Abstract
Advanced glycation end products (AGEs) have been reported to contribute to aging and cardiovascular complications. In the present study, the immunoreactivity of AGEs in human serum samples of healthy older subjects (n = 31), senile diabetic patients without cardiovascular complications (n = 33), senile diabetic patients with cardiovascular complications (n = 32), senile non-diabetic patients with cardiovascular complications (n = 30) ,and healthy young subjects (n = 31) were investigated. The patients were selected on clinical grounds from the National Institute of Cardiovascular Disease, Karachi and the Jinnah Postgraduate Medical Centre, Karachi, Pakistan. Fasting blood glucose, HbA1C and serum fructosamine levels were significantly (P < 0.001) increased in senile diabetic patients with and without cardiovascular complications as compared to non-diabetic senile patients with cardiovascular complications and healthy older subjects. Additionally, serum AGEs were found to be significantly (P < 0.001) increased in senile diabetic patients with cardiovascular complications and senile non-diabetic patients with cardiovascular complications, followed by diabetic patients without cardiovascular complications as compared to healthy older subjects and young control subjects. However, no significant difference was found in the senile diabetic patients without cardiovascular complications and senile non-diabetic patients with cardiovascular complications. In contrast to all four senile groups, serum AGEs were significantly (P < 0.001) lower in young control subjects. The AGEs distribution in the senile groups corroborates the hypothesis that the advanced glycation process might play a role in the development of cardiovascular complications, which are more severe in diabetic patients compared with non-diabetic patients with cardiovascular complications.
Keywords: Advanced glycation end products, Cardiovascular complications, Senile, Diabetes, Aging
Introduction
Diabetes is very common among senile persons, and the number of senile men and women is increasing rapidly in Pakistan (Saleheen and Frossard 2004). Essentially, chronic hyperglycemia is involved in the pathogenesis of diabetic micro- and macro-vascular complications in diabetes. Cardiovascular disease is the leading cause of death among people with diabetes, yet much of the population remains unaware of the risk. In the developed world, the risk of cardiovascular disease is increased two- to four-fold among diabetic patients compared with non-diabetic persons within corresponding population groups. The formation of advanced glycation end products (AGEs) correlates with glycemic control. AGEs have been linked to premature cardiovascular complications in diabetic patients as well as in nondiabetic subjects (Saleheen and Frossard 2004; Lyons 1993). AGEs are a heterogeneous group of compounds that have multiple biological effects on various cell types, including endothelial cells, macrophages, and smooth muscle cells (Schmidt et al. 1992; Yan et al. 2006). The presence of AGEs have also been reported in atherosclerotic plaques (Xanthis et al. 2007), and the cross-linking abilities of AGEs may contribute to the increased stiffening of collagen and possibly to vascular hypertrophy (Soldatos and Cooper 2006; Sims et al. 1996). Another possible mechanism by which AGEs may contribute to the development of atherosclerosis is by activating the transcription nuclear factor κB (NF-κB) through RAGE binding, resulting in induction of cellular adhesion molecule expression and cytokine activation (Bierhaus et al. 1997), or through glycoxidation of lipoproteins and increased foam cell formation (Baynes and Thorpe 2000).
There exist studies that reports increased serum AGEs in diabetic and nondiabetic patients with cardiovascular disease (Kanauchi et al. 2001) but, to our knowledge, none of these studies has so far reported the correlation of increased AGEs in senile non-diabetic subjects with cardiovascular complications. Moreover, the level of serum AGEs could be considered as a marker for the development of cardiovascular complications in senile diabetic, as well as non-diabetic, patients. The aim of this study was to evaluate AGEs immunoreactivity in human serum of senile diabetic and non-diabetic cardiovascular disease patients and to compare it with non-diabetic senile and young subjects.
Research design and methods
Subjects and sample collection
The study population consisted of senile type 2 diabetic patients with cardiovascular complications (n = 32), senile type 2 diabetic patients without cardiovascular complications (n = 33), senile non-diabetic patients with cardiovascular complications (n = 30), healthy older subjects (n = 31) ,and normal young subjects (n = 31). Blood samples were collected from the subjects during the period March 2004 to December 2007. The local ethical committee approved the protocol, and written informed consent was obtained from each patient after the nature of the study had been fully explained. The senile subjects selected were over 60 years of age, and young (age ranging from 20 to 25 years), apparently healthy, people were selected as control subjects. Sex, weight, duration of diabetes, duration of complications in diabetic and non-diabetic patients, type of diabetes and type of treatments received were also recorded. A physical examination was made including measurement of blood pressure. Individuals were classified as having diabetes mellitus if any of the following criteria were met (Gabir et al. 2000): fasting serum glucose levels of 7.0 mmol/L or more, random glucose levels of more than 11.1 mmol/L, and/or current use of medications prescribed to treat diabetes (e.g., insulin or drugs). Senile diabetic patients with more than one complication were excluded from the study. Senile diabetic and non-diabetic patients having cardiovascular complications such as angina, myocardial infarction and hypertension were investigated. Macrovascular disease was considered to be present if there was a history of myocardial infarction, angina, stroke, intermittent claudication, vascular surgery or amputation for atherosclerotic disease or one or more absent foot pulses upon examination. Patients were selected on clinical grounds from the National Institute of Cardiovascular Disease, Karachi and the Jinnah Postgraduate Medical Centre, Karachi, Pakistan.
Blood was collected in fasting state after a 10-h overnight fast. Samples were withdrawn by venous puncture and distributed equally into three tubes containing EDTA (for HbA1C), heparin (for glucose estimation) or no anti-coagulant (for serum collection). The samples were then immediately stored on ice until processed. Clotted blood was centrifuged at 1,500 rpm for 30 min and the serum was separated and frozen at −70°C until analysis. Blood glucose was determined by the glucose oxidase method, glycosylated hemoglobin (HbA1C) was determined calorimetrically using an HbA1C kit (Bio Systems Reagents and Instruments, Barcelona, Spain). The serum fructosamine was determined calorimetrically using a fructosamine kit (Randox, Antrim, UK).
Pretreatment of serum samples for AGE measurement
To 100 μl serum diluted with 100 mM phosphate-buffered saline, pH 7.2 (PBS), 100 μl 0.6% SDS/10 mM Tris-HCl saline, pH 7.4 and 5 μl 2 M NaBH4/50 mM NaOH were added. The mixture was immediately heated at 100°C for 10 min. After cooling in ice water, 800 μl PBS was added and the samples were then used for AGE assay.
Generation of AGE-BSA standard
AGE-BSA (bovine serum albumin) was prepared by incubating 5 g BSA with glucose (0.56 M) in PBS under sterile conditions for 16 weeks at 37°C. Samples were dialysed against PBS and stored at −70°C, protected from light until use.
AGE measurement by ELISA
The amount of AGE was determined by non-competitive ELISA using rabbit polyclonal antibodies to AGE (Abcam, Cambridge, UK) (Ono et al. 1998). A 96-well microplate was coated with 200 μl sample or corresponding control in 50 mM sodium bicarbonate buffer (pH 9.6) and kept at 4°C overnight. After overnight incubation, the wells were washed four times with PBS containing 0.05% Tween-20 (PBST). Each well was blocked for 2 h with blocking buffer, washed four times with PBST and incubated with 200 μl 1:104 diluted anti-AGE antibodies for 2 h. After washing wells four times, 200 μl 1:2,000 diluted HRP-anti-rabbit immunoglobulin (Abcam) was added to each well and incubated for 2 h. Wells were washed five times, then 200 μl 3,3′,5,5′-tetramethylbenzidine (TMB) solution was added to each well and incubated for 30 min; absorbance at 650 nm was then measured. Results are expressed as arbitrary AGE units (1 mU AGE corresponds to 4 μg AGE-BSA standard).
Statistical analysis
Data was analyzed using the Statistical Package for Social Sciences (SPSS, v 10.0; SPSS, Chicago, IL). The results are presented as mean ± SEM. The statistical significance of the difference between two means of various parameters between different groups was evaluated by one-way analysis of variance (ANOVA). Bonferroni’s post hoc test was used to determine group mean differences. With this test, SPSS automatically adjusts the significance level for multiple comparisons to avoid spurious significant differences being identified (any values below the level of 0.05 was considered as significant).
Results
Fasting blood glucose, HbA1C and serum fructosamine were significantly increased in senile diabetic patients with or without cardiovascular complications as compared to senile non-diabetic patients with cardiovascular complications and healthy older subjects. The increase in the fasting blood glucose level in all senile diabetic patients with and without cardiovascular complications correlated significantly with glycosylated hemoglobin and serum fructosamine concentrations. Also, no differences in fasting blood glucose, glycosylated hemoglobin and serum fructosamine were found between senile diabetic patients with cardiovascular complications and those without cardiovascular complications. When compared with age-matched normal subjects, senile non-diabetic patients with cardiovascular complications showed no significant differences in levels of fasting blood glucose, glycosylated hemoglobin and serum fructosamine.
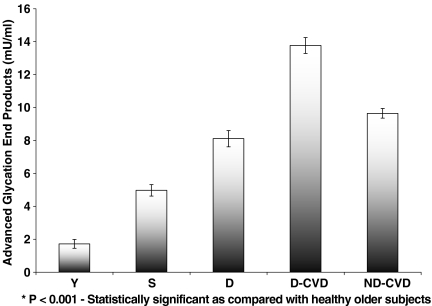
Figure 1 demonstrates that the level of serum AGEs is significantly (P < 0.001) higher in senile diabetic patients with and without cardiovascular complications and in senile non-diabetic patients with cardiovascular complications (13.77 ± 0.30 mU/ml, 8.11 ± 0.49 mU/ml, 9.65 ± 0.28 mU/ml, respectively) than in healthy older subjects (4.97 ± 0.34 mU/ml) or young control subjects (1.72 ± 0.26 mU/ml). In addition, compared with the normal young subjects, the healthy older subjects show the advancement of biological aging by increased AGEs values; healthy older subjects showed a significant increase in AGEs (P < 0.001) , having levels ∼34.6% higher than normal young subjects.
Fig. 1.
Serum levels of advanced glycation end products (AGEs) in senile diabetic and non-diabetic patients with and without cardiovascular complications. Bars represent mean ± SEM for young normal (Y; n = 31), healthy older subjects (S; n = 31), senile diabetics without cardiovascular complications (D; n = 33), senile diabetics with cardiovascular complications (D-CVD; n = 32) and senile non-diabetics with cardiovascular complications (ND-CVD; n = 30)
Among the age-matched subjects, serum AGEs were found to be significantly higher in senile diabetic patients with cardiovascular complications (P < 0.001) followed by senile non-diabetic subjects with cardiovascular complications (P < 0.001). In contrast, senile diabetic patients without cardiovascular complications, showed a significant difference in the level of AGEs (P < 0.001) compared with age-matched subjects, but this level was lower than in groups with cardiovascular complications with or without diabetes (Fig. 1).
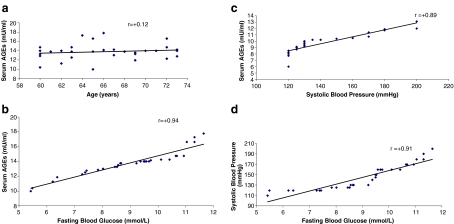
Correlation of age and serum AGEs (r = 0.12), systolic blood pressure and serum AGEs (r =0.03) and diastolic blood pressure and serum AGEs (r = 0.03) was not significant in senile diabetic patients with cardiovascular complications. However, a significant correlation between fasting blood glucose and serum AGEs (r = 0.94) and fasting blood glucose and systolic blood pressure (r = 0.91) was observed in these patients (Fig. 2).
Fig. 2.
a Correlation of age vs serum AGEs in diabetic patients with cardiovasular complications. b. Correlation of fasting blood glucose vs serum AGEs in diabetic patients with cardiovasular complications. c Correlation of systolic blood pressure vs serum AGEs in diabetic patients with cardiovascular complications. d Correlation of fasting blood glucose vs systolic blood pressure in diabetic patients with cardiovascular complications
Discussion
Diabetes mellitus is a major health problem of Pakistan. Over 12% of the Pakistani population in the age group of 25 years and above suffers from this disease, and about 10% from impaired glucose tolerance (Shera 1998). Diabetics are prone to long-term complications such as cardiovascular diseases; development of such complications is a major cause of morbidity and mortality and is an ever-increasing burden to healthcare authorities in both developed and developing nations (Veiraiah 2005). Epidemiological studies have confirmed that hyperglycemia is the most important factor in the onset and progress of vascular complications in diabetes (Yamagishi and Imaizumi 2005). The formation of AGEs correlates with glycemic control. The AGE hypothesis proposes that accelerated chemical modification of proteins by glucose during hyperglycemia contributes to the pathogenesis of diabetic complications, including atherosclerosis (Yamagishi et al. 2007). Glycation has both physiological and pathophysiological significance. Under physiological conditions, glycation can be detected in the ageing process, and the reactions are more rapid and more intensive with frequently increased glucose concentrations (Vlassara and Palace 2003). The AGE concept proposes that chemical modification and crosslinking of tissue proteins, lipids and DNA affect their structure and function. This in turn contributes to a gradual decline in tissue function and to the pathogenesis of cardiovascular complications in diabetic and in nondiabetic patients (Brownlee 2001; Schleicher and Friess 2007). AGEs have previously been shown to accumulate in many tissues with age, independently of diabetes (Peppa et al. 2004; Ulrich and Cerami 2001). Since the body does not contain any single enzyme capable of AGE structure degradation, AGEs accumulate during the biological life of the proteins on which they had been formed (Chuyen 2006).
The use of high-titer polyclonal anti-AGE antibodies in an ELISA assay has been applied successfully in this study. In the present study, a non-competitive AGE ELISA method with a polyclonal anti-AGE antibody was developed to quantify AGE products in serum samples collected from senile diabetic patients with and without cardiovascular complications and non-diabetic patients with cardiovascular complications. This antibody has been reported to cross-react mostly with N-(carboxymethyl)lysine (CML) and slightly with N-(carboxyethyl)lysine (CEL), pentosidine, argpyrimidine and imidazolones. In contrast, the antibody did not cross-react with Amadori products, which were reduced by NaBH4/50 mM NaOH during pretreatment of the serum samples and therefore were not detected by our assay (Ono et al. 1998). CML—a major product of oxidative modification of glycated proteins—has been suggested to represent a general marker of oxidative stress and long-term damage to proteins in aging, atherosclerosis, cataract and diabetes (Ahmed 2005). In order to correlate the s-AGE with the advancing age, the values from young healthy subjects (age ranging from 20 to 25 years) with no other medical complications were compared.
In this study, it was found that serum AGEs levels were related linearly with age. In the diabetic group with cardiovascular complications, a significantly higher level of AGEs was observed compared with non-diabetic individuals with cardiovascular complications, in accordance with earlier reports (Kanauchi et al. 2001; Basta et al. 2004; Uribarri et al. 2007). The fact that tissue levels of AGEs correlate with prevailing serum concentrations of glucose, fructosamine, and glycated hemoglobin points to a role for hyperglycemia, yet there is good evidence that other carbohydrates such as ascorbate, pentoses and metabolic intermediates may act as potent glycating agents (Dyer et al. 1991). Uribarri et al. (2007) showed positive correlation with serum AGEs and oxidative stress markers. It was also observed that fasting blood glucose, HbA1C and serum fructosamine levels are significantly increased in senile diabetic patients with and without complications as compared with senile non-diabetic patients with the same complications and healthy older subjects. These observations are similar to those of other workers (Khaw et al. 2001; Valeri et al. 2004). Serum fructosamine concentration correlates closely with HbA1C because it reflects glycemic control that lasts 2–3 weeks, and HbA1C reflects glycaemic control lasting 4–6 weeks. Any reduction in HbA1C is likely to reduce the risk of complications, with the lowest risk being in those with HbA1C in the normal range (Valeri et al. 2004).
In addition to diabetic patients, serum AGEs were also found to be higher in senile non-diabetic patients with cardiovascular complications compared to senile diabetic patients without cardiovascular complications; however, this increase was not significant. Both the structural and functional implications of this finding indicate a potentially important role for AGEs in diabetic and non-diabetic patients. Environmental conditions can result in the formation of various AGEs by a variety of chemical reactions, and formation of such structures under non-diabetic conditions is difficult to explain. Studies have suggested a role for oxidative stress in the formation of AGEs structures; therefore, it might be postulated that reactive oxygen intermediates may accelerate the rate of AGEs formation through reactive oxoaldehydes, or, vice versa, AGEs might induce oxidative stress through chemical and cellular mechanisms (Schleicher and Friess 2007; Wright et al. 2006). In addition to monosaccharides, AGEs have also been reported to be produced from dicarbonyl compounds derived from the Maillard reaction, autoxidation of sugars and other metabollic pathways, e.g., glycolysis, and this can account for the increase in s-AGE in non-diabetic patients with cardiovascular complications (Miyata et al. 2003). The present study shows increased levels of serum AGEs in senile groups as compared with that of young normal subjects. This indicates that these changes are correlated with the advancement of age.
The results of the present study thus demonstrate a positive correlation of the levels of serum AGEs with cardiovascular complications in senile patients with and without diabetes. This study seems to favor the use of serum AGEs as a biomarker for cardiovascular complications that might enable more effective diagnosis in senile populations.
Acknowledgment
This work was financially supported by Pakistan Science Foundation grant.
References
- Ahmed N (2005) Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract 67:3–21 doi:10.1016/j.diabres.2004.09.004 [DOI] [PubMed]
- Basta G, Del Turco S, De Caterina R (2004) Advanced glycation end products: implications for accelerated atherosclerosis in diabetes. Recent Prog Med 95:67–80 [PubMed]
- Baynes JW, Thorpe SR (2000) Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med 28:1708–1716 doi:10.1016/S0891-5849(00)00228-8 [DOI] [PubMed]
- Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T et al (1997) Advanced glycation end product-induced activation of NF-kB is suppressed by a-lipoic acid in cultured endothelial cells. Diabetes 46:1481–1490 doi:10.2337/diabetes.46.9.1481 [DOI] [PubMed]
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 doi:10.1038/414813a [DOI] [PubMed]
- Chuyen NV (2006) Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs. Mol Nutr Food Res 50:1140–1149 doi:10.1002/mnfr.200600144 [DOI] [PubMed]
- Dyer DG, Blackledge JA, Thorpe SR, Baynes JW (1991) Formation of pentosidine during nonenzymatic browning of protein by glucose: identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem 266:11654–11660 [PubMed]
- Gabir MM, Roumain J, Hanson RL, Bennett PH, Dabelea D, Knowler WC et al (2000) The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 23:1108–1112 doi:10.2337/diacare.23.8.1108 [DOI] [PubMed]
- Kanauchi M, Tsujimoto N, Hashimoto T (2001) Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care 24:1620–1623 doi:10.2337/diacare.24.9.1620 [DOI] [PubMed]
- Khaw K, Wareham N, Luben R, Bingham S, Oakes S, Welch A et al (2001) Glycated hemoglobin, diabetes and mortality in men in Norfolk Cohort of European prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 322:1–6 doi:10.1136/bmj.322.7277.15 [DOI] [PMC free article] [PubMed]
- Lyons TJ (1993) Glycation and oxidation: a role in the pathogenesis of atherosclerosis. Am J Cardiol 71:26B–31B doi:10.1016/0002-9149(93)90142-Y [DOI] [PubMed]
- Miyata T, Ishikawa N, van Ypersele de Strihou C (2003) Carbonyl stress and diabetic complications. Clin Chem Lab Med 41:1150–1158 doi:10.1515/CCLM.2003.178 [DOI] [PubMed]
- Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y (1998) Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract 41:131–137 doi:10.1016/S0168-8227(98)00074-6 [DOI] [PubMed]
- Peppa M, Uribarri J, Vlassara H (2004) The role of advanced glycation end products in the development of atherosclerosis. Curr Diabetes Rep 4:31–36 doi:10.1007/s11892-004-0008-6 [DOI] [PubMed]
- Saleheen D, Frossard P (2004) CAD risk factors and acute myocardial infarction in Pakistan. Acta Cardiol 59:417–424 doi:10.2143/AC.59.4.2005208 [DOI] [PubMed]
- Schleicher E, Friess U (2007) Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl 106:S17–S26 doi:10.1038/sj.ki.5002382 [DOI] [PubMed]
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J et al (1992) Isolation and characterization of binding proteins for advanced glycosylation end products from lung tissue which are present on the endothelial cell surface. J Biol Chem 267:14987–14997 [PubMed]
- Shera S (1998) Prevalence and prevention. Diabetes Dig 12:7–8
- Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ (1996) The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 39:964–951 doi:10.1007/BF00403914 [DOI] [PubMed]
- Soldatos G, Cooper ME (2006) Advanced glycation end products and vascular structure and function. Curr Hypertens Rep 8:472–478 doi:10.1007/s11906-006-0025-8 [DOI] [PubMed]
- Ulrich P, Cerami A (2001) Protein glycation, diabetes, and aging. Recent Prog Horm Res 56:1–21 doi:10.1210/rp.56.1.1 [DOI] [PubMed]
- Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G et al (2007) Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress and aging. J Gerontol A Biol Sci Med Sci 62:427–433 [DOI] [PMC free article] [PubMed]
- Valeri C, Pozzilli P, Leslie D (2004) Glucose control in diabetes. Diabetes Metab Res Rev 20:S1–S8 doi:10.1002/dmrr.512 [DOI] [PubMed]
- Veiraiah A (2005) Hyperglycemia, lipoprotein glycation, and vascular disease. Angiology 56:431–438 doi:10.1177/000331970505600411 [DOI] [PubMed]
- Vlassara H, Palace MR (2003) Glycoxidation: the menace of diabetes and aging. Mt Sinai J Med 70:232–241 [PubMed]
- Wright E Jr, Scism-Bacon JL, Glass LC (2006) Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract 60:308–314 doi:10.1111/j.1368-5031.2006.00825.x [DOI] [PMC free article] [PubMed]
- Xanthis A, Hatzitolios A, Koliakos G, Tatola V (2007) Advanced glycosylation end products and nutrition-a possible relation with diabetic atherosclerosis and how to prevent it. J Food Sci 72:125–129 doi:10.1111/j.1750-3841.2007.00508.x [DOI] [PubMed]
- Yamagishi S, Imaizumi T (2005) Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 11:2279–2299 doi:10.2174/1381612054367300 [DOI] [PubMed]
- Yamagishi S, Matsui T, Ueda S, Nakamura K, Imaizumi T (2007) Advanced glycation end products (AGEs) and cardiovascular disease (CVD) in diabetes. Cardiovasc Hematol Agents Med Chem 5:236–240 [DOI] [PubMed]
- Yan SF, Yan SD, Herold K, Ramsamy R, Schmidt AM (2006) Receptor for advanced glycation end products and the cardiovascular complications of diabetes and beyond: lessons from AGEing. Endocrinol Metab Clin North Am 35:511–524 doi:10.1016/j.ecl.2006.06.003 [DOI] [PubMed]