Abstract
There is evidence for two subpopulations among circulating endothelial progenitor cells (EPCs), i.e., CD34+-EPCs and CD14+-EPCs. Prior studies on the relationship between the level of EPCs and coronary artery disease (CAD), either did not distinguish between the two types of EPCs or studied only CD34+-EPCs. We therefore investigated whether the number of circulating CD14+-EPCs correlates with either CAD and/or cardiovascular risk factors. Circulating CD14+-EPCs—as defined by the surface markers CD14+KDR+—were analyzed by flow cytometry in 100 individuals [34 control subjects, 41 patients with stable CAD and 25 patients with acute coronary syndromes (ACS)]. The level of circulating CD14+-EPCs was not significantly different in patients with normal coronary arteries compared to those with stable CAD or ACS. Neither was there any association between the severity of CAD or risk factors and the number of circulating CD14+-EPCs. Thus, the number of circulating CD14+-EPCs was not significantly correlated either with the severity of coronary disease or with cardiovascular risk factors.
Keywords: Endothelial progenitor cells, Coronary disease, Risk factors, CD14+KDR+ cells, Monocytes
Preventing atherosclerosis and cardiovascular disease is one of the most important health issues worldwide, with cardiovascular disease being the leading cause of death in industrialized countries. The accumulation of cardiovascular risk factors such as age, diabetes, hypertension, smoking and hyperlipidemia, impair the ability of the vascular endothelium to produce vasodilatory and anti-adhesion moieties, and increase production of vasoconstrictor, proadhesion, and pro-thrombotic molecules, leading to elevated vascular tone, enhanced cell adhesion, proliferation of media smooth muscle cells, and propensity toward thrombosis (Drexler and Hornig 1999; Rubanyi 1993). Given the role of endothelial cell loss in the pathogenesis of vascular diseases, attention has recently turned to the development of strategies to enhance rapid endothelial recovery (Losordo et al. 2003; Dimmeler and Zeiher 2004). It was demonstrated that circulating bone marrow-derived endothelial progenitor cells (EPCs) may gravitate to sites of vascular injury and contribute to neoangiogenesis (Asahara et al. 1999; Urbich and Dimmeler 2004). The capacity of circulating EPCs to repair vascular damage suggests that they may play a key role in maintaining homeostasis of the endothelium. It follows that the number of circulating EPCs may reflect the “vascular health” of an individual, and thus represent a marker by which to assess cardiovascular disease risk. However, the lack of a uniform EPC definition complicates cross-study comparisons and may contribute to the apparent paradox of some studies that suggest that EPCs are reduced in the presence of cardiovascular risk factors and coronary artery disease (CAD) (Vasa et al. 2001; Hill et al. 2003; Schmidt-Lucke et al. 2005), whereas others suggest that numbers are increased in those with obstructive CAD (George et al. 2004; Massa et al. 2005; Güven et al. 2006).
Currently, a widely accepted consensus defines cells positive for the surface markers CD34 and vascular endothelial growth factor receptor-2 (VEGFR2 or KDR in humans) as EPCs (Werner and Nickenig 2006). Nevertheless, it has been convincingly demonstrated that EPCs bear various other cell surface markers, including markers of the monocytic cell lineage such as CD14 (Rehman et al. 2003; Gulati et al. 2003; Romagnani et al. 2005; Burger et al. 2002; Fernandez Pujol et al. 2000; Schmeisser et al. 2001). Importantly, both types of EPCs showed comparable vasculogenic capacity in vivo (Hur et al. 2004). More importantly, there are data indicating that the vast majority of EPCs actually arise from the CD14+ subpopulation of peripheral blood mononuclear cells (PBMCs) (Rehman et al. 2003; Gulati et al. 2003; Romagnani et al. 2005). However, previous studies focusing on the relationship between either CAD or cardiovascular risk factors and the level of EPCs either did not distinguish between the two types of EPCs (Vasa et al. 2001; Hill et al. 2003; George et al. 2004) or studied only CD34+-EPCs (Schmidt-Lucke et al. 2005; Massa et al. 2005). Therefore, the question of whether the level of CD14+-EPCs correlates with either CAD or cardiovascular risks remains unresolved.
In this prospective study, circulating EPCs arising from the CD14+ subpopulation of PBMCs were defined by the surface markers CD14+KDR+ and analyzed by flow cytometry. We explored the relationship between the level of circulating CD14+KDR+ cells and both the severity of CAD and cardiovascular risk factors.
Methods
Control subjects and patients
The protocol was approved by the Institutional Review Board at Chongqing University of Medical Sciences. The study population comprised 100 in-patients who underwent angiography between May 2006 and June 2008. The sample size was estimated on the basis of a previous study showing a significant decrease in circulating CD34+-EPC numbers in patients with risk factors for CAD (Vasa et al. 2001). Assuming that the magnitude of the effect would be lower, we enlarged the size of the patient and control samples by approximately 50%. Control subjects were defined as having angiographically normal coronary arteries. Patients with stable CAD met the following criteria: (1) history of transient episodes of typical chest pain on effort, lasting unchanged for more than 3 months before blood samples were drawn and not associated with rest angina; (2) having at least one coronary stenosis producing ≥ 50% diameter reduction. Patients with acute coronary syndromes (ACS) were defined as having at least one coronary stenosis producing ≥ 50% diameter reduction and with unstable angina (UA) or myocardial infarction (MI) within the past 2 weeks. Inclusion criteria included the ability to give informed consent and referral for coronary angiography for the evaluation of CAD. Exclusion criteria included immune suppression, acute-chronic inflammatory diseases, history of renal, hepatic or hematologiccoagulative disorders, malignancies, retinopathy, concomitant other heart diseases (e.g., cardiomiopathy), the presence of symptomatic peripheral vascular disease or cerebral vascular disease, the presence of ACS prior to the past 2 weeks and within the past 3 months, history of hemorrhage (including menses) or blood-transfusion within the past 2 weeks.
Definition of risk factors for CAD
To determine the overall risk factor load of an individual subject, we calculated a risk score including male sex, hypertension (BP ≥140/90 mmHg or on antihypertensive medication), hypercholesterolemia (LDL cholesterol ≥ 3.4 mmol/L or 130 mg/dL), low HDL cholesterol (HDL cholesterol <1.04 mmol/L or 40 mg/dL), cigarette smoking (a history of smoking in the last 3 months), age (male ≥45 and female ≥55 years of age), family history of premature CAD (MI or sudden death in a first relative, male <55 and female <65 years) and pathoglycemia (impaired glucose tolerance/impaired fasting glucose/diabetes mellitus) (American Diabetes Association 2004). HDL cholesterol ≥1.56 mmol/L (60 mg/dL) counts as a “negative” risk factor, its presence removes one risk factor from the total count.
Angiographic assessment
Diagnostic angiography was performed with the Judkins technique with a digital angiography system (Innova 2000, GE; http://www.gehealthcare.com). Standardized angiographic projections that showed the coronary artery stenosis at its most severe were recorded in every patient. Interpretation of the coronary angiograms was made by two cardiologists. Percentage of coronary narrowing was calculated by comparison of the minimum diameter of the segment involved to the diameter of an adjacent angiographically normal coronary segment. CAD was evaluated with the vessel score, i.e., the number of vessels with a significant stenosis (≥50% reduction in lumen diameter). Scores ranged from 0 to 3, depending on the number of vessels involved. Left main artery stenosis was scored as 2-vessel disease. The severity of CAD was assessed by using Gensini score (Gensini 1983).
Flow cytometry
After placement of an arterial sheath, 3–5 mL blood was wasted and then 1 mL whole blood was taken in an evacuated tube containing EDTA as the anticoagulant. A volume of 100 μL peripheral blood was evaluated by flow cytometry (fluorescence-activated cell sorter; Calibur, Becton Dickinson Biosciences, Franklin Lakes, NJ) within 3 h after drawing. All procedures were similar to those previously described (Fadini et al. 2006). In brief, before being stained with specific monoclonal antibodies, cells were treated with fetal calf serum for 10 min, and the samples were then washed with buffer containing PBS and 0.5% bovine albumin. This approach is used to saturate sites for nonspecific binding. Blood cells were then stained with FITC-conjugated anti-human CD14 monoclonal antibody (Jingmei Biotech, Shenzhen, China) and PE-conjugated anti-human KDR monoclonal antibody (R&D Systems, Minneapolis, MN). Appropriate isotype controls were used for each staining procedure. The frequency of peripheral blood cells positive for the aforementioned reagents was determined by a 2-dimensional side-scatter fluorescence dot-plot analysis. CD14/KDR double positive cells were identified by the expression of KDR in the CD14 gate. After incubation, cells were lysed and washed with PBS. Data were processed with use of the CellQuest software program (Becton Dickinson). The same trained operator, who was blinded to the subjects’ characteristics, performed all of the tests throughout the study.
Statistical analysis
Data are expressed as mean ± SD. Continuous variables were tested for normal distribution with the Kolmogorov-Smirnov test. The number of risk factors (risk score) was considered as a continuous variable. Normally distributed variables were compared by means of one-way analysis of variance (ANOVA). In the case of non-normal distribution, nonparametric tests were used (Mann-Whitney U test or Kruskal-Wallis test). Comparison of categorical variables was generated by the Pearson χ2 test. Linear regression analysis and nonparametric bivariate correlation (Spearman’s rank correlation coefficient) were used to correlate circulating CD14+KDR+ cell counts with cardiovascular risk factors. To identify independent determinants of CD14+KDR+ cell numbers, a multivariate linear regression analysis for various cardiovascular risk factors was performed. Statistical significance was assumed if a null hypothesis could be rejected at Ρ ≤ 0.05. All statistical analysis was performed with SPSS 13.0 (http://www.spss.com).
Results
Patients’ clinical characteristics
According to coronary angiographic results and whether complicating ACS was present, the subjects were divided into three groups: group A, 34 patients who had normal coronary angiogram served as control subjects; group B, 41 patients who had stable CAD; and group C, 25 patients who had ACS. The clinical characteristics of the 100 subjects are summarized in Table 1. As expected, patients with CAD had a significantly higher number of risk factors, were slightly but significantly older, and were more frequently treated with statins, platelet inhibitors, and ACE inhibitors/AT-1 receptor blockers (ACEI/ARB). Patients with ACS differed from patients with stable CAD with respect to hyperglycemia, risk score and Gensini score, which were all higher in patients with ACS.
Table 1.
Patients’ clinical characteristics. CAD Coronary artery disease, ACS acute coronary syndromes, ACEI/ARB ACE inhibitors/AT-1 receptor blockers
| Characteristic | Group A: Control (n = 34) | Group B: Stable CAD (n = 41) | Group C: ACS (n = 25) | A vs B and C, P Value |
|---|---|---|---|---|
| Age | 60 ± 11 | 70 ± 10 | 69 ± 9 | <0.001 |
| Body mass index | 23.98 ± 3.63 | 23.29 ± 3.79 | 23.7 ± 3.36 | 0.712 |
| Male gender, n (%) | 17 (50) | 20 (48.8) | 18 (72) | 0.142 |
| Hypertension, n (%) | 13 (38.2) | 17 (41.5) | 14 (56) | 0.363 |
| Hyperglycemia, n (%) | 7 (20.6) | 8 (19.5) | 14 (56) | 0.002 |
| Smoking, n (%) | 8 (23.5) | 4 (9.8) | 8 (32) | 0.074 |
| Family history, n (%) | 1 (2.9) | 2 (4.9) | 0 | 0.530 |
| LDL, mmol/L | 2.67 ± 0.78 | 2.68 ± 0.84 | 2.61 ± 1.28 | 0.951 |
| HDL, mmol/L | 1.36 ± 0.29 | 1.24 ± 0.33 | 1.28 ± 0.33 | 0.266 |
| Risk score | 2.2 ± 1.6 | 2.4 ± 1.5 | 3.4 ± 1.4 | 0.009 |
| Vessel score | 0 | 1.6 ± 0.8 | 2 ± 0.8 | <0.001 |
| Gensini score | 0 | 22.28 ± 21.74 | 52.38 ± 42.95 | <0.001 |
| Statins therapy, n (%) | 8 (23.5) | 17 (41.5) | 12 (48) | 0.029 |
| ACEI/ARB, n (%) | 6 (17.6) | 16 (39) | 16 (64) | 0.001 |
| Aspirin, n (%) | 12 (35.3) | 25 (61) | 16 (64) | 0.038 |
Determinants of circulating CD14+-EPCs levels
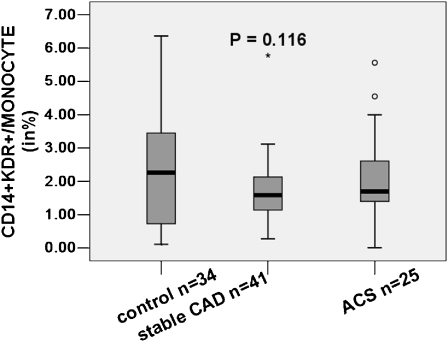
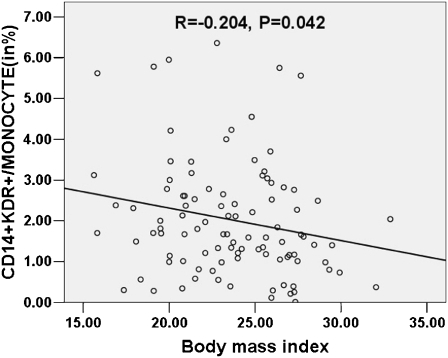
We directly determined the number of CD14+KDR+ cells in the peripheral blood by flow cytometry (Fig. 1). Levels of CD14+KDR+ cells did not differ significantly in control subjects compared with patients with stable CAD and patients with ACS (2.36% ± 1.83%, 1.69% ± 0.96% and 2.1% ± 1.31%, respectively; see Fig. 2). By univariate analysis for the entire cohort, the classic risk factors of male sex, age, hypertension, smoking, dyslipidemia and hyperglycemia, as well as disease activity and extent of coronary atherosclerotic involvement, were not correlated with the number of circulating CD14+KDR+ cells. Statistical analyses revealed that medications had no influence on CD14+KDR+ cell counts. We found only a weak negative correlation between body mass index (BMI) and CD14+KDR+ cell levels (Table 2). We did not analyze the relationship between family history of premature CAD and CD14+KDR+ cell counts, because only three subjects had a positive family history. By multivariate analysis, BMI remained the only significant independent predictor of a reduced number of CD14+KDR+ cells (P = 0.042, β = −0.204, R2 = 0.042, see Fig. 3).
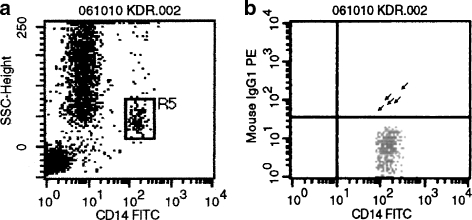
Fig. 1.
CD14+KDR+ cells were detected in peripheral blood by flow cytometry. a Sideward scatter with monocyte gate indicated. b Double fluorescence with PE-labeled KDR and FITC-labeled CD14 antibodies. Quadrants were set on the basis of isotype controls. Arrows CD14+KDR+ cells. A representative blot is shown (n = 100)
Fig. 2.
Percentage of circulating CD14+KDR+ cells/monocytes in control subjects, patients with stable coronary artery disease (CAD) and patients with acute coronary syndromes (ACS)
Table 2.
Univariate correlation between cardiovascular risk factors, the severity of coronary disease, drugs, and CD14+KDR+ cells in 100 individuals
| Variable | R | P Value |
|---|---|---|
| Age | −0.104 | 0.304 |
| BMI | −0.204 | 0.042 |
| Sex | 0.036 | 0.723 |
| Hypertension | −0.121 | 0.23 |
| Hyperglycemia | 0.103 | 0.306 |
| Smoking | −0.032 | 0.755 |
| HDL | −0.033 | 0.747 |
| LDL | 0.039 | 0.697 |
| Risk score | 0.028 | 0.755 |
| Vessel score | −0.039 | 0.703 |
| Gensini score | −0.011 | 0.912 |
| Statin therapy | 0.047 | 0.643 |
| ACEI/ARB | −0.031 | 0.759 |
Fig. 3.
Number of CD14+KDR+ cells is negatively correlated with body mass index (BMI)
Discussion
Previous reports have indicated a correlation between circulating CD34+KDR+ cells and both cardiovascular risk factors and the severity of coronary lesions (Vasa et al. 2001; Schmidt-Lucke et al. 2005; Massa et al. 2005). However, to the best of our knowledge, this is the first study to address the relationship between circulating CD14+KDR+ cells and CAD. The principal finding of this study is the lack of association between the level of CD14+KDR+ cells and either cardiovascular risk or the severity of CAD as assessed by coronary angiography. We detected no significant augmentation of CD14+KDR+ cells in patients with ACS. For the purpose of this study, we selected patients with various degrees of cardiovascular risk, normal or mild-to-severe stenosis, 0-vessel disease to 3-vessel disease, stable lesions to active lesions, representing diverse aspects of the spectrum of atheromatous CAD. Although the study has limited power, it shows that the level of circulating CD14+KDR+ cells is not a useful surrogate marker for cumulative cardiovascular risk and vascular function.
EPCs have been studied in a variety of patients, including those with vascular disease and those with risk factors for vascular disease. Vasa et al. (2001) observed an inverse correlation between the number of circulating EPCs and both atherosclerotic risk factors and angiographically documented CAD. Hill et al. (2003) described a reduction in EPC colony forming units in healthy individuals with risk factors for cardiovascular disease. These two groups came to similar conclusions by diverse experimental methodology, one group culturing mononuclear cells initially adherent to fibronectin and another group discarding the adherent cells and culturing those initially nonadherent. Massa et al. (2005) observed no significant difference in the number of circulating CD34+KDR+ cells in patients with stable CAD compared with healthy controls. Güven et al.(2006) reported that the number of EPC colonies was increased in proportion with the severity of CAD. In addition, the level of EPCs in that study did not vary significantly with cardiovascular risk factors. George et al. ( 2004) found higher number of EPC colonies in patients with UA as compared with stable angina. Massa et al. (2005) showed that the number of CD34+KDR+ cells in the blood was increased in patients with acute MI compared with healthy controls or patients with stable CAD. Schmidt-Lucke et al. (2005) detected a significant decreased level of CD34+KDR+ cells in patients with ACS compared with healthy controls. Thus the relationship between circulating EPCs and vascular diseases, particularly atherosclerosis, remains controversial and incompletely understood.
According to their initial discovery, EPCs were defined as cells positive for both hematopoietic stem cell markers such as CD34 and the endothelial marker protein KDR (Asahara et al. 1997). However, increasing evidence suggests that myeloid cells can also give rise to endothelial cells (Rehman et al. 2003; Gulati et al. 2003; Romagnani et al. 2005; Burger et al. 2002; Fernandez Pujol et al. 2000; Schmeisser et al. 2001). Also, a recently identified population of peripheral blood-derived CD34−CD133+ KDR+ cells may differentiate into CD34+CD133+KDR+ and eventually mature EC (Friedrich et al. 2006). Moreover, Ingram et al. (2004) provided data to support a hierarchy of EPCs that can be assayed in limiting dilution. These data support the notion that it will be difficult to define “true” EPCs. To date there is no uniform method or criteria for defining an EPC despite extensive research activity. Some methods of characterization are based on in vitro behavior, including the ability to form endothelial colonies with the incorporation of acetylated low-density lipoprotein and binding of lectins (Vasa et al. 2001; Hill et al. 2003; George et al. 2004; Güven et al. 2006). The majority of acetylated LDL (+) lectins (+) cells express monocyte/macrophage markers such as CD14, and a much lower percentage of cells express CD133 (Rehman et al. 2003). However, EPCs defined in this way represent a mixed population since it has become clear that at least two types of endothelial-lineage cells can be obtained from the in vitro culture of mononuclear cells (Rehman et al. 2003; Hur et al. 2004). Early EPCs, termed circulating angiogenic cells (CACs), are derived mainly from monocytic cells co-expressing some endothelial markers (Rehman et al. 2003; Romagnani et al. 2005; Fernandez Pujol et al. 2000; Schmeisser et al. 2001; Hur et al. 2004). Early EPCs secrete large amounts of angiogenic growth factors (Rehman et al. 2003; Hur et al. 2004). Alternatively, late EPCs possess high proliferation potency and express CD34 (Hur et al. 2004; Lin et al. 2000; Reyes et al. 2002). In addition, ex vivo cultivation procedures may confound the influence of cardiac risk factors on the number of EPCs, whereas the direct measurement of EPCs might more closely resemble in vivo conditions (George et al. 2006). In this study, we directly determined the number of CD14+KDR+ cells, which correspond to early EPCs, in peripheral blood using flow cytometry. CD14 is an extremely important monocyte surface molecule, and KDR expression is believed to be restricted exclusively to endothelial cells. CD14 and KDR are expressed simultaneously by EPCs arising from monocytes but CD14 is completely lost by EPCs as they differentiate (Fernandez Pujol et al. 2000; Schmeisser et al. 2001; Eggermann et al. 2003). Mature monocytes do express VEGFR-1 but are negative for KDR (Sawano et al. 2001). Thus we chose these two markers as they would not involve mature endothelial cells or monocytes. Most importantly, previous studies showed that CD14+KDR+ cells exhibit the potential to differentiate into cells with endothelial characteristics both in vitro and in vivo (Nowak et al. 2004; Elsheikh et al. 2005). Our study showed CD14+KDR+ cells constituting approximately 2.02% ± 1.41% of the total population of monocytes in the blood, which is very closely to the observation of Elsheikh et al. (2005) (2% ± 0.5%). The number of CD14+KDR+ cells in the blood was not significantly different in controls compared with patients with stable CAD or ACS. Although medications differed among the three groups, univariate analysis revealed no relationship between medication and CD14+KDR+ cell levels. Neither was any correlation found between CD14+KDR+ cell levels and either the severity of coronary atheroma or cardiac risk. The results of the current study are in agreement with those of Güven et al. (2006), who also found no significant correlation between CACs and angiographically documented CAD or cardiac risk. Schmidt-Lucke et al. (2005) detected no significant augmentation of EPCs in patients with ACS, which likely reflects the early blood sampling. In our study, most of the blood samples from patients with ACS were obtained on day 3–10 after onset, and thus there would have been enough time for cell mobilization. We did not detect a significant augmentation of CD14+KDR+ cells in patients with ACS. We found that the number of CD14+KDR+ cells inversely correlates, albeit weakly, with BMI. The clinical significance of this observation needs to be evaluated in future research.
Taken together, there are three possible factors that might explain disparities in these data. First, George et al. (2006) found that, in healthy individuals, there was no correlation between the various methods used for estimating EPC numbers. Thus, there are fundamental methodological differences that likely account for the different results. Second, these data are consistent with the emerging concept that several blood cell populations with angiogenic activity (e.g., CD14+KDR+ cells and CD34+KDR+ cells) exist that have different roles in neovasculogenesis and are regulated by different mechanisms. The present data do not conflict with those of Vasa et al. (2001) since these authors actually investigated cell lines that are even more closely related to conventional stem cells, i.e., CD34+KDR+ cells. Third, albeit less likely, the absolute number of CD34+KDR+ cells is so small that minute fluctuations could cause enormous changes in proportion.
Study limitations
In this study we simply measured the number of CD14+KDR+ cells without examining their functional activity such as proliferative potential, secretary capacity or migratory capacity. It could be that risk factors and CAD significantly affects the functional activity of CD14+KDR+ cells rather than their number. We used a rigorous definition of family history of premature CAD for eliminating likely earlier misdiagnosis. Only 3 out of 100 patients had a positive family history of premature CAD, which could be another limitation. Namely, the number of subjects in our study was relatively small, and some group comparisons may have lacked power to detect significant differences for select variables.
References
- American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care 27(Suppl 1):S5–S10 doi:10.2337/diacare.27.2007.S5 [DOI] [PubMed]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967 doi:10.1126/science.275.5302.964 [DOI] [PubMed]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228 [DOI] [PubMed]
- Burger PE, Coetzee S, McKeehan WL, Kan M, Cook P, Fan Y et al (2002) Fibroblast growth factor receptor-1 is expressed by endothelial progenitor cells. Blood 100:3527–3535 doi:10.1182/blood.V100.10.3527 [DOI] [PubMed]
- Dimmeler S, Zeiher AM (2004) Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis. J Mol Med 82:671–677 doi:10.1007/s00109-004-0580-x [DOI] [PubMed]
- Drexler H, Hornig B (1999) Endothelial dysfunction in human disease. J Mol Cell Cardiol 31:51–60 doi:10.1006/jmcc.1998.0843 [DOI] [PubMed]
- Eggermann J, Kliche S, Jarmy J, Hoffmann K, Mayr-Beyrle U, Debatin KM et al (2003) Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiovasc Res 58:478–486 doi:10.1016/S0008-6363(03)00252-9 [DOI] [PubMed]
- Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S (2005) Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood 106:2347–2355 doi:10.1182/blood-2005-04-1407 [DOI] [PubMed]
- Fadini GP, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A et al (2006) Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke 37:2277–2282 doi:10.1161/01.STR.0000236064.19293.79 [DOI] [PubMed]
- Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML et al (2000) Endothelial-like cells derived from human CD14 positive monocytes. Differentiation 65:287–300 doi:10.1046/j.1432-0436.2000.6550287.x [DOI] [PubMed]
- Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N (2006) CD34−/CD133+/VEGFR−2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res 98:e20–e25 doi:10.1161/01.RES.0000205765.28940.93 [DOI] [PubMed]
- Gensini GG (1983) A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51:606 doi:10.1016/S0002-9149(83)80105-2 [DOI] [PubMed]
- George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A et al (2004) Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J 25:1003–1008 doi:10.1016/j.ehj.2004.03.026 [DOI] [PubMed]
- George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A (2006) Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng 12:331–335 doi:10.1089/ten.2006.12.331 [DOI] [PubMed]
- Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG et al (2003) Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93:1023–1025 doi:10.1161/01.RES.0000105569.77539.21 [DOI] [PubMed]
- Güven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC (2006) The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol 48:1579–1587 doi:10.1016/j.jacc.2006.04.101 [DOI] [PubMed]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600 doi:10.1056/NEJMoa022287 [DOI] [PubMed]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK et al (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24:288–293 doi:10.1161/01.ATV.0000114236.77009.06 [DOI] [PubMed]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K et al (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104:2752–2760 doi:10.1182/blood-2004-04-1396 [DOI] [PubMed]
- Lin Y, Weisdorf D, Solovey A, Hebbel RP (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105:71–77 doi:10.1172/JCI8071 [DOI] [PMC free article] [PubMed]
- Losordo DW, Isner JM, Diaz-Sandoval LJ (2003) Endothelial Recovery. The next target in restenosis prevention. Circulation 107:2635–2637 doi:10.1161/01.CIR.0000071083.31270.C3 [DOI] [PubMed]
- Massa RV, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM et al (2005) Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105:199–206 doi:10.1182/blood-2004-05-1831 [DOI] [PubMed]
- Nowak G, Karrar A, Holmen C, Nava S, Uzunel M, Hultenby K et al (2004) Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation 110:3699–3707 doi:10.1161/01.CIR.0000143626.16576.51 [DOI] [PubMed]
- Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169 doi:10.1161/01.CIR.0000058702.69484.A0 [DOI] [PubMed]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marku PH, Verfaillie CM (2002) Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 109:337–346 [DOI] [PMC free article] [PubMed]
- Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F et al (2005) CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res 97:314–322 doi:10.1161/01.RES.0000177670.72216.9b [DOI] [PubMed]
- Rubanyi GM (1993) The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol 22(Suppl 4):S1–S14 doi:10.1097/00005344-199304000-00002 [DOI] [PubMed]
- Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T et al (2001) Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97:785–791 doi:10.1182/blood.V97.3.785 [DOI] [PubMed]
- Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J et al (2001) Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel (R) under angiogenic conditions. Cardiovasc Res 49:671–680 doi:10.1016/S0008-6363(00)00270-4 [DOI] [PubMed]
- Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U et al (2005) Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111:2981–2987 doi:10.1161/CIRCULATIONAHA.104.504340 [DOI] [PubMed]
- Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353 doi:10.1161/01.RES.0000137877.89448.78 [DOI] [PubMed]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H et al (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7 doi:10.1161/hh1301.093953 [DOI] [PubMed]
- Werner N, Nickenig G (2006) Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med 10:318–332 doi:10.1111/j.1582-4934.2006.tb00402.x [DOI] [PMC free article] [PubMed]