Abstract
Dietary caloric restriction (CR) slows aging, extends lifespan, and reduces the occurrence of age-related diseases in short-lived species. However, it is unclear whether CR can exert similar beneficial effects in long-lived species, like primates. Our objective was to determine if CR could attenuate purported age-related changes in the 24-h release of adrenal steroids. To this end, we examined 24-hour plasma profiles of cortisol, and dehydroepiandrosterone sulfate (DHEAS) in young and old, male and female rhesus macaques (Macaca mulatta) subjected to either ad libitum (AL)-feeding or CR (70% of AL) for 2–4 years. Hormone profiles from young monkeys showed pronounced 24-h rhythms. Cortisol concentrations were higher in old males but not females, whereas DHEAS rhythms were dampened with age in both sexes. The cortisol rhythms of old CR males resembled that of young control males. However, CR failed to prevent age-related declines in DHEAS and further dampened DHEAS rhythms in both sexes. Apart from the partial attenuation of the age-related cortisol elevation in the old males, 24-h adrenal steroid rhythms did not benefit from late-onset CR.
Keywords: Adrenal steroids, Aging, Caloric restriction, Circadian, Cortisol, DHEAS, Endocrine rhythms, Primate
1. Introduction
To date, dietary caloric restriction (CR) remains the only reproducible, nongenetic intervention shown to limit or delay deleterious aging processes in mammals. A 30–50% reduction in caloric intake, without malnutrition, results in the extension of average and maximal lifespans, reduction in the incidence of age-related diseases, and the maintenance of healthy physiological function later into life in short-lived species [50–52]. The first report of the lifespan extending effects of CR appeared over 70 years ago [27]. Since then, numerous studies have advanced CR research, and have generated progress toward elucidating possible mechanisms underlying the anti-aging benefits of CR [3, 9, 11]. Although the fundamental mechanisms contributing to extended longevity with CR remain to be fully understood, much attention has been given to the associated decline in steady-state oxidative damage related to lower mitochondrial free radical generation [9, 11, 42]. Despite the large body of evidence describing the benefits of CR in short-lived species, including yeast, worms, flies, fish, and rodents [2, 51, 52], it remains to be determined whether CR is relevant to human or nonhuman primate aging [18, 20, 24, 25, 34, 41, 50].
Cross sectional and longitudinal studies of the effects of CR on longevity and the aging process in the rhesus monkey (Macaca mulatta) began in 1987 [12]. However, with a maximum lifespan of ~40 years [4, 5, 10, 41, 44], several more years of study will be necessary before a consensus is reached on possible CR effects on rhesus lifespan. Therefore, relevant biomarkers of aging are critical to furthering the understanding of the effects of CR on the aging process, especially to assess effects in long-lived species like primates [14, 28, 29].
There are several hormones that can serve as biomarkers of aging because their overall levels, as well as their rhythms, are thought to be disrupted or dampened with advancing age [19, 26, 40, 46]. A rich area of study in the CR field has been to utilize these biomarkers, or hormonal indicators, to better understand if CR has a beneficial effect on primates [19, 39, 46]. One such biomarker is the adrenal steroid, dehydroepiandrosterone sulfate (DHEAS), which is a prohormone correlated with protective effects against several diseases associated with advancing age, including diabetes [15], heart disease [1], and cancer [53]. In both humans and nonhuman primates, overall circulating concentrations of this important adrenal-derived sex-steroid precursor show a postmaturational decline with age [16, 19, 31, 32, 39, 41, 46]. Conversely, circulating concentrations of the stress-related glucocorticoid, cortisol, which is also produced by the adrenal cortex, do not decline with age but rather appear to become elevated [8, 23, 48]. Moreover, elevated circulating cortisol has been suggested to play a role in neurodegeneration [8, 23, 33]. Earlier work in rats [43] and monkeys [46] suggested that CR might prevent an age-related elevation in circulating glucocorticoids.
Previous studies of the rhesus monkey found that 3–6 years of CR could significantly attenuate the rate of age-related decline in circulating DHEAS [19, 21, 24]. However, these studies focused only on young adults, and so it is unclear whether the effect of CR is maintained through to old age. Although attempts have been made previously to evaluate the effect of CR on various hormones in old nonhuman primates [19, 40], the previous findings were equivocal because the studies utilized samples collected at single time points under anesthesia after an overnight fast, thereby not adequately controlling for the diurnal variation in release pattern of these hormones. To overcome this problem, we based our analysis on blood samples that were collected serially throughout the night and day using a remote sampling system. Possible age-related changes in the 24-h patterns of the circadian hormones examined in the present study may help to explain the manifestation of disrupted and dampened hormonal and behavioral rhythms observed in many old humans and nonhuman primates [4, 7, 45, 49]. The objective was to address the question of whether CR can retard purported age-related disruptions in the overall release and 24-h patterns of circadian adrenal hormones that may contribute to age-related diseases in a long-lived species, such as the rhesus monkey.
In the present study, we examined young and old, male and female rhesus monkeys under approximately ad libitum-fed and 30% CR conditions, to address the effects of age, CR, and age in combination with CR on the overall levels and 24-h patterns of circulating adrenal hormones thought to be relevant to the maintenance of normal daily rhythms in circulating sex-steroids and physical activity and rest. In doing so, we have extended our previous pilot study, which comprised a total of only 16 males [46], by including additional males and also by now including females as well. Our aim was to test the hypothesis that 30% CR initiated late in life would prevent age-related elevations and decreases in 24-hour cortisol and DHEAS, respectively.
2. Materials and methods
2.1. Experimental animals
A total of forty-two adult rhesus macaques (Macaca mulatta) were utilized in the following experiments, which were approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC). The monkeys were housed in a temperature controlled environment (24°C), under a fixed 12L:12D photoperiod (lights on from 0700 h – 1900 h) with ad libitum access to drinking water. Specially formulated monkey chow (Agway, Ithaca, NY), as previously described [12, 13, 26], was provided at 0800 h and 1500 h each day, and monkeys were supplemented with daily fresh fruits or vegetables (10–40 calories). Animal care was provided by the ONPRC in accordance with the NIH Guide for the Care and Use of Laboratory Animals [30]. Daily health checks were preformed to ensure that each animal’s behavior, chow consumption, and waste production was normal. Additionally, routine physical examinations, hematological studies, fecal parasite checks, tuberculin testing, and dental cleaning were performed periodically. Daily menstrual records were established for each female based on close inspection of the monkey’s perineum and cage pan for signs of menstrual bleeding. All females were determined to have regular monthly cycles of ~28 days. Only data obtained from healthy monkeys, as determined by the ONPRC veterinary staff, were included in the statistical analyses.
2.1.1. Calorie Restriction (CR) protocol
Monkeys were matched for initial body weight and age. About half of the monkeys from each experimental group were subjected to a continuous 30% CR diet as previously described [12, 13, 26]. Diet composition did not differ between groups but monkeys under CR conditions received 30% fewer calories than the controls, which were fed approximately ad libitum. This diet formulation was previously determined to provide the appropriate caloric intake for rhesus monkeys based on sex, age and bodyweight [12, 13, 30]. To avoid any deficiencies in essential nutrients in the CR monkeys, the diet was enriched with additional vitamins and minerals.
2.1.2. Experiment 1: Effects of age and CR on 24-h hormone profiles in male rhesus monkeys
Ten young adult (10.6 ± 0.1 years old; 9.45 ± 0.56 kg) and 10 old (26.5 ± 0.7 years old; 7.64 ± 0.72 kg) male rhesus monkeys were used to examine the effects of age and CR on circulating cortisol and DHEAS levels and 24-h patterns. The experimental animals comprised a subgroup of monkeys from a CR study conducted at the Laboratory of Experimental Gerontology (LEG) of the National Institute on Aging (Baltimore, MD). Four to five monkeys from each age group had been subjected to continuous 30% CR for 4 years, and 5–6 served as age-matched ad libitum controls. The male monkeys (n=20) were transferred to the ONPRC for the present study and were allowed to acclimate for at least 3 months, while still undergoing caloric manipulation. They were then used for the remote 24-h blood sampling.
2.1.3. Experiment 2: Effects of age and CR on 24-h hormone profiles in female rhesus monkeys
Analogous to Experiment 1, 8 young adult (11.5 ± 0.1 years old; 6.64 ± 0.43 kg) and 14 old (22.3 ± 0.6 years old; 6.68 ± 0.30 kg) female rhesus monkeys were used to examine the effects of age and CR on circulating cortisol and DHEAS levels and 24-h patterns. The monkeys were obtained from the LEG and were used in the present study after an acclamation period of more that 3 months at the ONPRC. The CR protocol and animal husbandry used for the female monkeys in Experiment 2 were identical to those used in Experiment 1 barring one exception. The inclusion of female monkeys in the current more comprehensive study was an addendum to the original experimental design, which utilized males exclusively [46]. This extension of the experimental design was initiated after the male study had begun. For this reason, the duration of CR experienced by the females was 2 years rather than the 4 years experienced by the males. Also, there were cohort differences between the sexes most notably, the old females (all premenopausal) were ~4 years younger than the old male group.
2.2. Remote 24-h blood sampling
At least 1 week prior to blood sampling, each monkey was fitted surgically with an indwelling subclavian vein catheter, and allowed a 2 week adjustment period; during this time the monkeys became accustomed to wearing a protective nylon mesh jacket, as previously described [47]. The sampling set-up consisted of a swivel at the top of each monkey’s cage, a protective stainless-steel flexible tether, and a nylon mesh jacket (Lomir Biomedical In., Malone NY) to protect the catheter tubing exiting from each monkey’s back. To maintain catheter patency, heparinized saline (4 IU/ml) was continuously infused (~1 ml/h) using a peristaltic pump (Gilson Medical Electronics, Middeton, WI). Serial blood samples were collected via the vascular line and swivel remotely from an adjacent room, thereby ensuring that the monkeys remained undisturbed during the entire sampling procedure. Beginning at 1900 h, blood (1ml) was collected into EDTA-coated borosilicate glass tubes at 1-h intervals across the night and day for 24 hours. The samples were centrifuged at 4°C and the plasma supernatant was stored at −20°C until assay. In each experiment, blood sampling was performed at approximately the same time of the year.
2.3. Hormone assays
2.3.1. Cortisol and DHEAS
Plasma concentrations of cortisol and DHEAS were determined at the ONPRC Endocrine Services Laboratory using validated monkey reagents as previously described [6, 46]. Cortisol levels were determined by electrochemiluminescence (ECL) using the Elecsys 2010 Platform (Roche Diagnositics, Indianapolis, IN). DHEAS levels were determined by radioimmuoassay (RIA), which included a highly specific antibody (Endocrine Sciences, Tarzana, CA) raised against DHEAS-17-(O-carboxymethly)oxime-BSA and [3H]DHEAS (SA, 22 Ci/mmlol). The intra- and inter-assay coefficients of variation were less than 10% for each assay and the assay detection limits were 3 ng/ml.
2.4. Statistical analysis
Group mean (Mean) hormone values were determined by taking the overall mean of the individual hormone values spanning the entire 24-h sampling period. Group maximum (Max) hormone values were determined by first identifying the maximum and adjacent values spanning five hours for an individual and then taking the mean of those individual Max values. Similarly, the group minimum (Min) values were determined by taking the mean of the five adjacent minimum values spanning a five-hour period. Additionally, the mean 24-h area under the curve (AUC) of cortisol and DHEAS concentrations were determined. Two-way analysis of variance (ANOVA) followed by the Newman-Keuls test was used to evaluate group differences (using age and diet as variables) in Mean, Max, Min, and mean 24-h AUC hormone concentrations. A p-value < 0.05 was accepted as being statistically significant.
3. Results
3.1. Experiment 1: Effects of age and CR on 24-h adrenal steroid hormone profiles in male rhesus monkeys
3.1.1. Effects of age on 24-h plasma cortisol and DHEAS profiles in male rhesus monkeys
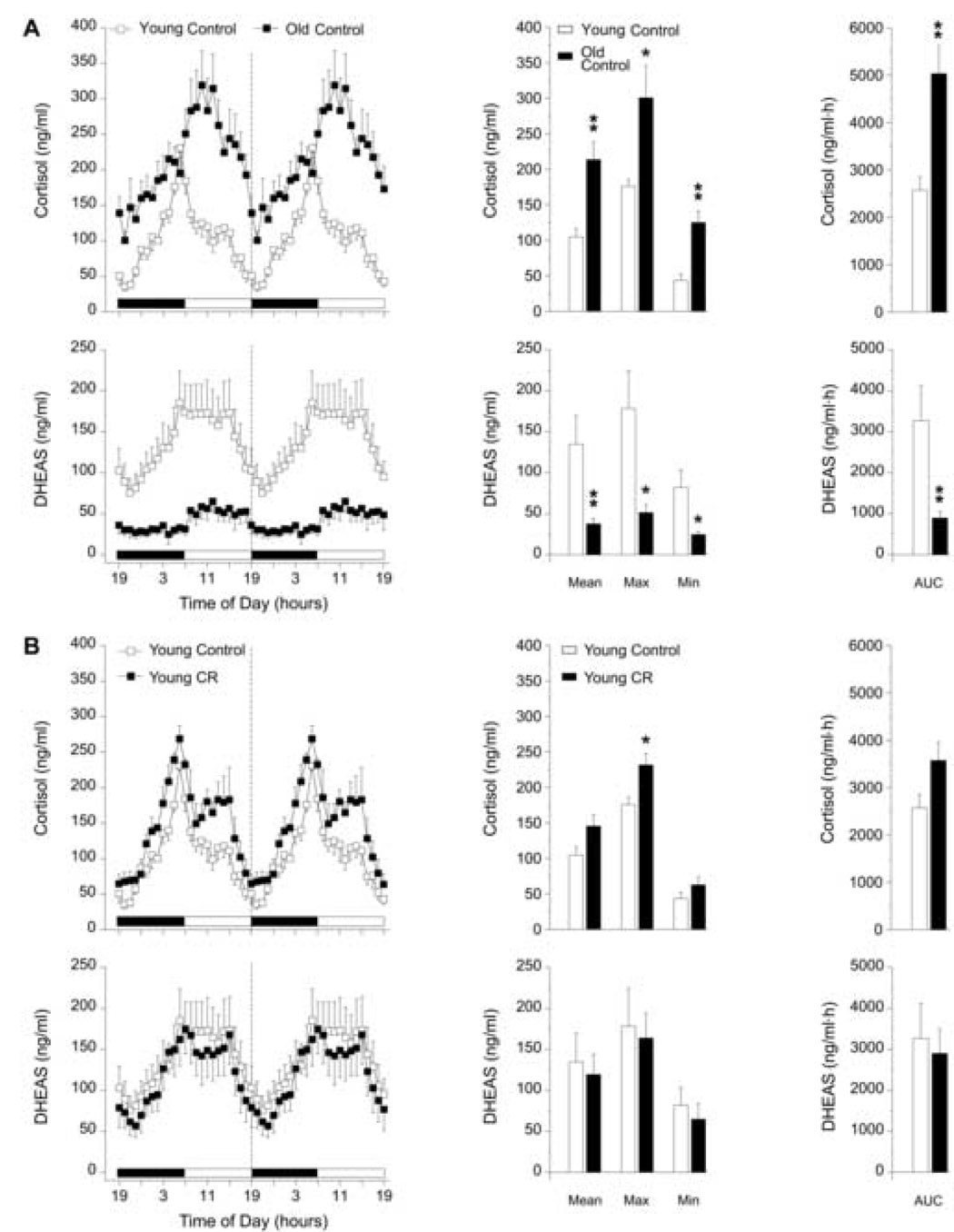
Twenty-four-hour plasma profiles (left panels) of cortisol and DHEAS, and the corresponding analyses (center and right panels) are shown in Figure 1. Young males showed robust diurnal variations in the 24-h profiles of cortisol and DHEAS, regardless of diet (Figs. 1A and 1B). The overall Mean (p < 0.01), Max (p < 0.05), Min (p < 0.01), and mean 24-h AUC (p < 0.01) values of cortisol concentrations in the old control males were significantly higher and the peak of the cortisol rhythm appeared to be slightly phase-delayed compared to that of the young control males. Conversely, the old control males showed a diurnal variation in DHEAS that was markedly dampened (Fig. 1A). Mean (p < 0.01), Max (p < 0.05), Min (p < 0.05), and mean 24-h AUC (p < 0.01) values of DHEAS in the old males were statistically lower than in the young males.
Fig. 1.
Effect of age (A), and four years of CR (B, C), and age in combination with four years of CR (D) on circulating 24-h hormone patterns in male rhesus monkeys. Left panels: mean plasma cortisol and DHEAS 24-h hormone profiles from (A) young control (~10 years old, n = 5) and old control (~26 years old, n = 6) males; (B) young control (~10 years old, n = 5) and young CR (~10 years old, n = 5) males; (C) old control (~26 years old, n = 6) and old CR (~26 years old, n = 4) males; (D) young control (~10 years old, n = 5) and old CR (~26 years old, n = 4) males. Although the blood samples were collected over 24 hours, from 1900 – 1900 h, the data have been double plotted (separated by a vertical dashed line) to aid in the visualization of the night and day variations in hormone concentrations. The horizontal black and white bar on the abscissas correspond to the 12L:12D lighting schedule. Center panels: analyses of age, CR, and age in combination with CR-related differences in mean (Mean), maximum (Max), and minimum (Min) hormone values. Right panels: analyses of age, CR, and age in combination with CR-related differences in the mean 24-h area under the curve (AUC) of cortisol and DHEAS concentrations. Values are expressed as mean ± SEM. *p < 0.05, **p < 0.01.
3.1.2. Effects of four-year CR on 24-h plasma cortisol and, DHEAS profiles in male rhesus monkeys
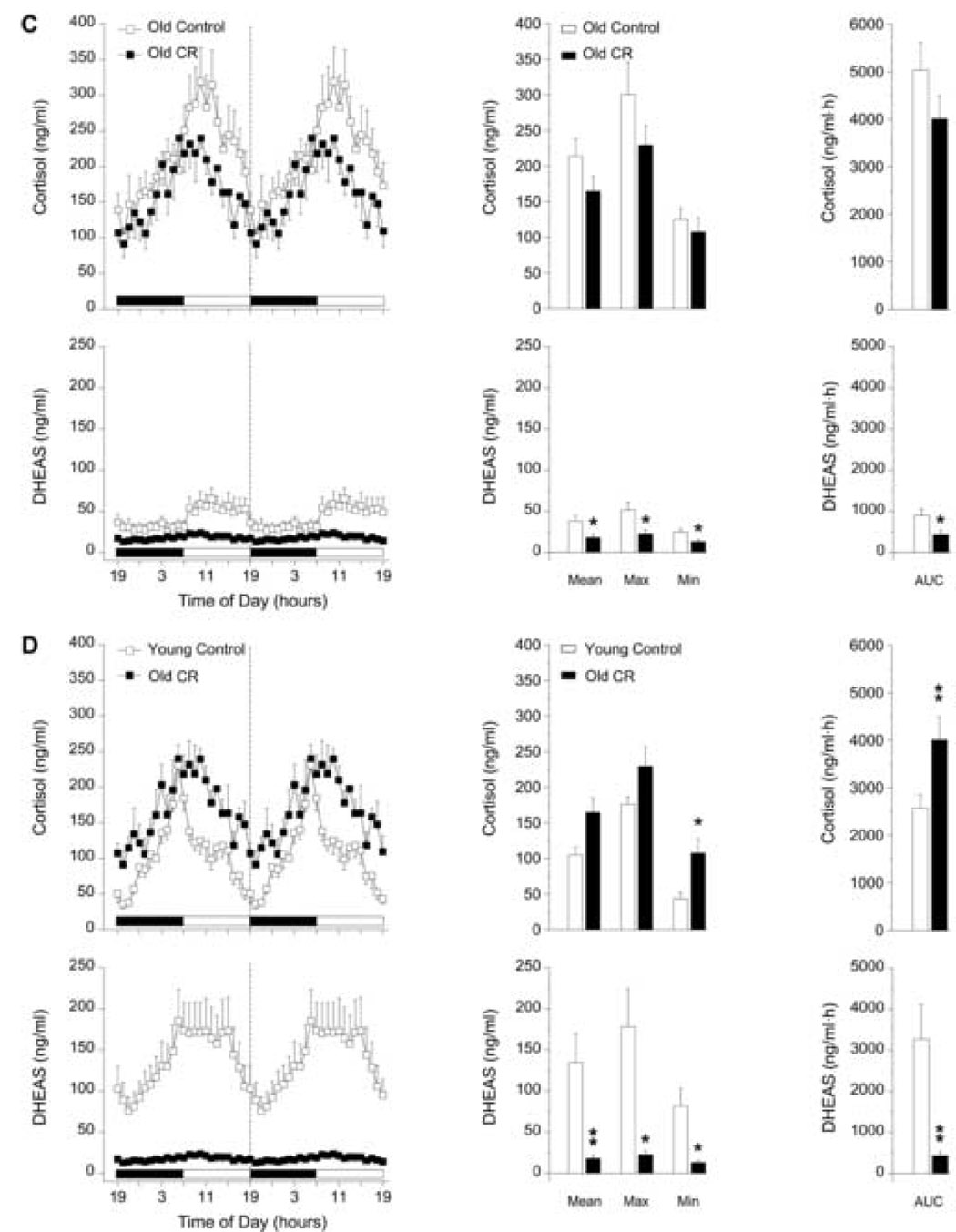
Figure 1 (B–D) illustrates the effects of 4 years of CR on 24-h plasma profiles of cortisol and DHEAS in male rhesus monkeys. CR had little effect on the 24-h rhythm of cortisol and no significant effect on the 24-h rhythm of DHEAS in the young males. The Max concentration of cortisol in CR young males was significantly higher (p < 0.05) than that of age-matched controls but this difference did not contribute to an overall elevation in the Mean or mean 24-h AUC of cortisol (Fig 1B). The Mean, Max, Min and mean 24-h AUC values of cortisol did not significantly change with CR in the old males (Fig 1C). However, there appeared to be a non-significant trend for all of the cortisol values to decrease under CR in the old males. Interestingly, Figure 1C illustrates that the 24-h rhythm of DHEAS in the old males subjected to 4 years of CR was further dampened (p < 0.05) than what was evident with the effects of age alone.
To some extent the age-effects previously observed in the diurnal variation of plasma cortisol were prevented in the old males subjected to 4 years of CR (Fig. 1D). The age-related phase-delay in the cortisol rhythm was partially prevented and the Mean and Max concentrations of cortisol in old males under CR were not significantly different compared to young controls. However, CR did not prevent the age-effects on Min (p < 0.05) and mean 24-h AUC (p < 0.01) values of cortisol, which remained significantly higher than in the young controls (Fig. 1D). Four years of late-onset CR in the old males completely failed to prevent the age-effects on the 24-h rhythm of DHEAS (Fig. 1D). Mean (p < 0.01), Max (p < 0.05), Min (p < 0.05), and mean 24-h AUC (p < 0.01) values of DHEAS in the old CR males were statistically lower compared to the DHEAS values of the young males.
3.2. Experiment 2: Effects of age and CR on 24-h adrenal steroid hormone profiles in female rhesus monkeys
3.2.1. Effects of age on 24-h plasma cortisol and DHEAS profiles in female rhesus monkeys
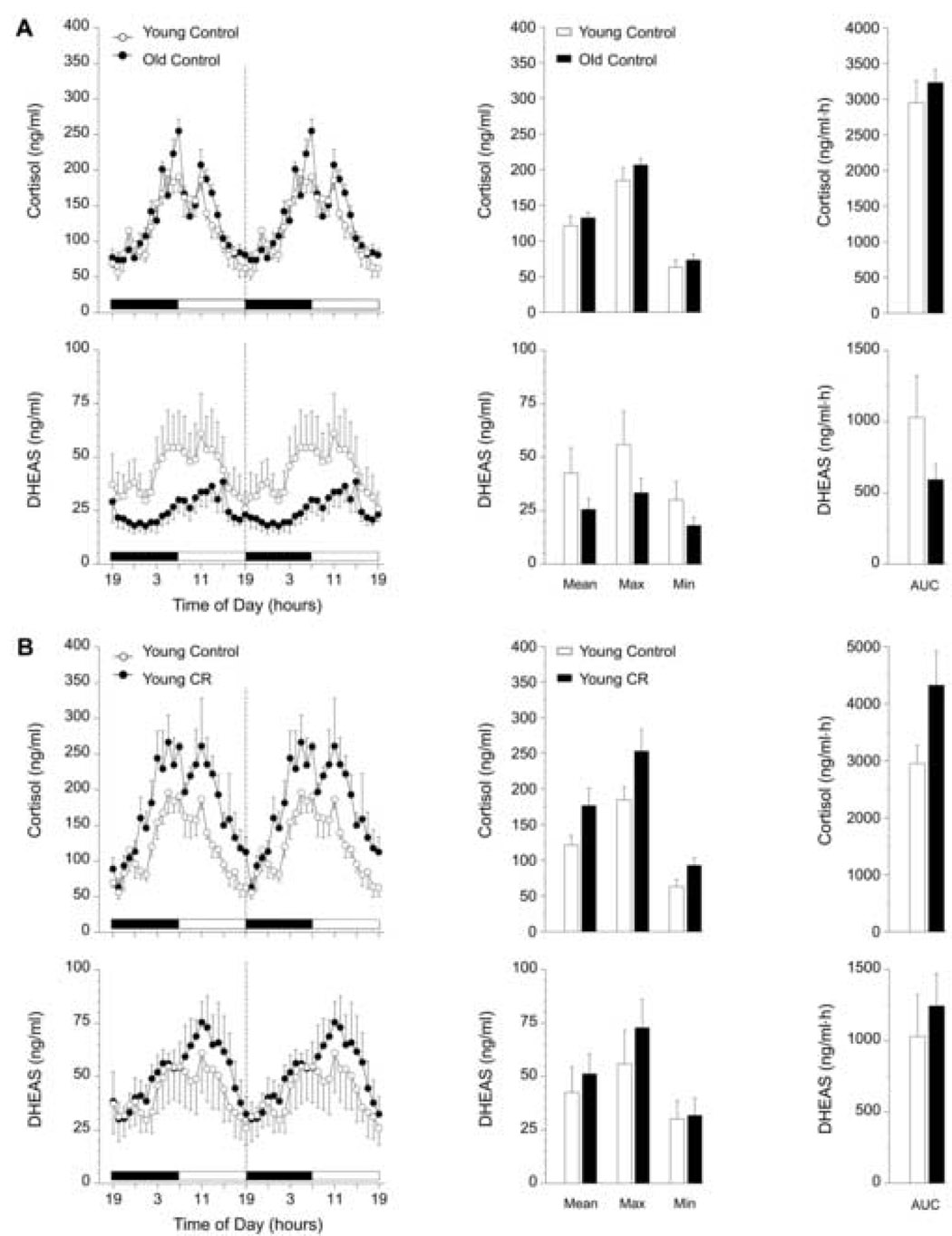
Figure 2A shows the effects of age on 24-h hormone profiles (left panels) and the corresponding analyses (center and right panels) of cortisol and DHEAS in female rhesus monkeys on the control diet. The 24-h patterns of plasma cortisol from young and old control female monkeys were qualitatively and quantitatively indistinguishable. Conversely, the diurnal pattern of plasma DHEAS in old females appeared to be dampened compared to the young group, although this trend was not statistically significant.
Fig. 2.
Effect of age (A), and 2 years of CR (B, C), and age in combination with 2 years of CR (D) on circulating 24-h hormone patterns in female rhesus monkeys. Left panels: mean plasma cortisol and DHEAS 24-h hormone profiles from (A) young control (~12 years old, n = 4) and old control (~22 years old, n = 7) females; (B) young control (~12 years old, n = 4) and young CR (~12 years old, n = 4) females; (C) old control (~22 years old, n = 7) and old CR (~22 years old, n = 7) females; (D) young control (~12 years old, n = 4) and old CR (~22 years old, n = 7) females. Although the blood samples were collected over 24 hours, from 1900 – 1900 h, the data have been double plotted (separated by a vertical dashed line) to aid in the visualization of the night and day variations in hormone concentrations. The horizontal black and white bar on the abscissas correspond to the 12L:12D lighting schedule. Center panels: analyses of age, CR, and age in combination with CR-related differences in mean (Mean), maximum (Max), and minimum (Min) hormone values. Right panels: analyses of age, CR, and age in combination with CR-related differences in the mean 24-h area under the curve (AUC) of cortisol and DHEAS concentrations. Values are expressed as mean ± SEM. *p < 0.05.
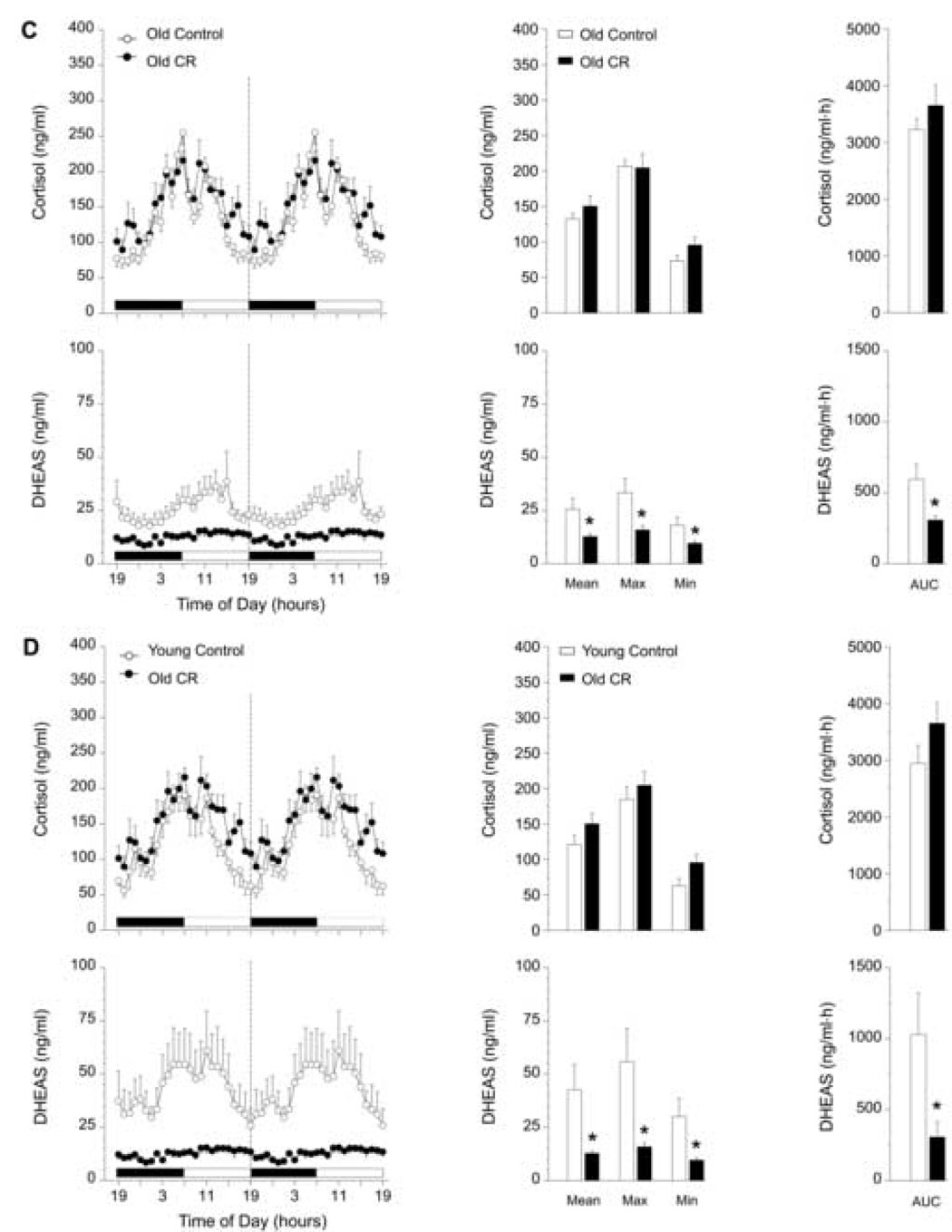
3.2.2. Effects of two-year CR on 24-h plasma cortisol and DHEAS profiles in female rhesus monkeys
Mean, Max, Min, and mean 24-h AUC values of the twenty-four-hour cortisol profiles from the young and old female monkeys subjected to CR continuously for two years did not differ significantly from age-matched controls (Figs. 2B and 2C). Moreover, CR did not have an effect on circulating DHEAS concentrations in the young females. In contrast, old females subjected to CR had significantly lower (p < 0.05) Mean, Max, Min, and mean 24-h AUC values of DHEAS compared to old control females (Fig. 2C). Two years of late-onset CR clearly did not prevent the age-related declining trend in DHEAS concentrations depicted in Figure 2A, but caused a further attenuation of DHEAS in old female monkeys (Fig. 2D).
Discussion
Thus far, the responses to CR in the rhesus monkey have been markedly similar to that of the rodent. Monkeys and rodents subjected to CR experience delayed sexual [38] and skeletal [22] maturation, decreased body temperature [26], and decreased plasma insulin levels and altered glucose regulation [17, 26]. However, the effects of CR on the longevity of the rhesus monkey will take several more years of investigation to determine. For this reason, it is essential to identify appropriate biomarkers of aging that are useful under dietary manipulations for furthering the understanding of CR’s effects on long-lived species such as the rhesus monkey. The effects of CR on purported age-related changes in hormonal indicators such as the adrenal steroids, cortisol and DHEAS, may provide critical insight regarding the benefit of CR in long-lived species.
In the present study, we revealed distinct qualitative and quantitative age-related differences in the circulating 24-h cortisol and DHEAS rhythms of male monkeys and a similar age-related trend in the circulating 24-h DHEAS rhythm of female rhesus monkeys. Levels of cortisol increased with age in males, but no age-related change was observed in the cortisol rhythm of old females. Additionally, the phase relationship of the 24-h cortisol rhythm changed with age in males. Although the qualitative characteristics of the circadian wave shape were preserved in the 24-h cortisol rhythm of old males, the peak of the rhythm and ensuing decline were delayed nearly 3 hours. It is possible that the age-related elevation in cortisol levels that we observed in the male but not in the female monkeys was attributable to cohort differences between sexes. The old females studied were ~4 years younger than the old males studied, and the young females were ~2 years older than the young male group. This age difference is noteworthy, as the median lifespan of captive rhesus monkeys is ~25 years; thus females age-matched to the males (~26 years old) would presumably exhibit more advanced aging characteristics such as the onset of menopause and age-associated pathologies [10, 41, 44]. It is possible that an even older female group would show similar changes to those observed in the old males. The age-related changes we observed in males but not in females were consistent with previous findings in humans indicating that prior to menopause, women have lower 24-h mean cortisol levels than age-matched men [48]. Unlike the 24-h rhythm of cortisol, we observed age-related changes in 24-h plasma DHEAS in both sexes. DHEAS levels throughout the day and night markedly declined with age in the males and a similar non-significant trend was evident with age in the females. The age-related decline in DHEAS in the males was very pronounced but not significantly so in the females in part due to lower pre-existing DHEAS levels in the young females (mean 24-h AUC: young females = 1029.7 ± 293.2 ng/ml·h, young males = 3270.7 ± 849.4 ng/ml·h). These findings support and extend previous works [16, 19, 26, 39, 41, 46] by showing a marked age-related decline in the magnitude of the 24-h DHEAS rhythm in the males and a similar trend in the female monkeys, and by showing an elevation and slight shift in the 24-h cortisol rhythm of aged male monkeys. The dramatic age-related decline in DHEAS levels that we observed in the males matches previous reports in other primate species, including humans [16, 19, 31, 32, 39, 41, 46].
Regarding the influence of CR on the hormonal parameters examined in our study, there were also age effects in the male and female monkeys that merit discussion. An elevation in circulating corticosterone is one of the hallmarks of CR in young adult rodents, but in old rodents CR reduces corticosterone levels [43]. This is in contrast to our observations of 24-h cortisol levels in young and old monkeys subjected to CR. The effects of CR on the 24-h cortisol rhythms of the monkeys we observed were unimpressive. The young male monkeys subjected to CR for 4 years had a modest increase in Max cortisol levels that did not result in any change in Mean or mean 24-h AUC values of cortisol. Among young female monkeys, 2 years of CR appeared to elevate cortisol levels, but this difference was not confirmed statistically. Likewise, the old females on CR had similar cortisol rhythms compared to age-matched controls. The circulating cortisol levels of old male monkeys subjected to CR did not differ statistically with the control old males although, qualitatively, they appeared lower. Conversely, under CR conditions, a partial relief of the age-associated elevation and of the delay in the 24-h cortisol rhythm was evident when the old CR males were compared with the young controls.
A previous report from the NIA study of aging in male rhesus monkeys documented that CR attenuated the age-related decline in DHEAS levels [38]. In the current study, we did not observe an attenuation of the postmaturational decline in DHEAS under CR. Conversely, we observed an age-dependant decline in DHEAS that was furthered by CR. In sharp contrast to the previous NIA report, the old females subjected to CR in the present study had a more pronounced dampening of 24-h DHEAS concentrations compared to what was observed with age alone. As with the old females subjected to 2 years of CR, the age effect on the DHEAS rhythm of the old males was not attenuated by 4 years of CR. Instead, the DHEAS rhythm was further dampened by CR than by age alone.
Several factors could account for the dampening effect of CR on the aged monkeys’ DHEAS rhythms. First, the timing of initiation and duration of CR intervention may be critical in determining the hormonal responses of a long-lived species, like the rhesus monkey. Typically, studies involving short-lived species have initiated CR very early in life and have continued it throughout life into old age [50–52], and longer intervention or CR initiated earlier in life may reveal similar benefits in the monkey. Second, DHEAS may be an inappropriate biomarker of aging under CR conditions. This possibility is supported by studies showing that short-term changes in diet alone can affect serum and urinary DHEAS levels in humans [35–37]. Consequently, one can not exclude the possibility that the CR manipulation itself may have directly caused a dampening of DHEAS levels, by a mechanism that is unrelated to aging.
In general, apart from a limited attenuation of an age-related change in the 24-h cortisol rhythm experienced by the old male monkeys, our results did not support the hypothesis that CR imposed over a 2–4 year period could prevent or limit age-related changes in the 24-h rhythms of circadian adrenal hormones. However, our suggestion is not that CR may have deleterious effects on the aging process per se but rather, it may be that the timing and duration of CR are critical factors in determining any benefit. A longer duration and earlier initiation of CR may attenuate age-related changes while a short-term and late-onset CR may exacerbate the effects of aging. Additionally, the particular endocrine markers examined in the current study may be poor indicators of advancing age under dietary manipulations and the examination of other better-suited biomarkers may be necessary to determine if short-term, late-onset CR is truly unbeneficial. Overall, the intervention of 2–4 years of CR in the rhesus monkeys did not result in an impressive reversal of the age-effects on the circulating 24-h patterns of adrenal steroids.
Acknowledgements
The authors are grateful for the technical expertise of Vasilios T. Garyfallou, Dr. Frank Koegler, Lindsay Pranger, and Dr. David Hess at the ONPRC, and the ONPRC Department of Animal Resources for their animal care. This work was supported by NIH grants: AG-023477, AG-19914, HD-29186 & RR-00163.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors confirm that no actual, or potential, conflicts of interest exist.
References
- 1.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315(24):1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 2.Barrows CH, Kokkonen GC. Dietary restriction and life extension, biological mechanisms. In: Moment GB, editor. Nutritional approaches to aging research. Boca Raton, FL: CRC Press Inc; 1982. pp. 219–243. [Google Scholar]
- 3.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 4.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3rd Edition. Philadelphia: WB Saunders; 2000. pp. 26–42. [Google Scholar]
- 5.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J Gerontol. 2003;58A(3):212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 6.Ducsay CA, Hess DL, McClellan MC, Novy MJ. Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in baboons. J Clin Endo Metab. 1991;73(2):385–395. doi: 10.1210/jcem-73-2-385. [DOI] [PubMed] [Google Scholar]
- 7.Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- 8.Ferrari E, Arcaini A, Gornati R, Pelanconi L, Cravello L, Fioravanti M, Solerte SB, Magri F. Pineal and pituitary-adrenocortical function in physiolocial aging and in senile dementia. Exp Gerontol. 2000;35(9–10):1239–1250. doi: 10.1016/s0531-5565(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 9.Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146(9):3713–3717. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- 10.Hansen BC, Bodkin NL, Ortmeyer HK. Calorie restriction in nonhuman primates: mechanisms of reduced morbidity and mortality. Toxicol Sci. 1999;52(2 supp):56–60. doi: 10.1093/toxsci/52.2.56. [DOI] [PubMed] [Google Scholar]
- 11.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 12.Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45(5):B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- 13.Ingram DK, Lane MA, Cutler RG, Roth GS. Longitudinal study of aging in monkeys: effects of diet restriction. Neurobiol Aging. 1993;14:687–688. doi: 10.1016/0197-4580(93)90072-j. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 15.Kameda W, Daimon M, Oizumi T, Jimbu Y, Kimura M, Hirata A, Yamaguchi H, Ohnuma H, Igarashi M, Tominaga M, Kato T. Association of decrease in serum dehydroepiandrosterone sulfate levels with the progression to type 2 diabetes in men of a Japanese population: The Fungata Study. Metabolism. 2005;54:669–676. doi: 10.1016/j.metabol.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez J-L, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: Its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 17.Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, Read V, Roth GS. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am J Physiol. 1995;268(5 Pt 1):E941–E948. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- 18.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Ann N Y Acad Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 19.Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by caloric restriction. J Clin Endo Metab. 1997 doi: 10.1210/jcem.82.7.4038. 82-2093-6. [DOI] [PubMed] [Google Scholar]
- 20.Lane MA, Ingram DK, Roth GS. Beyond the rodent model: calorie restriction in rhesus monkeys. Age. 1997;20:45–56. doi: 10.1007/s11357-997-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane MA, Ingram DK, Roth GS. Nutritional modulation of aging in nonhuman primates. J Nutr Health Aging. 1999;3:69–76. [PubMed] [Google Scholar]
- 22.Lane MA, Reznick AZ, Tilmont EM, Lanir A, Ball SS, Read V, Ingram DK, Cutler RG, Roth GS. Aging and food restriction alter some indices of bone metabolism in male rhesus monkeys (Macaca mulatta) J Nutr. 1995;125(6):1600–1610. doi: 10.1093/jn/125.6.1600. [DOI] [PubMed] [Google Scholar]
- 23.Magri F, Locatelli M, Balza G, Molla G, Cuzzoni G, Fioravanti M, Solerte SB, Ferrari E. Changes in endocrine circadian rhythms as markers of physiological and pathological brain aging. Chronobiol Int. 1997;14(4):385–396. doi: 10.3109/07420529709001459. [DOI] [PubMed] [Google Scholar]
- 24.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 25.Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging. 2005;26:1117–1127. doi: 10.1016/j.neurobiolaging.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 27.McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 28.Nakamura E, Lane MA, Roth GS, Cutler RG, Ingram DK. Evaluating measures of hematology and blood chemistry in male rhesus monkeys as biomarkers of aging. Exp Gerontol. 1994;29:151–177. doi: 10.1016/0531-5565(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura E, Lane MA, Roth GS, Ingram DK. A strategy for identifying biomarkers of aging: Further evaluation of hematology and blood chemistry data from a caloric restriction study in rhesus monkeys. Exp Gerontol. 1998;35:421–443. doi: 10.1016/s0531-5565(97)00134-4. [DOI] [PubMed] [Google Scholar]
- 30.National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. Washington: National Academy Press; 1996. [Google Scholar]
- 31.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 32.Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75(4):1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 33.Porter NM, Landfield PW. Stress hormones and brain aging: adding injury to insult? Nat Neurosci. 1998;1(1):3–4. doi: 10.1038/196. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 35.Remer T, Manz F. Role of nutritional status in the regulation of adrenarche. J Clin Endocrinol Metab. 1999;84(11):3936–3944. doi: 10.1210/jcem.84.11.6093. [DOI] [PubMed] [Google Scholar]
- 36.Remer T, Pietrzik K, Manz F. Short-term inpact of a lactovegitarian diet on adrenocortical activity and adrenal androgens. J Clin Endocrinol Metab. 1998;83(6):2132–2137. doi: 10.1210/jcem.83.6.4883. [DOI] [PubMed] [Google Scholar]
- 37.Remer T. Adrenarche and nutritional status. J Pediatr Endocrinol Metab. 2000;13 Suppl 5:1253–1255. [PubMed] [Google Scholar]
- 38.Roth GS, Blackman MR, Ingram KD, Lane MA, Ball SS, Cutler RG. Age-related changes in androgen levels of rhesus monkeys subjected to diet restriction. Endocrine J. 1993;1:227–234. [Google Scholar]
- 39.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 40.Roth GS, Lesnikov V, Lesnikov M, Ingram DK, Lane MA. Dietary caloric restriction prevents the age-related decline in plasma melatonin levels of the rhesus monkey. J Clin Endo Metab. 2001;86:3292. doi: 10.1210/jcem.86.7.7655. [DOI] [PubMed] [Google Scholar]
- 41.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 42.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996 doi: 10.1126/science.273.5271.59. 273-59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart J, Meaney MJ, Aitken D, Jensen L, Kalant N. The effects of acute and life-long food restriction on basal and stress-induced serum corticosterone levels in young and aged rats. Endocrinology. 1988;123(4):1934–1941. doi: 10.1210/endo-123-4-1934. [DOI] [PubMed] [Google Scholar]
- 44.Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15(3):263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- 45.Tobler I. Napping and polyphasic sleep in mammals. In: Dinges DF, Broughton RJ, editors. Sleep and Alertness: Chronobiological, Behavioral, and Medical Aspects of Napping. New York: Raven Press; 1989. pp. 9–30. [Google Scholar]
- 46.Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann NY Acad Sci. 2004;1019:1–5. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- 47.Urbanski HF, Garyfallou VT, Kohama SG, Hess DL. Alpha-adrenergic receptor antagonism and N-methyl-d-aspartate (NMDA) induced luteinizing hormone release in female rhesus macaques. Brain Res. 1997;744:96–104. doi: 10.1016/s0006-8993(96)01083-9. [DOI] [PubMed] [Google Scholar]
- 48.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endo Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 49.Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using Etho Vision. Neurobiol Aging. 2006;27(10):1477–1483. doi: 10.1016/j.neurobiolaging.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337(14):986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, Il: Charles C. Thomas; 1988. [Google Scholar]
- 52.Yu BP. Modulation of aging processes by dietary restriction. Boca Raton: CRC Press; 1994. [Google Scholar]
- 53.Zumoff B, Levin J, Rosenfeld RS, Markham M, Strain GW, Fukushima DK. Abnormal 24-hr mean plasma concentrations of dehydroisoandrosterone and dehydroisoandrosterone sulfate in women with primary operable breast cancer. Cancer Res. 1981;41(9 Pt 1):3360–3363. [PubMed] [Google Scholar]