Abstract
Erectile dysfunction (ED) is a highly prevalent disease affecting millions of men worldwide with a tendency for widespread increase. ED is now considered an early manifestation of atherosclerosis and, consequently, a precursor of systemic vascular disease. Atherosclerosis and ED share potentially modifiable risk factors, as smoking or high-fat food intake, but it is unclear how regular consumption of anti-oxidant rich drinks, which exhibit recognised anti-atherosclerotic features, affects ED progression. The objective of this study was to evaluate the modulating effects of chronic consumption of catechin-rich beverages on the vascular structure of the rat corpus cavernosum, and how this could contribute to delay or prevention of the onset of ED. Male Wistar rats aged 12 months were treated with green tea (GT) or a green tea extract solution (GTE) as the only liquid source for 6 months. Consumption of GT and GTE led to decreased plasma androgen levels without any significant change in plasma lipid levels. A reduction in corpus cavernosum intracellular storage of lipids, associated with decreased expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR2 in endothelial cells, was observed. Taken together, these results suggest diminished atherosclerotic progression in cavernous tissue. However, functional studies will be necessary to elucidate if catechin-rich beverages are useful compounds in the prevention of deleterious vascular events associated with ED. It was also demonstrated that regular consumption of catechins reduces atherosclerotic progression and mortality due to cardiovascular disease. The results reported here suggest diminished atherosclerotic progression in cavernous tissue in aged rats following chronic ingestion of catechin-rich beverages.
Keywords: Aging, Catechins, Erectile dysfunction, Testosterone, VEGF, VEGF receptors
Introduction
Erectile dysfunction (ED) is a highly prevalent disease affecting millions of men worldwide, with a tendency for widespread increase (Ayta et al. 1999). For many years, ED was seen as an age-related complication of cardiovascular disease, diabetes mellitus and arterial hypertension, but this dysfunction is now considered as an early manifestation of atherosclerosis and, consequently, a precursor of systemic vascular disease (Cheitlin 2004). Therefore, several authors consider that all men with ED should be considered at risk of cardiovascular disease until proven otherwise (Blumentals et al. 2004; Vlachopoulos et al. 2005).
The small vessels of the penis are very sensitive to structural and functional changes (Kaya et al. 2006). Vasculogenic ED, which occurs in two-thirds of cases, includes both arteriogenic ED (cavernosal artery insufficiency) and veno-occlusive ED, which are commonly discussed separately but often coexist in the same patient (Azadzoi 2006). Vasculogenic ED is frequently caused by vascular-associated impairment of the relaxation of the cavernous smooth muscle, which is considered an endothelial dysfunction (Goldstein 2003; Guay 2007) and the etiologic connection between ED and systemic vascular disease. Endothelial dysfunction is defined as incapacity of vasodilatation in endothelium-dependent way, and it is due to impaired bioavailability of nitric oxide (NO) that is normally associated with the diminished synthesis of NO or its enhanced degradation in perivascular smooth muscle. In addition, endothelial NO specifically exerts vasoprotective effects, preventing the development of atherosclerosis and decreasing cardiovascular risk (Busse and Fleming 1996; Gewaltig and Kojda 2002).
Cavernosal angiogenic deficit also contributes to ED, and it has been shown that intracavernosal injection of vascular endothelial growth factor (VEGF), the main angiogenic growth factor, facilitates the recovery of erectile function in experimental rat models of ED (Lee et al. 2002; Gholami et al. 2003; Rogers et al. 2003; Park et al. 2004). On the other hand, vasculogenic ED associated with corpora cavernosa atherosclerosis could be considered a hypoxia/ischemia pathology per se, thus acting as an angiogenesis inducer (Egami et al. 2006).
The underlying pathology of cardiovascular disease is usually atherosclerosis, which gradually develops over the years before being manifested as ED, heart attack or stroke (Jackson 2004). Atherosclerosis itself is strongly related to risk factors such as hypertension, high fat intake, cigarette smoking and high blood cholesterol (De Backer et al. 2003). The same risk factors are also associated with an increased risk of ED progression (Esposito and Giugliano 2005; Bacon et al. 2006; Greenfield and Donatucci 2007). The mechanism behind the action of such different factors is not known, but there is a strong belief that reactive oxygen species play an important role (Halliwell and Gutteridge 1999) suggesting that anti-oxidants may have protective effects in this situation. In fact, it was found that the ingestion of pomegrate juice, a beverage rich in anti-oxidants, preserved erectile tissue function and prevented cavernous fibrosis in arteriogenic ED in young rabbits (Azadzoi et al. 2005).
Green tea (GT), obtained from infusion of non-fermented Camellia sinensis leaves, is an anti-oxidant-enriched natural beverage containing substantial levels of catechins. Catechins are flavanoids belonging to a family of polyphenolic compounds, of which epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC) and epigallocatechin-3-gallate (EGCG) are the most abundant members in GT. The regular ingestion of GT (Riemersma et al. 2001; Stoclet et al. 2004; Kavantzas et al. 2006) or green tea extract (GTE) (Bursill et al. 2007) led to a reduction in plasma cholesterol and a decrease in aortic atherosclerosis plaques in cholesterol-fed experimental animals (Muramatsu et al. 1986). GT consumption also prevented dyslipidemia and cardiac dysfunction progression in diabetic rats (Anandh Babu et al. 2006). In aged humans, GT consumption was associated with ameliorated endothelial function (Woo et al. 1997) and reduced mortality from cardiovascular diseases (Kuriyama et al. 2006), which lends support to the existence of a strong vasculoprotective effect. The protection afforded by GT consumption may be due to an increase in plasma antioxidant potential (Tijburg et al. 1997; Zhu et al. 1999), but the decrease in VEGF expression in aortic atherosclerotic plaques following GT intake (Kavantzas et al. 2006) suggests the intervention of an additional anti-angiogenic mechanism.
Thus, we hypothesised that a catechin-associated improvement of vascular function and anti-atherosclerosis effect could delay, or even prevent, the onset of age-related ED. Such actions might be due to modulation of VEGF activity. To our knowledge, this is the first time that cavernous tissue has been studied in aged animals following chronic intake of catechin-rich natural solutions.
Materials and methods
Animals
Thirty 12-month-old male Wistar rats (Charles River Laboratories, Barcelona, Spain), weighing 893 ± 104 g, were individually housed and maintained under a 12-h light-dark cycle and standard temperature (20–22°C) with free access to food and a liquid source. Rats were randomly separated into three groups each consisting of 10 rats: (1) control (C) animals with access to tap water; (2) green tea (GT)-treated animals given an infusion of GT prepared from three tea bags (Lipton, 1.3 g/bag) in a litre of boiling water for 5 min as the only available liquid source, and (3) GT extract (GTE)-treated rats with free access to an aqueous solution containing 200 mg/l catechins extracted from GT but with a low EGCG content. The catechin composition of GT was as follows: EGCG 439.2 mg/l, (−)-epicatechin (EC) 264.1 mg/l, galocatechin-3-gallate (GCG) 32.1 mg/l and ECG 97.3 mg/l, in a total catechin concentration of 832.7 mg/l. The solution of GTE comprised 7.6 mg/l EGCG, 111.9 mg/l EC, 18.8 mg/l GCG and 61.7 mg/l ECG.
During the 6 months of treatment all rats had access to standard laboratory animal food (Letica, Barcelona, Spain). Bottles containing the beverages were protected from light to avoid oxidation of light-sensitive components and beverages were renewed every 2–3 days. Ad libitum ingestion of both food and fluid was monitored every other day and animal weight was recorded every week. All animal procedures adhered to European Community guidelines (86/609/EEC) and the Portuguese Act (129/92) for the use of experimental animals.
Tissue collection and preparation
At the end of treatment, all animals were anaesthetised (sodium pentobarbital; 80 mg/kg body weight, i.p.), blood was drawn from the left ventricle into heparinised tubes and plasma fractions were frozen at −80°C, until analysis. Penises were dissected from skin and surrounding fat, and excised.
Biochemical analysis
Plasma testosterone was measured by radioimmunoassay using antiserum against testosterone and 125I-testosterone (Testo-RIA-CT, Biosource Europe, Nivelles, Belgium). Plasma cholesterol, triglycerides and HDL cholesterol concentrations were determined by enzymatic colorimetric tests, using commercially available kits (Cholesterol, Triglycerides, HDL Cholesterol Direct, ABX Diagnostics, Shefford, UK), in an auto-analyser (Cobas Mira Plus, ABX Diagnostics).
Immunohistochemistry
Penis fragments were fixed in 10% buffered formaldehyde for 24 h and embedded in paraffin, oriented along the transversal axis. Sections, 5 μm thick, were cut with a Leica RM2145 microtome (Leica Microsystems, Wetzlar, Germany) and placed onto 0.1% poly-l-lysine coated microscopy slides for immunostaining. Sections were deparaffinised, hydrated, treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity, exposed to 1 M HCl for 30 min for epitope retrieval and neutralised with borax for 5 min. All slides were then incubated overnight with goat anti-VEGF (1/20 diluted, R&D Systems, Minneapolis, MN), goat anti-VEGFR1 (1/200 diluted, Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-VEGFR2 (1/500 diluted, Santa Cruz Biotechnology) primary polyclonal antibodies. After 30 min incubation with biotinylated secondary antibody and streptavidin-horseradish peroxidase complex (Vectastain, Vector, Burlingame, CA), sections were simultaneously reacted with 3,3′-diaminobenzidine tetrahydrochloride/H2O2 during the same period of time and counterstained with hematoxylin. All slides were examined using a Nikon bright-field microscope and images acquired on 100 ASA colour print film.
Immunofluorescence
Immunofluorescence detection of VEGF/VEGFR1 and VEGFR1/VEGFR2 was also performed, starting with a mix of rabbit anti-VEGFR1 (Lab Vision Corporation, Fremont, CA) with goat anti-VEGF (R&D Systems) or mouse anti-VEGFR2 (Santa Cruz Biotechnology) primary antibodies, followed by a suitable mix of secondary antibodies, anti-rabbit conjugated with Alexa 488 (green) with anti-goat or anti-mouse conjugated with Alexa 568 (red) both diluted 1/500. Sections were observed in a confocal microscope (Bio-Rad Laboratories, Richmond, CA) set at excitation wavelengths of 488 nm and 568 nm from a krypton-argon laser source.
Ultrastructural study by transmission electron microscopy
Corpus cavernosum fragments were fixed in 2.5% glutaraldehyde with 5 mM CaCl2, in 0.1 M cacodylate buffer, pH 7.2, for 2 h, rinsed in buffer with 7% sucrose overnight, post-fixed in 1% osmium tetroxide in cacodylate buffer, pH 7.2, at room temperature for 1 h, dehydrated in ascending concentrations of ethanol, and embedded in Epon. Ultrathin sections were stained with aqueous uranyl acetate for 5 min, lead citrate for 5 min, and observed in a Jeol 100B electron microscope (Jeol, Tokyo, Japan). Lipid-rich cells were counted and morphometric measurements of basal lamina were done on electron micrographs taken from randomly selected vessels from corpus cavernosum in a total of 30 measurements per experimental group.
Western blotting
Twenty micrograms of protein from each sample, homogenised in 50 mM Tris pH 7.2, 0.1M NaCl, 5 mM EDTA, 0,5% v/v Triton X-100 and 2% v/v Protease Inhibitor Cocktail P8340 (Sigma-Aldrich, Gillingham, UK) were loaded into 8% and 12% SDS-polyacrylamide gel (to quantify VEGF receptors and VEGF, respectively) and allowed to run for 1 h. After electrophoresis, separated peptides were transferred to a nitrocellulose membrane with pore of 0.45 μm (Bio-Rad) for 2 h. Membranes were incubated for 1 h with blocking solution (5% dried nonfat milk Molico, with 0.1% Tween 20 in Tris-buffered saline) and immunoreacted either with monoclonal mouse anti-VEGF (1/250 diluted, R&D Systems), goat anti-VEGFR1 (1/100 diluted, Santa Cruz Biotechnology), mouse anti-VEGFR2 (1/100 diluted, Santa Cruz Biotechnology) and monoclonal mouse anti-golgin-97 (1/200 diluted, Molecular Probes, Leiden, The Netherlands) for 24 h. After extensive washing and incubation with appropriated secondary antibody coupled to horseradish peroxidase for 1 h, labelled bands were visualised using chemiluminescent substrate (Kit SuperSignal, Pierce Biotechnology, Rockford, IL). Western blots (WB) were analysed using the ScionImage software version for Windows beta 4.0.3. (Scion, Frederick, MD). Total VEGF, VEGFR1 and VEGFR2 were normalised using golgin as an internal control. Results represent the variation in each protein for each treatment group (GT and EGT) compared with controls; each experiment was repeated five times.
Statistical analysis
Values are expressed as mean ± SEM throughout the text. All statistical analysis was performed employing absolute values using the Statistical Package for the Social Sciences (SPSS), version 13.0 for Windows (SPSS, Chicago, IL); the difference of mean values between groups was assessed using a two-tail t test. A value of P < 0.05 was considered significant.
Results
Body weight and food and fluid ingestion
No significant differences in body weight were found when C (965 ± 126 g), GT (962 ± 152 g) and GTE (946 ± 108 g) animals were compared. Also, no differences were found in the amount of food ingested or in the volume of liquid drunk by the three groups of rats. All animals presented in good health.
Biochemical results
The results of biochemical assays are listed in Table 1. In GT rats, the plasma levels of triglycerides, total cholesterol and HDL were not altered when compared to the C group. The GTE rats did not reveal significant changes and a similar lipid profile was observed compared with C. However, in both catechin-treated groups (GT and GTE) we detected a statistically significant decrease (P < 0.01) of plasma total testosterone levels (0.216 ± 0.093 and 0.153 ± 0.059 ng/ml, respectively) when compared to the C group (0.670 ± 0.408 ng/ml).
Table 1.
Plasma lipid and testosterone levels in rats treated with green tea (GT) or green tea extract (GTE) compared to control (C) rats. Values represent mean ± SEM of ten determinations
| C | GT | GTE | |
|---|---|---|---|
| Triglycerides (mg/dl) | 382.3 ± 129.7 | 333.6 ± 129.7 | 372.3 ± 160.0 |
| Cholesterol (mg/dl) | 135.0 ± 39.9 | 146.0 ± 44.9 | 137.9 ± 52.8 |
| HDL (mg/dl) | 20.2 ± 5.5 | 20.9 ± 4.8 | 21.6 ± 6.4 |
| Testosterone (ng/ml) | 0.670 ± 0.408 | 0.216 ± 0.093* | 0.153 ± 0.059* |
* P < 0.01 vs C
Morphological study of corpus cavernosum
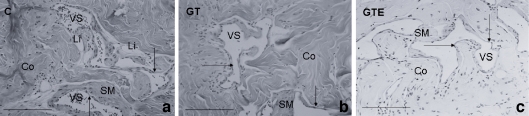
Under light microscope examination, the rat cavernous tissue had a sponge-like texture, exhibiting a mesh of interconnected cavernous spaces that were lined by vascular endothelium and separated by trabecula composed mainly of connective tissue (collagen fibres and fibroblasts). Fusiform smooth muscle fibres were restricted to endothelium periphery and apparently displayed similar thickness in all experimental groups of rats. While there were no marked differences in the tissue organisation between the three groups, C animals presented some clusters of lipid-rich cells close to vessel spaces (Fig. 1a) that were absent from the catechin-treated animals (Fig. 1b,c).
Fig. 1.
Hematoxylin and eosin staining of penile tissue from control (C) (a), green tea (GT) (b) and green tea extract (GTE) (c)-treated rats. No marked differences in the tissue organisation were observed between the three groups. Cavernous tissue has a sponge-like structure, with cavernous vascular spaces (VS) lined by endothelium (arrow) and separated by trabecula composed mainly of connective tissue (Co). Smooth muscle fibres are restricted to the endothelium periphery (SM). Note the clusters of lipid-rich cells close to vessel spaces in C animals (Li). Bar 50 μm
Immunohistochemical study of VEGF, VEGFR1 and VEGFR2 expression in corpus cavernosum
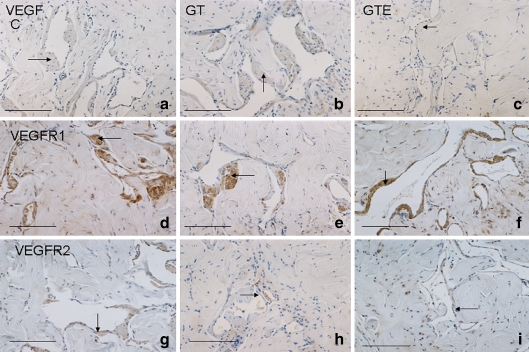
In all groups, VEGF presented a scattered distribution over the smooth muscle fibres, particularly in those surrounding the endothelium (Fig. 2a–c). VEGFR1 was also expressed in perivascular smooth muscle fibres (Fig. 2d–f), and co-localised with VEGF (Fig. 3a–c). No marked differences were observed in either VEGFR1 or VEGF expression between the C, GT and GTE groups. On the other hand, immunohistochemical detection of VEGFR2 was restricted to the endothelium in all experimental groups (Fig. 2g–i), showing notably decreased expression in both GT- and GTE-treated animals (Fig. 2h and i, respectively) as compared to C rats (Fig. 2g).
Fig. 2.
Immunohistochemical detection of vascular endothelial growth factor (VEGF) in the corpus cavernosum of control (C) (a), green tea (GT) (b) and green tea extract GTE (c) animals, presenting a scattered distribution over the smooth muscle fibres, particularly in those surrounding endothelium (arrow). As observed in (c) VEGF is decreased in the GTE group. VEGF receptor 1 (VEGFR1) was also detected by immunohistochemistry in perivascular smooth muscle fibres (arrow) and no marked differences were observed among the C (d), GT (e) and GTE (f) experimental groups. Immunohistochemical detection of VEGFR2 (g, h, i) was restricted to the endothelium (arrow) in all groups and expression was noticeably reduced in both GT- and GTE-treated animals (h and i) as compared to the C group (g). Bar 50 μm
Fig. 3.
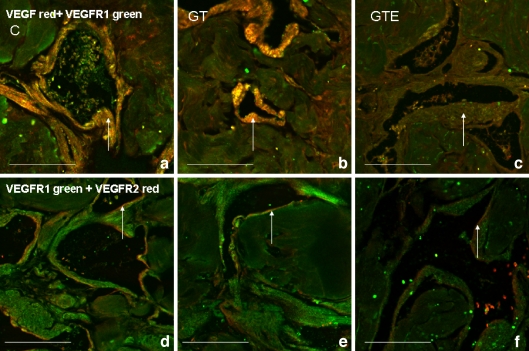
Confocal microscopy immunofluorescence of VEGF (red) and VEGFR1 (green) in corpus cavernosum of rats. Double-labelling was observed in the muscle layer at the vessel periphery (white arrow) in control (C) (a), green tea (GT) (b) and green tea extract (GTE) (c) groups of animals. An apparent decrease in VEGF expression was observed in the GTE group (c) as compared with the C (a) and GT (b) groups. Double-labelling of VEGFR1 (green) and VEGFR2 (red) demonstrated VEGFR2 expression in the endothelium (white arrow), with a substantial decrease in labelling in the corpus cavernosum of rats from the groups that had consumed catechin-rich beverages (GT and GTE) (e and f, respectively) when compared to C animals (d). Bar 100 μm
Co-expression of VEGF/ VEGFR1 and VEGFR1/ VEGFR2 in corpus cavernosum
The distribution of VEGF receptors in corpus cavernosum agrees with previous results obtained in aged rats (Neves et al. 2006). Double-labeling confocal microscopy showed that VEGF and VEGFR1 were found in the thick smooth muscle layer at the vessel periphery in all groups of rats (Fig. 3a–c). Simultaneous immunofluorescence detection of VEGFR1 and VEGFR2 revealed a substantial decrease in VEGFR2 expression in the corpus cavernosum of GT- and GTE-treated rats (Fig. 3e and f) when compared to C animals (Fig. 3d), consistent with immunocytochemical observations.
Ultrastructural study of corpus cavernosum
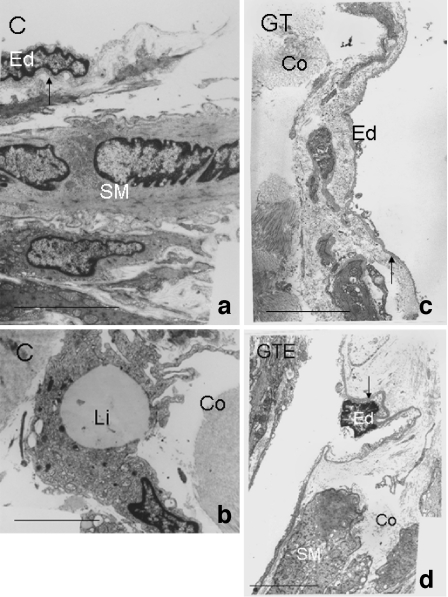
In all experimental groups, cavernous tissue endothelial cells were surrounded by smooth muscle fibres (Fig. 4a,c,d) and the subendothelial layer presented connective tissue containing disperse fibroblasts and an abundant extracellular matrix (Leeson and Leeson 1965). Close to the subendothelial surface of the endothelial cell membrane, a basal lamina was observed. While in C animals, the basal lamina had an average thickness of 61 nm (Fig. 4a), this was increased to an average value of 218 nm in the corpus cavernosum of rats ingesting GT (Fig. 4c), and to 221 nm in the GTE group (Fig. 4d; P < 0.01). Next to the basal membrane, the extracellular matrix consists of fibres structurally resembling organised collagen, i.e. fibrils of homogeneous diameter organised into small bundles and elastic fibrils, but although these presented as abundant deposits there were no major structural differences when comparing C to GT and GTE groups. We also observed immature newly synthesised collagen fibres that penetrated endothelial basement membranes in all experimental groups. In C animals there were frequent lipid droplet-rich cells in erectile tissue, localised close to vascular space (Fig. 4b), whereas in the GT group of rats these were very sparse and in GTE animals no lipid-rich cells were found.
Fig. 4.
Ultrastructure of cavernous tissue of rats from control (C) (a, b), green tea (GT) (c) and green tea extract (GTE) (d) animals. Endothelial cells (Ed) were separated by basal lamina (arrow) from the surrounding smooth muscle fibres (SM). Profuse collagen fibres (Co) were observed in all experimental groups studied. Note the increased average thickness of the basal lamina (arrow) in the corpus cavernosum of rats ingesting GT or GTE (c and d, respectively). In C animals there were frequent lipid droplet rich cells (Li) in erectile tissue, localised close to the vascular space. Bar 5 μm
Semi-quantification of VEGF, VEGFR1 and VEGFR2 expression by western blotting
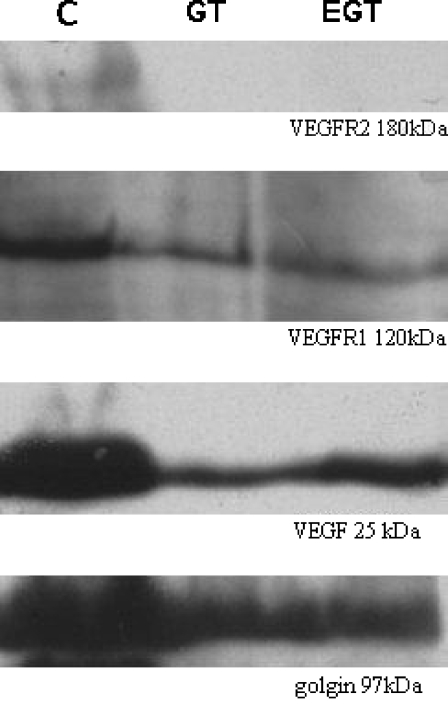
Chemiluminescent immunodetection of VEGF revealed a band with an apparent molecular weight of 25 kDa whose expression showed a quantitative reduction in samples from rats that had consumed catechin-rich beverages (52% in GT and 80% in GTE groups, relative to the control group, 100%) (Fig. 5). A similar variation was observed for the 120 kDa VEGFR1 monomer band (62% in GT and 79% in GTE groups, relative to the control group, 100%). However, the most noteworthy decrease was observed in expression of the 180 kDa VEGFR2 (5% in GT and 13% in GTE groups, relative to the control group, 100%).
Fig. 5.
Representative bands obtained by western blot analysis of VEGF (25 kDa), VEGFR1 (120 kDa) and VEGFR2 (180 kDa) in corpus cavernosum samples of control (C), green tea (GT) and green tea extract (EGT)-treated rats. Semi-quantitative analysis was carried out by calculating the fraction of pixels presented in the selected band relative to the golgin band in the same sample used as an internal control. All the studied molecules exhibited lower expression in the GT and GTE groups when compared with the control, the decrease in VEGFR2 expression being particularly evident
Discussion
In the present experiment, 12-month-old rats were submitted to the consumption of catechin-rich solutions for 6 months. The major difference between the GT and GTE beverages was the catechin content of the solution: GT rats had access to a high-content EGCG infusion, while GTE rats ingested an EGCG-depleted solution. We verified that the extended consumption of both catechin-rich solution led to a significant decrease in plasma testosterone levels in the 18-month-old animals. This finding contrasts with previous reports (Kao et al. 2000) where no reduction in serum levels of testosterone was observed after oral administration of EGCG during a short period in adult rats. In the same study it was found that only EGCG, but not other structurally similar catechins, affected hormone levels, but even this action was highly dependent on the route of administration (Kao et al. 2000). In the present study, the diminished testosterone levels may have resulted from additional unspecified compounds present in the beverage, and also from the flavonoids, which possess oestrogenic properties (Mazur et al. 1998; Geleijnse et al. 2000; Cheng 2006). Actually, as phyto-oestrogens, they bind with low affinity to oestrogen receptors (Mazur et al. 1998) and, for this reason, polyphenols have been proposed as an alternative hormonal replacement therapy for postmenopausal women (Atteritano et al. 2007; Kreijkamp-Kaspers et al. 2007). In addition, oestrogens are also known to act as anti-androgens in some tissues (Tindall et al. 1981) and are capable of influencing erectile physiology by multiple mechanisms (Zvara et al. 1995; Reilly et al. 1997; Traish and Kim 2005; Traish et al. 2007). This way, we hypothesise that the long-term consumption of GT and GTE results in a decreased synthesis of luteinizing hormone (LH), similar to the effect of catechin intraperitoneal administration on pituitary LH expression (Kao et al. 2000), which in turn leads to testosterone reduction. The exact action of the androgens in sexual function is unclear but it is known that testosterone controls male gonad development and enhances libido. However, there is no conclusive evidence for a causal relationship between altered levels of testosterone and erectile dysfunction (Nickel et al. 1984; Tenover 1992; Fahmy et al. 1999) and in fact, reduced serum testosterone levels does not impede normal erectile function (Rhoden et al. 2002).
Interestingly, we did not find significant variation in plasma lipids in catechin-fed animals when compared to age-matched controls. Moreover, controls presented higher levels of triglycerides (+ 140%), total cholesterol (+ 78%) and HDL (+ 44%) when compared to the levels displayed in 12-month-old Wistar rats from a previous study (Almeida et al. 1998). Although other studies have reported a reduction in plasma cholesterol due to the action of GT catechins (Muramatsu et al. 1986; Suzuki et al. 2005; Bursill et al. 2007), others found that GT consumption did not lower plasma cholesterol but tended to reduce atherosclerotic plaque formation, possibly due to a reduction in LDL oxidation (Tijburg et al. 1997). This observation is also important, considering the fact that we found a decrease in lipid-rich cells in the corpus cavernosum of GT- and GTE-fed rats when compared with the C group. The contribution of these cells for atheroma in the corpus cavernosum is still unknown, but the appearance of such cells in other tissues is recognised as one of the earliest signs of major vessel atheroma (Takahashi et al. 2002).
Severe testosterone deprivation in the young rat has a strong negative impact on the structure of penile tissues and erectile nerves (Brown et al. 1999; Neves et al. 2006); however, we observed no ultrastructural changes in the corpus cavernosum of any catechin-treated aged rats. Interestingly, the main structural changes were observed at the corpus cavernosum endothelium basement lamina, as its thickness increased approximately 3.6-fold in GT- and GTE-fed rats when compared to C animals, as we and others (Candiello et al. 2007) reported. This structural element acts as a support scaffold for epithelial cells and directly participates in wound repair, insulation and transport processes, as well as signalling events mediating cellular proliferation, migration, and apoptosis (Erickson and Couchman 2000). In general, the appearance of a basement membrane reflects the balance established between the biosynthesis and degradation of its components in a complex setting of cell-matrix interactions, involving protein degradation/modification via matrix metalloproteinases (Kalluri 2003). These proteins are zinc-dependent proteases that degrade extracellular matrix components and non-matrix substrates such as growth factors and cell surface receptors (Ra and Parks 2007). The interplay between matrix metalloproteinases and their natural antagonists, namely the tissue inhibitor of metalloproteinases, has a major role in the turnover of the basement membrane (Visse and Nagase 2003; Baum et al. 2007) contributing both to the pathogenesis of major cardiovascular diseases such as atherosclerosis, and to restenosis (Galis et al. 2002).
The increase in endothelial basement lamina thickness observed in the animals treated with catechin-enriched beverages (GT and GTE) is likely due to a diminished degradation of basement lamina components by matrix metalloproteinases (Oak et al. 2005). This hypothesis is supported by the finding that EGCG inhibited metallo-proteinase-2 and reduced basement lamina degradation in prostate carcinoma cells (Pezzato et al. 2004). In addition, EGCG has been demonstrated to possess potent anti-matrix metalloproteinase activity in cancer cell cultures, also blunting the angiogenesis crucial for the growth of solid tumours (Garbisa et al. 1999; Maeda et al. 2003). Further studies will be necessary in order to clarify the biological significance of the augmented basement lamina thickness in the endothelial cells of corpus cavernosum of catechin-treated rats.
Immunohistochemical and western blot studies demonstrated reduced VEGF expression in the corpus cavernosum of catechin-treated animals. This finding is in agreement with previous data showing that VEGF expression is prevented by GT polyphenols (Sartippour et al. 2002), and particularly by EGCG, in several types of cancer cell lines (Masuda et al. 2002). However, VEGF can be detected by immunohistochemistry in the corpus cavernosum of catechin-treated animals. The explanation for this finding is still unknown but it may be justified by the ability of extracellular matrix components to strongly bind EGCG and therefore entrap in it VEGF, as was previously described for platelet-derived growth factor (PDGF) (Doss et al. 2005). Then again, it is well established that VEGF binds specifically to membrane receptors VEGFR1 and VEGFR2, which have tyrosine kinase activity. These receptors are expressed mostly, but not exclusively, on endothelial cells, and most VEGF angiogenic effects result from VEGFR2 activation (Ferrara et al. 2003). Since EGCG is anchored firmly in the extracellular matrix, the transducing ability of the entrapped VEGF is abolished given that the molecule cannot move and therefore interact with its specific receptors (Tang et al. 2003). The immunohistochemical study in GT and GTE animals, strongly supported by molecular data, points to a reduction in expression of VEGFR2, likely to result from its downregulation due to the effect of EGCG on tyrosine kinase receptors (Lamy et al. 2002) or from the inability of VEGF to interact with VEGFR2 as a consequence of its entrapment in the extracellular matrix. The reduced expression of VEGF and VEGFR2 reported herein suggests decreased angiogenic activity and, consequently, a forestalling of atherosclerotic plaque formation ability (Kumamoto et al. 1995; Chen et al. 1999) for which the near absence of lipid-rich cells in the corpus cavernosum provides additional corroboration. In this context, it is worth recalling that atherosclerosis leads to arterial narrowing, impaired perfusion and hypoxia, known to exert stimulatory effects on VEGF and VEGF receptor expression (Ferrara and Davis-Smyth 1997; Semenza 2000) in particular that of VEGFR2 (González-Pacheco et al. 2006). Our data indicate that rats on a long-term catechin beverage ingestion do not exhibit relevant structural changes in corpus cavernosum vasculature, indicating that the blood is perfusing the tissue at non-hypoxic levels, which suggests that the anti-oxidant effect of the beverage is an additional contributor to VEGF downregulation.
Taken together, the data obtained from this study suggest that prolonged consumption of catechin-rich beverages has a favourable effect on atheroma prevention. This may result from the combination of the mild oestrogenic effect of flavonoids, the reduction in lipid cells at the perivascular space in the corpus cavernosum and the anti-angiogenic properties of the beverage compounds, as indicated by the decreased expression of angiogenic factors. More studies, particularly measurements of endothelial function and intracavernous pressure to evaluate erection functional capability will be necessary to elucidate if catechin-rich beverages are useful prophylactic tools for the prevention of the deleterious vascular events associated with ED.
Acknowledgements
The authors thank Dr. Conceição Gonçalves from the Laboratório Nobre of Faculty of Medicine of University of Porto for testosterone RIA assays. This work was supported by Fundação para a Ciência e a Tecnologia (FCT)–Unit 121/94 and SFRH/BD/19497/2004.
References
- Almeida H, Magalhães MC, Magalhães MM (1998) Age-related changes in the inner zone of the adrenal cortex of the rat—a morphologic and biochemical study. Mech Ageing Dev 105:1–18 [DOI] [PubMed]
- Anandh Babu PV, Sabitha KE, Shyamaladevi CS (2006) Green tea extract impedes dyslipidaemia and development of cardiac dysfunction in streptozotocin-diabetic rats. Clin Exp Pharmacol Physiol 33:1184–1189 [DOI] [PubMed]
- Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Mazzaferro S, Anna RD, Cannata ML, Gaudio A, Frisina A, Frisina N, Corrado F, Cancellieri F, Lubrano C, Bonaiuto M, Adamo EB, Squadrito F (2007) Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-years randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 92:3068–3075 [DOI] [PubMed]
- Ayta IA, Mckinlay JB, Krane RJ (1999) The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 84:50–56 [DOI] [PubMed]
- Azadzoi KM (2006) Vasculogenic erectile dysfunction: beyond the haemodynamic changes. BJU Int 97:11–17 [DOI] [PubMed]
- Azadzoi KM, Schulman RN, Aviram MA, Siroky MB (2005) Oxidative stress in arteriogenic erectile dysfunction: prophylatic role of antioxidants. J Urol 174:386–393 [DOI] [PubMed]
- Bacon C, Mittleman M, Kawachi I, Giovannucci E, Glasser D, Rimm E (2006) A prospective study of risk factors for erectile dysfunction. J Urol 176:217–221 [DOI] [PubMed]
- Baum O, Ganster M, Baumgartner I, Nieselt K, Djonov V (2007) Basement membrane remodeling in skeletal muscles of patients with limb ischemia involves regulation of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases. J Vasc Res 44:202–213 [DOI] [PubMed]
- Blumentals WA, Gomez-Caminero A, Joo S, Vannappagari V (2004) Should erectile dysfunction be considered as a marker for acute myocardial infarction? Results from a retrospective cohort study. Int J Impot Res 16:350–353 [DOI] [PubMed]
- Brown GR, Nevison CM, Fraser HM, Dixson AF (1999) Manipulation of postnatal testosterone levels affects phallic and clitoral development in infant rhesus monkeys. Int J Androl 22:119–128 [DOI] [PubMed]
- Bursill CA, Abbey M, Roach PD (2007) A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis 193:86–93 [DOI] [PubMed]
- Busse R, Fleming I (1996) Endothelial dysfunction in atherosclerosis. J Vasc Res 33:181–194 [DOI] [PubMed]
- Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H (2007) Biomechanical properties of native basement membranes. FEBS J 274:2897–2908 [DOI] [PubMed]
- Cheitlin CMD (2004) Erectile dysfunction: the earliest sign of generalized vascular disease. J Am Coll Cardiol 43:185–186 [DOI] [PubMed]
- Chen YX, Nakashima Y, Tanaka K, Shiraishi S, Nakagawa K, Sueishi K (1999) Immunohistochemical expression of vascular endothelial growth factor/vascular permeability factor in atherosclerotic intimas of human coronary arteries. Arterioscler Thromb Vasc Biol 19:131–139 [DOI] [PubMed]
- Cheng TO (2006) Why is green tea more cardioprotective in women than in men. Int J Cardiol 122:244 [DOI] [PubMed]
- De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats VM, Orth-Gomér K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D (2003) European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 24:1601–1610 [DOI] [PubMed]
- Doss MX, Potta SP, Hescheler J, Sachinidis A (2005) Trapping of growth factors by catechins: a possible therapeutical target for prevention of proliferative diseases. J Nutr Biochem 16:259–266 [DOI] [PubMed]
- Egami K, Murohara T, Aoki M, Matsuishi T (2006) Ischemia-induced angiogenesis: role of inflammatory response mediated by P-selectin. J Leukoc Biol 79:971–976 [DOI] [PubMed]
- Erickson AC, Couchman JR (2000) Still more complexity in mammalian basement membranes. J Histochem Cytochem 10:1291–1306 [DOI] [PubMed]
- Esposito K, Giugliano D (2005) Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res 17:391–398 [DOI] [PubMed]
- Fahmy AK, Mitra S, Blacklock ARE, Desai KM (1999) Is the measurement of serum testosterone routinely indicated in men with erectile dysfunction. Br J Urol Int 84:482–484 [DOI] [PubMed]
- Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25 [DOI] [PubMed]
- Ferrara N, Gerber HP, Couter JL (2003) The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed]
- Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E (2002) Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res 91:852–859 [DOI] [PubMed]
- Garbisa S, Biggin S, Cavallarin N, Sartor L, Benelli R, Albini A (1999) Tumor invasion: molecular shears blunted by green tea. Nat Med 5:1216 [DOI] [PubMed]
- Geleijnse JM, Witteman JCM, Launer LJ, Lamberts SWJ, Pols HAP (2000) Tea and coronary heart disease: protection through estrogen-like activity. Arch Intern Med 160:3328–3329 [DOI] [PubMed]
- Gewaltig MT, Kojda G (2002) Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc Res 55:250–260 [DOI] [PubMed]
- Gholami SS, Rogers R, Chang J, Ho HC, Grazziottin T, Lin CS, Lue TF (2003) The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol 169:1577–1581 [DOI] [PubMed]
- Goldstein I (2003) The association of ED (erectile dysfunction) with ED (endothelium dysfunction) in the international journal of impotence research: the journal of sexual medicine. Int J Impot Res 15:229–230 [DOI] [PubMed]
- González-Pacheco FR, Deudero JJP, Castellanos MC, Castilla MA, Álvarez-Arroyo MV, Yagüe S, Caramelo C (2006) Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol 291:1395–1401 [DOI] [PubMed]
- Greenfield JM, Donatucci CF (2007) Smoking, obesity, and sedentary lifestyle linked to erectile dysfunction. Nat Clin Pract Urol 4:16–17 [DOI] [PubMed]
- Guay AT (2007) ED2: erectile dysfunction = endothelial dysfunction. Endocrinol Metabol Clin N Am 36:453–463 [DOI] [PubMed]
- Halliwell B, Gutteridge JMC (1999) Free radicals, other reactive species and disease. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine, 3rd edn. Oxford Science Publications, Oxford, pp 617–783
- Jackson G (2004) Sex, the heart and erectile dysfunction. Taylor & Francis, London, p 10
- Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3:422–433 [DOI] [PubMed]
- Kao YH, Hiipakka RA, Liao S (2000) Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 141:980–987 [DOI] [PubMed]
- Kavantzas N, Chatziioannou A, Yanni AE, Tsakayannis D, Balafoutas D, Agrogiannis G, Perrea D (2006) Effect of green tea on angiogenesis and severity of atherosclerosis in cholesterol-fed rabbit. Vascul Pharmacol 44:461–463 [DOI] [PubMed]
- Kaya C, Uslu Z, Karaman I (2006) Is endothelial function impaired in erectile dysfunction patients. Int J Impot Res 18:55–60 [DOI] [PubMed]
- Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, van der Schouw YT (2007) Dietary phytoestrogen intake and cognitive function in older women. J Gerontol A Biol Sci Med Sci 62:556–562 [DOI] [PubMed]
- Kumamoto M, Nakashima Y, Sueishi K (1995) Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol 26:450–456 [DOI] [PubMed]
- Kuriyama S, Shimazu T, Ohmori K, Nakaya N, Nishino Y, Tsubono Y, Tsuji I (2006) Green tea consumption and mortality due to cardiovascular disease, cancer, all causes in Japan. JAMA 296:1255–1265 [DOI] [PubMed]
- Lamy S, Gingras D, Beliveau R (2002) Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res 62:381–385 [PubMed]
- Lee MC, El-Sakka AI, Graziottin TM, Ho HC, Lin CS, Lue TF (2002) The effect of vascular endothelial growth factor on a rat model of traumatic arteriogenic erectile dysfunction. J Urol 167:761–767 [DOI] [PubMed]
- Leeson TS, Leeson CR (1965) The fine structure of cavernous tissue in the adult rat penis. Invest Urol 3:144–154 [PubMed]
- Maeda K, Kuzuya M, Cheng XW, Asai T, Kanda S, Tamaya-Mori N, Sasaki T, Shibata T, Igushi A (2003) Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis 166:23–30 [DOI] [PubMed]
- Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB (2002) Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol 2:350–359 [DOI] [PubMed]
- Mazur WM, Wahala K, Rasku S, Salakka A, Hase T, Adlercreutz H (1998) Lignan and isoflavonoid concentrations in tea and coffee. Br J Nutr 79:37–45 [DOI] [PubMed]
- Muramatsu K, Fukuyo M, Hara Y (1986) Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol 32:613–622 [DOI] [PubMed]
- Neves D, Santos J, Tomada N, Almeida H, Vendeira P (2006) Ageing and orchidectomy modulate expression of VEGF receptors (Flt-1 and Flk-1) on corpus cavernosum of the rat. Ann N Y Acad Sci 1067:164–172 [DOI] [PubMed]
- Nickel JC, Morales A, Condra M, Fenemore J, Surridge DH (1984) Endocrine dysfunction in impotence: incidence and cost-effective screening. J Urol 1984 132:40–43 [DOI] [PubMed]
- Oak MH, El Bedoui JE, Schini-Kerth VB (2005) Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem 16:1–8 [DOI] [PubMed]
- Park K, Ahn KY, Kim MK, Lee SE, Kang TW, Ryu SB (2004) Intracavernosal injection of vascular endothelial growth factor improves erectile function in aged rats. Eur Urol 46:403–407 [DOI] [PubMed]
- Pezzato E, Sartor L, Dell’Aica I, Dittadi R, Gion M, Belluco C, Lise M, Garbisa S (2004) Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (−) epigallocatechin-3-gallate. Int J Cancer 112:787–792 [DOI] [PubMed]
- Ra HJ, Parks WC (2007) Control of matrix metalloproteinase catalytic activity. Matrix Biol 28:587–596 [DOI] [PMC free article] [PubMed]
- Reilly CM, Zamorano P, Stopper VS, Mills TM (1997) Androgenic regulation of NO availability in rat penile erection. J Androl 18:110–115 [PubMed]
- Rhoden EL, Telöken C, Mafessoni R, Vargas Souto CA (2002) Is there any relation between serum levels of total testosterone and the severity of erectile dysfunction. Int J Impot Res 14:167–171 [DOI] [PubMed]
- Riemersma RA, Rice-Evans CA, Tyrrell RM, Clifford MN, Lean MEJ (2001) Tea flavonoids and cardiovascular health. QJM 94:277–282 [DOI] [PubMed]
- Rogers S, Graziottin TM, Lin CS, Kan YW, Lue TF (2003) Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res 15:26–37 [DOI] [PubMed]
- Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN (2002) Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr 132:2307–2311 [DOI] [PubMed]
- Semenza GL (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14:1983–1991 [PubMed]
- Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, Bedoui JE, Chataigneau M, Schini-Kerth VB (2004) Vascular protection by dietary polyphenols. Eur J Pharmacol 500:299–313 [DOI] [PubMed]
- Suzuki J, Ogawa M, Izawa A, Sagesaka YM, Isobe M (2005) Dietary consumption of green tea catechins attenuate hyperlipidaemia-induced atherosclerosis and systemic organ damage in mice. Acta Cardiol 60:271–276 [DOI] [PubMed]
- Takahashi K, Takeya M, Sakashita N (2002) Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc 35:179–203 [DOI] [PubMed]
- Tang FY, Nguyen N, Meydani M (2003) Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int J Cancer 106:871–878 [DOI] [PubMed]
- Tenover JS (1992) Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metabol 75:1092–1098 [DOI] [PubMed]
- Tijburg LMB, Wiseman SA, Meijer GW, Weststrate JA (1997) Effects of green tea, black tea and dietary lipophylic antioxidants on LDL oxidizability and atherosclerosis in hypercholesterolemic rabbits. Atherosclerosis 135:37–47 [DOI] [PubMed]
- Tindall DJ, French FS, Nayfeh SN (1981) Estradiol-17 beta inhibition of androgen uptake, metabolism and binding in epididymis of adult male rats in vivo: a comparison with cyproterone acetate. Steroids 37:257–268 [DOI] [PubMed]
- Traish A, Kim N (2005) The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med 2:759–770 [DOI] [PubMed]
- Traish AM, Goldstein I, Kim NN (2007) Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 52:54–70 [DOI] [PMC free article] [PubMed]
- Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res 92:827–839 [DOI] [PubMed]
- Vlachopoulos C, Rokkas K, Ioakeimidis N, Aggeli C, Michaelides A, Roussakis G, Fassoulakis C, Askitis A, Stefanadis C (2005) Prevalence of asymptomatic coronary artery disease in men with vasculogenic erectile dysfunction: a prospective angiographic study. Eur Urol 48:996–1003 [DOI] [PubMed]
- Woo KS, McCrohon JA, Chook P, Adams MR, Robinson JTC, McCredie RJ, Lam CWK, Feng JZ, Celermajer DS (1997) Chinese adults are less susceptible than whites to age-related endothelial dysfunction. J Am Coll Cardiol 30:113–118 [DOI] [PubMed]
- Zhu QY, Huang Y, Tsang D, Chen ZY (1999) Regeneration of alpha-tocopherol in human low-density lipoprotein by green tea catechin. J Agric Food Chem 47:2020–2025 [DOI] [PubMed]
- Zvara P, Sioufi R, Schiper HM, Begin LR, Brock GB (1995) Nitric oxide mediated erectile activity is a testosterone dependent event: a rat erection model. Int J Impot Res 7:209–219 [PubMed]