9). The baseline prevalence of hypertension, hyperlipidemia, current smoking, and current MHT use were as follows: 24.0%, 29.0%, 11.6%, and 43.8%. History of diabetes in a first-degree relative and parental history of MI before age 60 years and was reported by 24.8% and 11.5% of women, respectively. The median (IQR) and mean (SD) of levels of HbA1c at study initiation were 4.99% (4.83, 5.17) and 5.03% (0.37), respectively.
Overall, the age-specific rates of diagnosed diabetes for women in this study of initially healthy women were lower than among women in the US population-at-large as estimated by the National Health Interview Survey (NHIS)26. In 1999, the year corresponding to median follow-up of our cohort, the estimated incidence per 1000 population for women aged 45–64 and 65–79 years in the NHIS were 8.2 and 9.0, respectively. Among WHS participants in the same age groups, diabetes incidence rates were 4.8 and 5.1 per 1000 person-years, respectively. Among 74 women with baseline HbA1c levels ≥ 7.0%, 81.1% (n=60) developed diabetes during the period of observation. The median follow-up for this category was identical to the rest of the cohort (10.1 vs. 10.1 years, p=0.8).
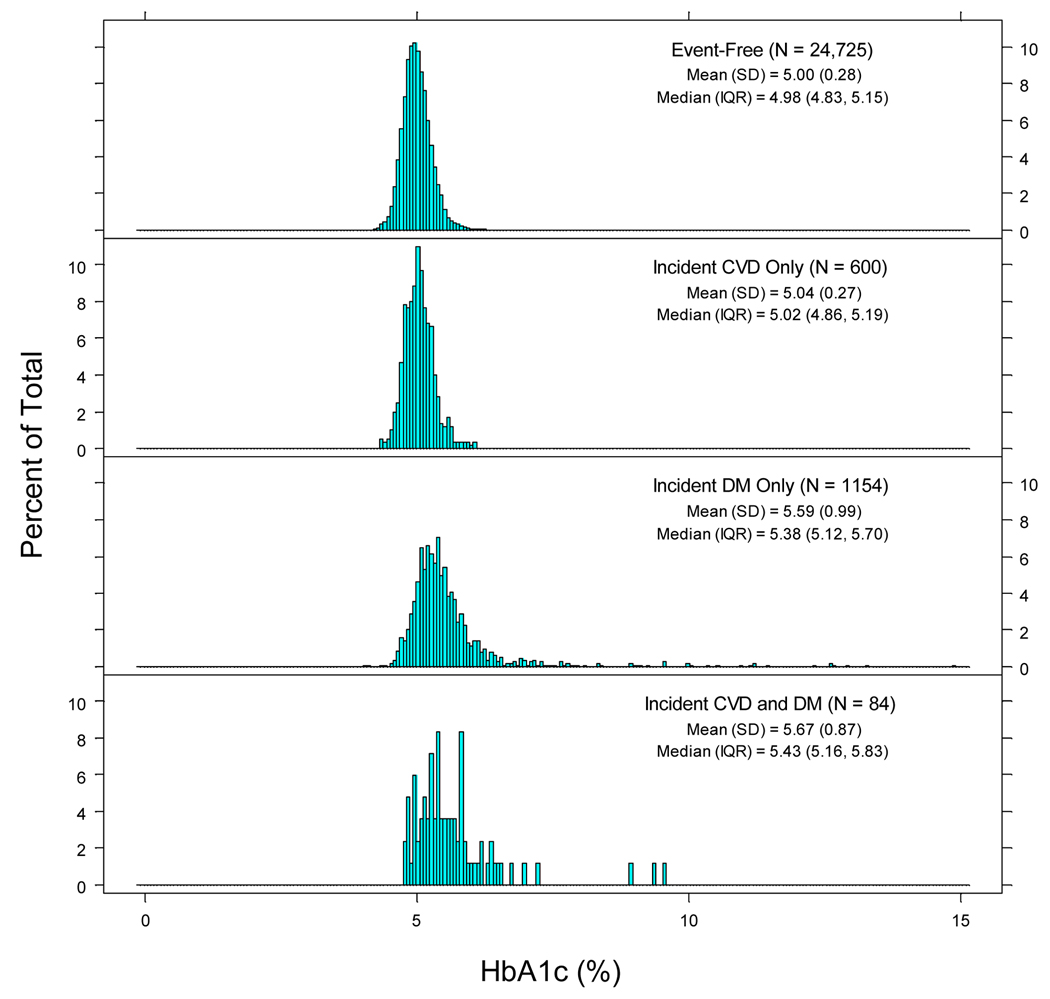
Figure 1 shows the distribution of HbA1c values according to disease categories: individuals remaining event-free, developing cardiovascular disease only, diabetes only, or both. HbA1c values appeared normally distributed among individuals remaining event-free but were rightward skewed in other subpopulations. Median HbA1c values were significantly lower in women remaining event-free when compared to all other subgroups (p<0.001 for all two-group comparisons).
Figure 1.
Histograms of HbA1c distribution according to four main groups: individuals remaining disease-free (N=24,725), developing incident cardiovascular disease (CVD) only (N=600), incident diabetes mellitus (DM) only (N=1,154), or both cardiovascular disease and diabetes mellitus (N=84).
Table 1 provides event rates and results of statistical models according to HbA1c quintiles. A graded risk increase was present in both age-adjusted and multivariable-adjusted models predicting clinical diabetes. Multivariable-adjusted RRs were 1.0, 1.1, 1.7, 2.6, 8.6 (p-trend<0.001). Exclusion of diabetes cases occurring within the first 2 years (n=175) had minimal influence on risk estimates; multivariable-adjusted RRs were 1.0, 1.3, 1.8, 2.8, and 8.2; p-trend<0.001. Cardiovascular disease incidence rose across quintiles of HbA1c. Age-adjustment weakened this association. Age-adjusted RRs were 1.0, 0.9, 1.1, 1.0, and 1.2 (p-trend=0.046) with an apparent increase in risk confined to women in the highest quintile. Results were not statistically significant in models additionally adjusting for cardiovascular risk factors. When modeled as a linear continuous term, there was no significant increase in risk of cardiovascular disease associated with a 1% increase in HbA1c (RR 1.10, p=0.28).
To examine threshold effects, analyses were repeated according to clinically expedient cutpoints of 0.5% increments above 5.0%, with the highest category defined by values ≥ 7.0%. For diabetes an increase in risk was noted in each category above 5.0% in both age-adjusted and multivariable models and after exclusion of cases diagnosed within 2 years or even 5 years of follow-up. Results were unchanged when analyses were limited to confirmed cases (N=406) occurring during 5 years of follow-up (data not shown). Because HbA1c, rather than reflecting ambient glucose levels, might indicate more widespread protein glycation27 and associated inflammation which may precede the development of both diabetes and cardiovascular disease28, we adjusted for baseline CRP and found similar results. In these analyses, the multivariable-adjusted RRs for incident diabetes across categories of HbA1c were 1.0, 2.9, 11.7, 27.8, 25.9, and 78.2 (95% CI for extreme categories: 57.3–106.8).
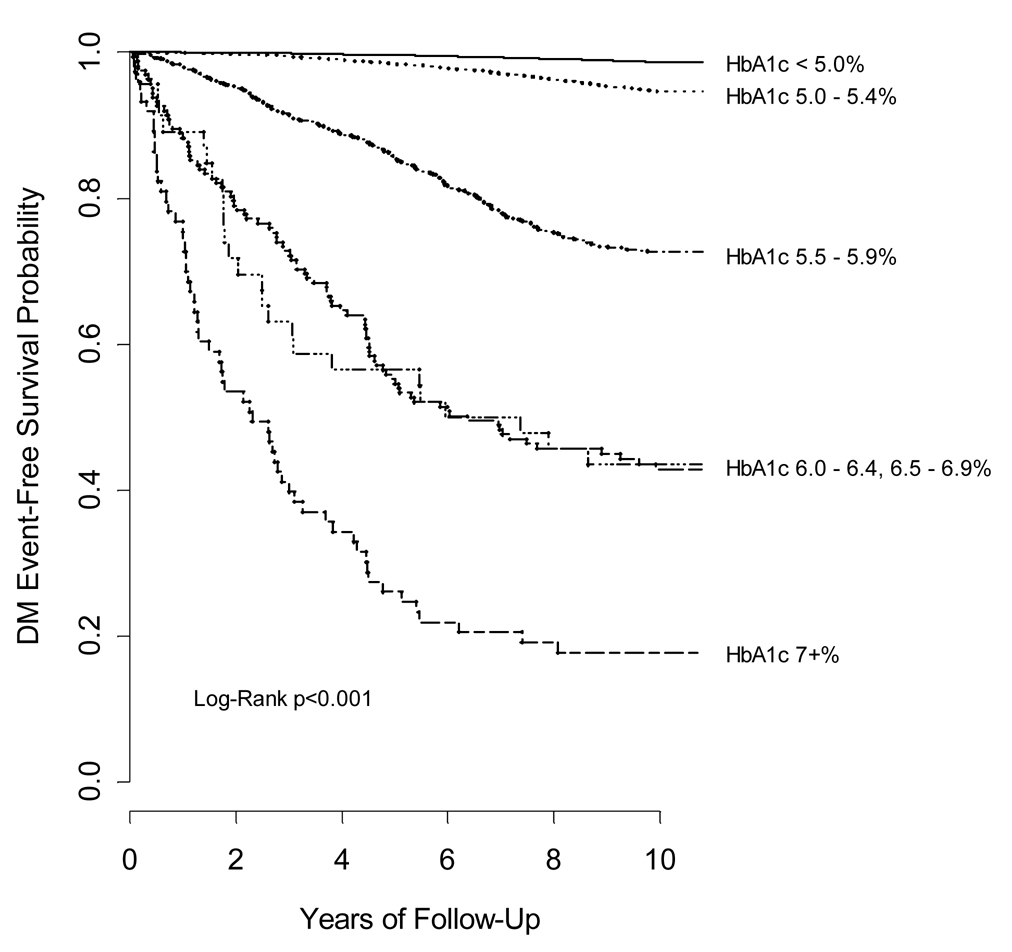
Figure 2 depicts Kaplan-Meier survival curves for diabetes according to HbA1c category. Event-free survival was significantly associated with baseline HbA1c (multi-group log-rank p<0.001). Importantly, curves appeared to diverge even among those with values of 5.0–5.4% and 5.5–5.9%.
Figure 2.
Kaplan-Meier curves for 10-year diabetes incidence according to baseline HbA1c category.
For incident cardiovascular disease, the risk associated with HbA1c was weaker than for diabetes (Table 2). The age-adjusted relative risk increased above a level of 5.0%; the RRs were 1.1, 1.6, 2.3, 2.7, and 2.3 for HbA1c categories 5.0–5.4%, 5.5–5.9%, 6.0–6.4%, 6.5–6.9%, and ≥ 7.0% as compared to a value below 5.0%. Risk estimates were statistically significant only in those higher HbA1c categories with relatively large numbers of events. In multivariable analyses, effect estimates were attenuated and no longer statistically significant. In analyses additionally adjusting for baseline CRP, the multivariable RR according to HbA1c category were 1.0, 0.9, 1.1, 1.5, 1.5, and 1.5 (95% CI for extreme categories: 0.6–4.1).
Table 2.
Relative risks (RRs) for diabetes and cardiovascular disease by HbA1c category
| HbA1c Categories (0.5% Increments) |
||||||
|---|---|---|---|---|---|---|
| < 5.0 | 5.0 – 5.4 | 5.5 – 5.9 | 6.0 – 6.4 | 6.5 – 6.9 | ≥ 7.0 | |
| N (%) | 13567 (51.1) | 11578 (43.6) | 1136 (4.7) | 162 (0.6) | 46 (0.2) | 74 (0.3) |
| Incident diabetes | ||||||
| Total Population | ||||||
| Events | 172 | 585 | 304 | 91 | 26 | 60 |
| Events/1000 person-years | 1.3 | 5.0 | 31.6 | 90.5 | 93.1 | 227.3 |
| Age-adjusted RR (95% CI) | 1.0 | 4.1 (3.5 – 4.9) | 25.6 (21.1 – 30.8) | 76.7 (59.4 – 99.1) | 77.6 (51.4 – 117.4) | 201.4 (149.7 – 271.1) |
| Multivariable-adjusted RR† (95% CI) | 1.0 | 2.9 (2.4 – 3.4) | 12.1 (10.0 – 14.8) | 29.3 (22.4 – 38.3) | 28.2 (18.5 – 43.0) | 81.2 (59.5 – 110.9) |
| Excluding cases of diabetes occurring during (a) the first two and (b) the first five years of follow-up | ||||||
| (a) Multivariable-adjusted RR‡ (95% CI) | 1.0 | 2.8 (2.4 – 3.4) | 10.8 (8.8 – 13.3) | 20.5 (14.9 – 28.2) | 16.9 (9.5 – 30.0) | 54.1 (35.3 – 82.9) |
| (b) Multivariable-adjusted RR§ (95% CI) | 1.0 | 3.0 (2.4 – 3.7) | 9.4 (7.2 – 12.1) | 12.5 (7.6 – 20.4) | 11.8 (5.2 – 27.0) | 29.2 (12.7 – 67.3) |
| Incident cardiovascular events* | ||||||
| Total population | ||||||
| Events | 288 | 325 | 53 | 11 | 3 | 4 |
| Events/1000 person-years | 2.2 | 2.9 | 4.8 | 7.1 | 6.9 | 5.7 |
| Age-adjusted RR (95% CI) | 1.0 | 1.1 (0.9 – 1.3) | 1.6 (1.2 – 2.1) | 2.3 (1.3 – 4.3) | 2.7 (0.9 – 8.5) | 2.3 (0.8 – 6.1) |
| Multivariable-adjusted RR† (95% CI) | 1.0 | 0.9 (0.8 – 1.1) | 1.2 (0.9 – 1.6) | 1.6 (0.9 – 3.0) | 1.7 (0.5 – 5.3) | 1.6 (0.6 – 4.5) |
Cardiovascular events: MI, CABG/PTCA, ischemic stroke, and cardiovascular death
Adjusted for same covariates as listed in Table 1 footnote.
After excluding women diagnosed with diabetes during the first two years of follow-up (N=175).
After excluding women diagnosed with diabetes during the first five years of follow-up (N=544).
COMMENT
In this large-scale prospective study of baseline HbA1c and 10-year incidence of type 2 diabetes and cardiovascular events in middle-aged and older American women, we found strong associations between asymptomatic glycemic exposure as quantified by HbA1c and incident diabetes. Our findings persisted in multivariable analysis after excluding early likely undiagnosed diabetes cases and in models assessing threshold effects. The risk gradient for incident diabetes was evident throughout the full range of baseline values even in categories minimally displaced from the population mean. Importantly, in this low-risk population, we observed an increased diabetes risk even among women with HbA1c levels between 5.0 and 5.5%, values falling within the normal reference range and not generally considered indicative of high risk in routine clinical practice. These findings support recent ADA recommendations to lower diagnostic thresholds for impaired fasting glucose.29 In contrast, in our study population the strength of association between HbA1c and cardiovascular events appeared weak and did not persist after accounting for established cardiovascular risk factors suggesting that these factors rather than dysglycemia itself may be more important for development of vascular events.
Prior studies of HbA1c as a predictor of diabetes have been largely confined to high-risk populations. Findings from longitudinal studies of Pima Indians,4, 6 Japanese5 and Chinese7 adults with baseline glucose intolerance or other diabetes risk factors suggest that in pre-diabetic individuals elevated HbA1c predicts progression to biochemical diabetes as determined by oral glucose tolerance testing. Among Pima Indians,4 glucose intolerant individuals with an elevated HbA1c (≥ 6.03%), a cutpoint 2 SDs above the mean for healthy Caucasian volunteers, had a 7-fold sex-adjusted increase in diabetes risk. In a later report from the same cohort,6 incorporation of HbA1c in a risk prediction algorithm allowed better identification of future diabetes than fasting or post-challenge glucose values. In this regard, a single measure of blood glucose has been shown to poorly characterize usual glycemia with large intraindividual variability, poor reproducibility, and potential for substantial misclassification.30–32 In contrast, HbA1c reflects the integrated average of glucose levels weighted proportionately toward more recent values.2 The test may be performed irrespective of prandial state, does not require glucose loading, and demonstrates good reproducibility on repeated measurements in non-diabetic subjects over time.33, 34 These favorable characteristics offer several practical advantages over other glycemic indicators.
Our findings demonstrate the potential prognostic importance of this biomarker at levels generally considered either normal or only mildly elevated in usual clinical care. We also chose to include individuals with HbA1c levels greater than 7.0% which was suggested to indicate biochemical diabetes in a meta-analysis comprising studies predominantly conducted in high-risk groups.25 It is important to note that diagnostic thresholds derived from high-risk populations may not be generalizable to lower risk groups as screening characteristics vary with underlying glucose frequency distributions.35, 36 In addition, while glycated hemoglobin levels are correlated with fasting and 2-hour blood glucose when glucose levels fall within the diabetic range, there is considerable overlap of HbA1c levels in milder forms of glucose intolerance.37, 38 Furthermore, in our low-risk population approximately 20% of those with HbA1c levels ≥ 7.0% did not develop clinical diabetes over a 10-year period and would have been incorrectly classified as diabetic based on this threshold criterion alone.
With regard to incident cardiovascular disease, prior studies of smaller size have demonstrated variable results. In the Rancho Bernardo cohort of 1,239 older non-diabetic adults, baseline HbA1c but not fasting or post-challenge glucose predicted cardiovascular mortality in women but not in men. A threshold effect was noted, such that women in the highest (≥ 6.7%) versus lower four quintiles had a near 3-fold elevation in adjusted risk.8 Subsequent reports from the Hoorn Study9, Framingham Offspring Study,11 and European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk)12 found significant associations when HbA1c was assessed on a linear basis as per 1.4% (2 SD units), per 0.7% (interquartile range), and per 1% increments, respectively. In particular, in the EPIC-Norfolk study a 1% increment in HbA1c was associated with a 21% increase in cardiovascular risk after multivariable adjustment in both men and women. However, when subjects with prior diabetes and cardiovascular disease were excluded this association was diminished and not statistically significant (RR 1.16, CI 0.99–1.36; p=0.08). In the Hoorn Study, which also presented categorical analyses with and without adjustment for traditional risk factors, the age-adjusted risk in the highest versus lowest category (≥ 6.5% versus < 5.2%), was 3.8 (95% CI 1.6–8.0). However, after additional adjustment for gender, hypertension, dyslipidemia, and smoking, this effect was attenuated and no longer statistically significant (RR 1.8; 95% CI 0.8–4.2). In our prior nested case-control study in the WHS cohort13, we similarly found that HbA1c levels were not predictive of cardiovascular events after adjustment for confounding effects of correlated cardiovascular risk factors.
Several limitations of our study merit further discussion. First, because our cohort comprised healthy predominantly non-Hispanic white women aged 45 years and older, our results may not be generalizable to other ethnic or racial groups, to men, or younger individuals who may otherwise be at risk for these disorders. Second, due to assay characteristics and specimen requirements, fasting glucose levels were not available. We were therefore unable to detect baseline mild unrecognized diabetes or lesser degrees of glucose intolerance. However, our results were similar in sensitivity analyses excluding individuals who developed clinical diabetes within 2 years and 5 years of follow-up. In addition, while type 2 diabetes may be unrecognized for many years in the general population, subjects in this study are health professionals who have regular access to medical care and therefore are less likely to remain undiagnosed. Nonetheless, given the likely inclusion of some subjects with undiagnosed diabetes and impaired glucose tolerance, our findings may not apply to those who are normoglycemic as assessed by more stringent metabolic criteria but importantly do apply to most clinic-based samples of asymptomatic individuals with no prior diagnosis of diabetes. Finally, we used a single baseline measurement of HbA1c. We therefore cannot evaluate the effects of changes in this parameter over time. However, glycated hemoglobin values have been found to reliably categorize glycemic status in non-diabetic subjects during a period of at least 4 to 6 years34 suggesting that exposure misclassification on this basis is likely to be small. Further, the distribution, mean and median values of HbA1c in our study are comparable to other referent populations with normal glucose tolerance.25, 39
In summary, we found that baseline HbA1c is an independent risk predictor for type 2 diabetes but not cardiovascular disease among healthy middle-aged and older women. We found evidence for a continuum of risk in the prediction of diabetes even at levels generally considered within the normal range. Although these data do not support the use of HbA1c as a single measure of diabetes risk, our results do suggest that the prognostic significance of elevated HbA1c may warrant a greater emphasis in primary prevention.
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (HL58755 and HL43851) and the National Cancer Institute (CA47988) and the Donald W. Reynolds Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976 Aug 19;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 2.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995 Apr;18(4):440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 3.Little RR. Glycated hemoglobin standardization--National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003 Sep;41(9):1191–1198. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- 4.Little RR, England JD, Wiedmeyer HM, et al. Glycated haemoglobin predicts progression to diabetes mellitus in Pima Indians with impaired glucose tolerance. Diabetologia. 1994 Mar;37(3):252–256. doi: 10.1007/BF00398051. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga H, Kosaka K. High glycosylated hemoglobin levels increase the risk of progression to diabetes mellitus in subjects with glucose intolerance. Diabetes Res Clin Pract. 1996 Mar;31(1–3):71–79. doi: 10.1016/0168-8227(96)01195-3. [DOI] [PubMed] [Google Scholar]
- 6.Narayan KM, Hanson RL, Pettitt DJ, Bennett PH, Knowler WC. A two-step strategy for identification of high-risk subjects for a clinical trial of prevention of NIDDM. Diabetes Care. 1996 Sep;19(9):972–978. doi: 10.2337/diacare.19.9.972. [DOI] [PubMed] [Google Scholar]
- 7.Ko GT, Chan JC, Tsang LW, Cockram CS. Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care. 2000 Dec;23(12):1770–1773. doi: 10.2337/diacare.23.12.1770. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Barrett-Connor E, Wingard DL, Shan J, Edelstein S. GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes. The Rancho Bernardo Study. Diabetes Care. 1996 May;19(5):450–456. doi: 10.2337/diacare.19.5.450. [DOI] [PubMed] [Google Scholar]
- 9.de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999 Aug;42(8):926–931. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 10.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk) BMJ. 2001 Jan 6;322(7277):15–18. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meigs JB, Nathan DM, D'Agostino RB, Sr, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002 Oct;25(10):1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 12.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004 Sep 21;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 13.Blake GJ, Pradhan AD, Manson JE, et al. Hemoglobin A1c level and future cardiovascular events among women. Arch Intern Med. 2004 Apr 12;164(7):757–761. doi: 10.1001/archinte.164.7.757. [DOI] [PubMed] [Google Scholar]
- 14.Ohlson LO, Svardsudd K, Welin L, et al. Fasting blood glucose and risk of coronary heart disease, stroke, and all-cause mortality: a 17-year follow-up study of men born in 1973. Diabet Med. 1986 Jan;3(1):33–37. doi: 10.1111/j.1464-5491.1986.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 15.Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998 Mar;21(3):360–367. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- 16.Scheidt-Nave C, Barrett-Connor E, Wingard DL, Cohn BA, Edelstein SL. Sex differences in fasting glycemia as a risk factor for ischemic heart disease death. Am J Epidemiol. 1991 Mar 15;133(6):565–576. doi: 10.1093/oxfordjournals.aje.a115928. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999 Feb;22(2):233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 18.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br Med J (Clin Res Ed) 1983 Sep 24;287(6396):867–870. doi: 10.1136/bmj.287.6396.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry IJ, Wannamethee SG, Whincup PH, Shaper AG. Asymptomatic hyperglycaemia and major ischaemic heart disease events in Britain. J Epidemiol Community Health. 1994 Dec;48(6):538–542. doi: 10.1136/jech.48.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005 Mar 31;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004 Sep 8;292(10):1188–1194. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 23.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997 Jul;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 24.Standards of medical care in diabetes--2006. Diabetes Care. 2006 Jan; Suppl 1:S4–S42. [PubMed] [Google Scholar]
- 25.Peters AL, Davidson MB, Schriger DL, Hasselblad V. A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels. Meta-Research Group on the Diagnosis of Diabetes Using Glycated Hemoglobin Levels. JAMA. 1996 Oct 16;276(15):1246–1252. [PubMed] [Google Scholar]
- 26.New Diabetes Data. [Accessed February, 2007];Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/diabetes/statistics/incidence/table5.htm.
- 27.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994 Jun;43(6):836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002 Jun;23(11):831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 29.Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2005 Jan;28 suppl_1:S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 30.Ganda OP, Day JL, Soeldner JS, Connon JJ, Gleason RE. Reproducibility and comparative analysis of repeated intravenous and oral glucose tolerance tests. Diabetes. 1978 Jul;27(7):715–725. doi: 10.2337/diab.27.7.715. [DOI] [PubMed] [Google Scholar]
- 31.Cummings ST, Fraser CG. Variability of capillary plasma glucose in healthy individuals in repeated 75 g oral glucose tolerance tests. Ann Clin Biochem. 1988 Nov;25(Pt 6):634–637. doi: 10.1177/000456328802500606. [DOI] [PubMed] [Google Scholar]
- 32.Ko GT, Chan JC, Woo J, et al. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998 Jan;35(Pt 1):62–67. doi: 10.1177/000456329803500107. [DOI] [PubMed] [Google Scholar]
- 33.Dunn PJ, Cole RA, Soeldner JS, Gleason RE. Reproducibility of hemoglobin AIc and sensitivity to various degrees of glucose intolerance. Ann Intern Med. 1979 Sep;91(3):390–396. doi: 10.7326/0003-4819-91-3-390. [DOI] [PubMed] [Google Scholar]
- 34.Meigs JB, Nathan DM, Cupples LA, Wilson PW, Singer DE. Tracking of glycated hemoglobin in the original cohort of the Framingham Heart Study. J Clin Epidemiol. 1996 Apr;49(1):411–417. doi: 10.1016/0895-4356(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 35.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000 Feb;23(2):187–191. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 36.Anand SS, Razak F, Vuksan V, et al. Diagnostic strategies to detect glucose intolerance in a multiethnic population. Diabetes Care. 2003 Feb;26(2):290–296. doi: 10.2337/diacare.26.2.290. [DOI] [PubMed] [Google Scholar]
- 37.Modan M, Halkin H, Karasik A, Lusky A. Effectiveness of glycosylated hemoglobin, fasting plasma glucose, and a single post load plasma glucose level in population screening for glucose intolerance. Am J Epidemiol. 1984 Mar;119(3):431–444. doi: 10.1093/oxfordjournals.aje.a113761. [DOI] [PubMed] [Google Scholar]
- 38.Davidson MB, Schriger DL, Peters AL, Lorber B. Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA. 1999 Apr 7;281(13):1203–1210. doi: 10.1001/jama.281.13.1203. [DOI] [PubMed] [Google Scholar]
- 39.Simon D, Senan C, Balkau B, Saint-Paul M, Thibult N, Eschwege E. Reproducibility of HbA1c in a healthy adult population: the Telecom Study. Diabetes Care. 1999 Aug;22(8):1361–1363. doi: 10.2337/diacare.22.8.1361. [DOI] [PubMed] [Google Scholar]