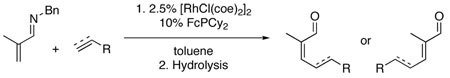
Abstract
The stereoselective alkylation of α,β-unsaturated imines via C-H activation followed by imine hydrolysis produces tri- and tetrasubstituted α,β-unsaturated aldehydes. In the presence of a rhodium catalyst, α,β-unsaturated N-benzyl imines derived from methacrolein, crotonaldehyde, and tiglic aldehyde undergo directed C-H activation at the β-position and react with terminal alkenes and alkynes to form the tri- and tetrasubstituted α,β-unsaturated imines with very high stereoselectivity. Hydrolysis to provide α,β-unsaturated aldehydes can be performed under carefully controlled conditions that maintains the stereochemistry of the β-alkylated imine products. Alternatively, for β-alkylation products of the N-benzyl imine of methacrolein, hydrolysis can be performed under conditions that provide complete isomerization to the E isomer.
The formation of carbon-carbon (C-C) bonds via the directed activation of carbon-hydrogen (C-H) bonds is actively being investigated with a number of reports describing the alkylation of aromatic C-H bonds.1,2 In contrast, the alkylation of olefinic C-H bonds has seen very limited development. In 1995 Trost and Murai independently reported the Ru-catalyzed C-H activation and alkylation of 1-acetylcyclohexene and α,β-unsaturated esters with alkenes such as silylethylenes and styrene.3a,b Unfortunately, this chemistry was not amenable to isomerizable olefins, and the scope of the α,β-unsaturated carbonyl moiety was also limited. α,β-Unsaturated carbonyl compounds are extremely versatile synthetic intermediates and are extensively used in natural product, drug and materials synthesis. We envisioned that the generality of olefinic C-H activation could be broadened considerably by the alkylation of α,β-unsaturated aldimines, which after hydrolysis would provide the corresponding α,β-unsaturated aldehydes as versatile synthetic intermediates.4 The alkylation of aromatic imines has shown broader alkene scope relative to the corresponding carbonyl compounds.2a–d Herein, we report the successful execution of this useful and fundamentally new approach for the first direct stereoselective synthesis of several classes of functionalized tri- and tetra-substituted α,β-unsaturated aldehydes.
An initial challenge in implementing this approach was identifying practical methods for the preparation and isolation of imines from minimally substituted α,β-unsaturated aldehydes due to their susceptibility to hydrolysis and their propensity to oligomerize under the reaction conditions. However, we found that aldehydes possessing an α-substituent readily condense with benzylamine in the presence of molecular sieves to form the corresponding aldimine products, which can then easily be obtained in analytically pure form by Kugelrohr distillation. Aldehydes possessing only a β-substituent require slightly milder reaction conditions and are isolated in lower yield due to the formation of oligomers.5 In this case, the condensation must be carried out at low temperatures using potassium carbonate as the desiccant. Utilizing these methods, we were able to synthesize and isolate the N-benzyl imines of tiglic aldehyde, methacrolein, and crotonaldehyde in 48 – 89% yield (eq 1).
 |
(1) |
We began our study on α,β-unsaturated imine alkylation by examining the reaction of imine 1 with n-hexene in the presence of Wilkinson’s catalyst at 150 °C (Table 1). While alkylation proceeded under these conditions, only a 34% yield was observed by NMR at 16 hours, and the E:Z selectivity was poor (entry 1). A short survey of reaction conditions was then undertaken. Reducing the number of equivalents of phosphine from 3 to 2 provided a 70% yield in 8 hours (entry 2). The use of trialkylphosphines such as tricyclohexylphosphine showed improved reactivity, providing complete consumption of starting material and a 66% yield of product with an E:Z isomeric ratio of 10:1 by NMR in less than one hour (entry 3). When the temperature was decreased to 100 °C the reaction still proceeded very quickly, and the E:Z ratio was not substantially improved. At 50 °C the reaction required substantially more time to go to completion however, the use of such mild temperatures allowed the Z isomer to be obtained exclusively (entry 5). Because improved reactivity was seen with tricyclohexylphosphine, the more electron-donating (dicyclohexylphosphinyl)ferrocene (FcPCy2) ligand was next employed. The resulting catalyst showed similar reactivity, providing clean conversion of the starting material to the Z isomer exclusively in 12 hours (entry 6).6 This catalyst system proved to be active even at room temperature, producing solely the Z isomer in excellent yield (entry 7). However, because the reaction times at 50 °C were much more convenient (12 h versus 60 h), all other substrates were run at this temperature. With more challenging olefin substrates, such as styrene, the superior reactivity of FcPCy2 was required to obtain reasonable reaction times with little isomerization (vide infra). For this reason, FcPCy2 was used in all subsequent experiments.
Table 1.
Optimization of Alkylation Conditions.
 | |||||
|---|---|---|---|---|---|
| Entry | Rh Source | Added Liganda | Temp (°C) | Time (h) | % Yield (Z:E)b |
| 1 | RhCl(PPh3)3 | - | 150 | 16 | 34 (1:10) |
| 2 | [RhCl(coe)2]2 | PPh3 | 150 | 8 | 70 (1:8) |
| 3 | [RhCl(coe)2]2 | PCy3 | 150 | 1 | 66 (1:10) |
| 4 | [RhCl(coe)2]2 | PCy3 | 100 | 4 | 86 (2:1) |
| 5 | [RhCl(coe)2]2 | PCy3 | 50 | 12 | 100 (95:5) |
| 6 | [RhCl(coe)2]2 | FcPCy2 | 50 | 12 | 100 (95:5) |
| 7 | [RhCl(coe)2]2 | FcPCy2 | 23 | 60 | 100 (95:5) |
10 mol % ligand was added.
All yields were determined by NMR integration relative to 2,6-dimethoxytoluene as an internal standard.
Several alkene coupling partners were examined. Alkenes with halide (entry 4) or ester functionality (entry 5) proved to be viable substrates for 1 and provided the trisubstituted olefins in good yields as exclusively the Z isomer prior to hydrolysis. Styrene also reacted cleanly with 1 with only minimal isomerization being seen under the reaction conditions for this less reactive substrate (entry 7). Furthermore, although terminal alkynes have been shown to undergo dimerization to form ene-yne products in the presence of rhodium catalysts,7 the mild conditions utilized by our chemistry allowed the alkenylation of 1 to compete with dimerization to provide the alkenylated product in excellent yield and stereoselectivity (entry 8).
Initial attempts to hydrolyze the imine provided a mixture of E and Z isomers. Indeed, in the presence of aqueous acid, hydrolysis with complete isomerization to the thermodynamically favored E isomer was observed, providing convenient access to this stereoisomer (entries 3 and 6). Preparation of the less stable Z enal isomer, which cannot be directly synthesized by other methods, could also be accomplished using milder hydrolysis conditions. Concomitant hydrolysis and purification on an activity III neutral alumina chromatography column provided the enal product in excellent yield with high stereoselectivity (entries 1, 2, 4, 5, 7, 8). In this manner, a separate hydrolysis step can be omitted, and good isomeric ratios favoring the Z isomer can be obtained for all products.
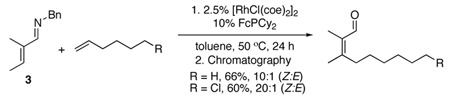
β,β-Disubstituted carbonyl compounds are very difficult to prepare stereoselectively. For this substitution pattern, common alkene synthesis methods such as Wittig, Horner Wadsworth Emmons, and olefin cross-metathesis are not stereoselective. Preliminary results indicate that β-substituted imines 2 and 3 undergo alkylation with 1-hexene and 6-chloro-1-hexene to provide the tri- (eq 2) and tetrasubstituted (eq 3) imines as single
 |
(2) |
 |
(3) |
stereoisomers. When the chlorinated alkene was employed, catalyst decomposition was problematic, and a second 0.05 equiv of catalyst was added 10 hours into the reaction. Subsequent chromatography on activity III neutral alumina then provided the corresponding tri- and tretrasubstituted α,β-unsaturated aldehydes in high yields and with only a modest reduction in stereochemical purity.
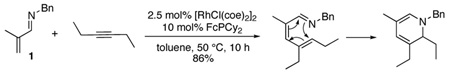
Reaction of 1 with other terminal and internal alkynes was also explored. The reaction of 1 with 3-hexyne did not provide the expected conjugated product, but instead reacted further in-situ to afford the dihydropyridine product (eq 4).8 This exciting reactivity has the potential to provide a variety of highly substituted pyridine and piperidine derivatives, and the scope of this chemistry is the focus of current research efforts.
 |
(4) |
In conclusion, the stereoselective alkylation of α,β-unsaturated imines via the directed activation of a C-H bond by a rhodium catalyst has been achieved. Use of the electron-rich (dicyclohexylphosphinyl)ferrocene ligand provides a catalyst that is active at mild temperatures, producing the Z isomer exclusively prior to hydrolysis, and allowing for good functional group compatibility, including the use of alkynes to form alkenylated products. Further progress on the synthesis of β,β-trisubstituted and tetrasubstituted enals is currently underway. This method promises to provide a straightforward route to these valuable synthetic intermediates.
Supplementary Material
Complete experimental details and spectral data for all compounds are described. This material is available free of charge via the Internet at http://pubs.acs.org.
Table 2.
Alkylation of α,β-Unsaturated Imines.
 | |||||
|---|---|---|---|---|---|
| Entry | Alkene(yne) | Temp. (°C) | Time (h) | Imine Z:Ea | % Yield (Z:E)b,c |
| 1 | 50 | 12 | >95:<5 | 91 (10:1) | |
| 2 | 23 | 60 | >95:<5 | 96 (10:1) | |
| 3 | 50 | 12 | >95:<5 | 77 (<5:>95)d | |
| 4 | 50 | 8 | >95:<5 | 80 (10:1) | |
| 5 |  |
50 | 4 | >95:<5 | 78 (5:1) |
| 6 |  |
50 | 4 | >95:<5 | 73 (<5:>95)d |
| 7 |  |
50 | 24 | 10:1 | 74 (5:1) |
| 8 |  |
50 | 4 | >95:<5 | 86 (20:1) |
Determined by NMR of the crude reaction mixture.
All yields indicated are isolated yields.
NOESY spectra were obtained to establish the stereochemistry of each aldehyde product.
Crude imine was stirred at a concentration of 0.1 M in a 5:5:2 solution of tetrahydrofuran : acetic acid : water for 16 hours prior to isolation.
Acknowledgement
This work was supported by NIH Grant GM069559 to J.A.E., an NSF predoctoral fellowship to D.A.C., and the Director and Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy, under Contract DE-AC03-76SF00098 to R.G.B.
References
- 1.For reviews on catalytic C-H activation see: Kakiuchi F, Murai S. In: Topics in Organometallic Chemistry. Murai S, editor. Vol. 3. Berlin Heidelberg: Springer-Verlag; 1999. pp. 47–79.Crabtree RH. J. Chem. Soc., Dalton Trans. 2001:2437.Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731. doi: 10.1021/cr0104330.Kakiuchi F, Murai S. Acc. Chem. Res. 2002;35:826. doi: 10.1021/ar960318p.
- 2.For recent examples of alkylations of aryl C-H bonds see: Thalji RK, Ellman JA, Bergman RG. J. Am. Chem. Soc. 2004;126:7192. doi: 10.1021/ja0394986.Lim S-G, Ahn J-A, Jun C-H. Org. Lett. 2004;6:4687. doi: 10.1021/ol048095n.Vo-Thanh G, Lahrache H, Loupy A, Kim I-J, Chang D-H, Jun C-H. Tetrahedron. 2004;60:5539.Thalji RK, Ahrendt KA, Bergman RG, Ellman JA. J. Org. Chem. 2005;70:6775. doi: 10.1021/jo050757e.Lenges CP, Brookhart M. J. Am. Chem. Soc. 1999;121:6616.Jia C, Kitamura T, Fujiwara Y. Acc. Chem. Res. 2001;34:633. doi: 10.1021/ar000209h.Ferreira EM, Stoltz BM. J. Am. Chem. Soc. 2003;125:9578. doi: 10.1021/ja035054y.
- 3.Trost BM, Imi K, Davies IW. J. Am. Chem. Soc. 1995;117:5371.Kakiuchi F, Tanaka Y, Sato T, Chatani N, Murai S. Chem. Lett. 1995:79.For other examples of olefin C-H bond alkylations see:Lim Y-G, Kang J-B, Kim YH. Chem. Commun. 1996:585.Fujii N, Kakiuchi F, Yamada A, Chatani N, Murai S. Bull. Chem. Soc. 1998;71:285.Sato T, Kakiuchi F, Chatani N, Murai S. Chem. Lett. 1998:893.Kakiuchi F, Sato T, Igi K, Chatani N, Murai S. Chem. Lett. 2001:386.Jun C-H, Moon CW, Kim Y-M, Lee H, Lee JH. Tetrahedron Lett. 2002;43:4233.Chatani N, Kamitani A, Murai S. J. Org. Chem. 2002;67:7014. doi: 10.1021/jo026001m.
- 4.Recently, Odom reported the β-alkylation of the N-phenyl imine of 1-acetylcyclohexene, which was prepared in situ by Ti - mediated hydroamination of cyclohexenylacetylene. See: Cao C, Li Y, Shi Y, Odom AL. Chem. Commun. 2004:2002. doi: 10.1039/b405620e.
- 5.Whitesell JK, Whitesell MA. Synthesis. 1983;1983:517. [Google Scholar]
- 6.In order to ascertain whether the starting material or the product was isomerizing at higher temperatures, the reaction of 1 with 2.5% [RhCl(coe)2]2 and 10% FcPCy2 at 50 °C was carried out to 75% conversion to the Z isomer exclusively, and then the temperature was increased to 150 °C. After 1 hour, a 1:2 Z:E ratio was obtained, indicating that the product is susceptible to isomerization.
- 7.Ishikawa M, Matsuguchi A, Furumori K, Ohshita J. J. Org. Chem. 1990;55:3277. [Google Scholar]
- 8.A similar electrocyclization has been reported (see reference 4)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete experimental details and spectral data for all compounds are described. This material is available free of charge via the Internet at http://pubs.acs.org.