Abstract
Phosphorylation of the histone family is not only a response to cell signaling stimuli, but also an important indicator of DNA damage preceding apoptotic changes. While astrocytic degeneration, including DNA damage, has been reported in Alzheimer disease (AD), its pathogenetic significance is somewhat unclear. In an effort to clarify this, we investigated the expression of γH2AX as evidence of DNA damage in astrocytes to elucidate the role of these cells in the pathogenesis of AD. In response to the formation of double-stranded breaks in chromosomal DNA, serine 139 on H2AX, a 14-kDa protein that is a member of the H2A histone family and part of the nucleosome structure, becomes rapidly phosphorylated to generate γH2AX. Using immunocytochemical techniques, we found significantly increased levels of γH2AX in astrocytes in regions know to be vulnerable in AD, i.e., the hippocampal regions and cerebral cortex. These results suggest that astrocytes contain DNA damage, possibly resulting in functional disability, which in turn reduces their support for neurons. These findings further define the role of astrocyte dysfunction in the progression of AD.
Keywords: Alzheimer disease, Astrocytes, DNA damage, Neurodegeneration
Introduction
Astrocytosis is a sequential morphological change resulting from astrocytic reaction to various kinds of stresses, e.g., oxidative stress (Iida et al. 2004), in the brain. In one study, a significant correlation between astrocytosis (or astrogliosis) and astrocyte degeneration (or astrodegeneration) was observed in cases of frontotemporal dementia (FTD), such that brain tissue expressing minimal to abundant degrees of astrogliosis also showed minimal to severe nuclear degeneration, respectively (Martin et al. 2001). Interestingly, it has also been shown that most regressive GFAP-positive astrocytes are apoptotic in AD (Kobayashi et al. 2002). In fact, DNA damage in astrocytes has been reported in FTD (Martin et al. 2001; Su et al. 2000), human alcoholic brains (Ikegami et al. 2003), and Pick’s disease (Gleckman et al. 1999), as evidenced mostly by increased TUNEL positivity in astrocytes.
While the pathogenic role of astrocytic DNA damage or apoptosis is unclear, previous findings support the idea that cellular degeneration during the course of disease progress in FTD and AD is not limited to neurons (Kobayashi et al. 2002; Martin et al. 2001) and, therefore, glial degeneration may be a concomitant process with neurodegeneration. Since the supportive role of glial cells, especially astrocytes, for neurons is very well known, astrocytic DNA damage and resultant apoptosis or degeneration may contribute to the pathogenesis of AD through the loss of supportive functions.
Phosphorylation of H2AX (γH2AX) at Ser139 is known to play a very early and important role in the cellular response to DNA double-strand breaks and is mediated by ataxia telangiectasia mutated kinase (ATM) (Burma et al. 2001; Rogakou et al. 1998; Sedelnikova et al. 2003). The 14-kDa protein H2AX is a member of the H2A histone family and part of the nucleosome structure. Within minutes following DNA damage, γH2AX localizes to sites of DNA damage at subnuclear foci. Phosphorylation of ubiquitinated H2AX may be a factor in the structural change in chromatin seen following induction of apoptotic cell death (Enomoto et al. 2004). The amino-terminal tails of core histones also undergo various post-translational modifications, including acetylation, phosphorylation and methylation (Cheung et al. 2000). These modifications occur in response to cell signaling stimuli and have a direct effect on gene expression. Phosphorylation of H2AX is also known to precede the translocation of phosphatidylserine to the outer cell membrane and the appearance of internucleosomal DNA fragments during apoptosis (Rogakou et al. 2000).
Nevertheless, astrocytic DNA damage involving γH2AX, an important marker of early DNA damage, has never been reported in neurodegenerative disorders such as AD. To address this point, we performed an immunocytochemical staining for γH2AX on age-matched control and AD brains to characterize DNA damage in astrocytes in AD.
Materials and methods
Tissue samples
Hippocampal tissue samples with adjacent temporal cortex were obtained at autopsy and fixed in either formalin or methacarn (methanol:chloroform:acetic acid; 6:3:1) and embedded in paraffin. Paraffin sections were cut at 6 μm using a microtome and placed on coated slides. For this study, 13 cases of AD [ages 71–95 years, mean 85; and with a mean post-mortem interval (PMI) of 17 h], 5 younger control cases (ages 15–37 years, mean 26; mean PMI of 14 h) and 8 aged-matched controls (ages 57–86, mean 71; mean PMI of 22 h) were examined. The clinical diagnosis was confirmed pathologically for all patients using CERAD criteria (Khachaturian 1985; Mirra et al. 1991). Table 1 lists detailed information about each of the cases used for this study.
Table 1.
Detailed case information. PMI Post-mortem interval
| Age (years) | PMI (h) | Gender | Cause of death |
|---|---|---|---|
| Alzheimer disease (AD) | |||
| 71 | 13 | F | AD (8 year history) |
| 76 | 47 | F | AD (advanced) |
| 78 | 3 | M | AD (severe) |
| 81 | 5 | F | AD (Braak III/IV) |
| 82 | 15 | M | AD (Braak III) |
| 85 | 3 | M | AD |
| 85 | 22 | F | AD (moderate) |
| 87 | 5 | F | AD (Braak V) |
| 89 | 9a | M | AD |
| 91 | 41 | F | AD |
| 91 | 7 | F | AD (Braak V) |
| 95 | 7 | M | Alzheimer (12 year history) |
| 95 | 46a | F | AD (probable) |
| Controls | |||
| 15 | 3 | F | Cystic fibrosis |
| 17 | 15 | M | Accident |
| 31 | 4 | F | Cystic fibrosis |
| 31 | 24 | M | NAc |
| 37b | 24 | F | Pleural sepsis |
| 57 | 15 | M | Cirrhosis |
| 61 | 5 | F | Lymphoma |
| 65 | 17 | M | Aortic aneurysm |
| 66 | 46 | M | Diabetes |
| 74b | 24 | M | Cancer |
| 78 | 18 | M | Accident |
| 82 | NA | F | Pneumonia |
| 86 | 10 | M | Heart disease |
aNegative Alzheimer disease case
bPositive control case
cNot available
Immunocytochemistry
Immunocytochemical analysis was performed using the peroxidase-anti-peroxidase method (Ogawa et al. 2003b; Sternberger 1986). Briefly, paraffin sections were deparaffinized in two changes of xylene and rehydrated through a graded series of ethanol to Tris -buffered saline (TBS) (50 mM Tris, 150 mM NaCl, pH 7.6). Endogenous peroxidases were inactivated with a 30 min incubation in 3% H2O2 and, thereafter, sections were incubated in 10% normal goat serum (NGS) in TBS and then in primary antibodies overnight at 4°C. After rinsing in 1% NGS, and 10 min in 10% NGS, goat-anti-rabbit or mouse was applied for 30 min. Again, after rinsing, rabbit or mouse specific peroxidase-anti-peroxidase complexes were applied for 1 h at room temperature. Immunostaining was visualized using 3′-3′-diaminobenzidine as chromagen. All sections were counterstained with haematoxylin and eosin. Sections were dehydrated and mounted.
For double-label immunocytochemistry, the second primary antibody was developed using the alkaline-phosphatase method with Fast Blue as chromagen. Stained sections were mounted with crystal mount. Antibodies including anti-γH2AX (Cell Signaling, Danvers, MA; rabbit polyclonal), anti-activated microglia (clone TAL1B5; Dako, Carpinteria, CA; mouse monoclonal), anti-GFAP (MP Biomedicals, Solon, OH; mouse monoclonal), and AT8 recognizing phosphorylated tau (Endogen, Woburn, MA; mouse monoclonal) were used for immunocytochemistry.
Immunostained hippocampal sections were examined throughout the entire area, including gray and white matters, under a light microscope. Double immunostained slides were analyzed qualitatively to verify the type of cells showing γH2AX immunopositivity.
Results
γH2AX immunoreactivity in AD and control brains
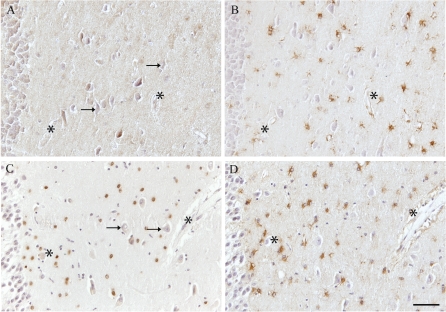
Specific nuclear immunopositivity for γH2AX was seen in the gray and white matter in 11 out of 13 AD cases. The positive nuclei, morphologically characterized as those of glial cells, were found in both gray and white matter, and consistently in the cornus ammonus (CA) regions of the hippocampus rather than other adjacent areas in the brain (Fig. 1 shows the CA4 region). Interestingly, only 2 of the 13 control brains (ages 37 and 74 years) demonstrated significant immunoreactivity in glia, while all other control cases showed no immunoreactivity (Fig. 1). The two positive control cases (highlighted in Table 1), were a younger patient with pleural sepsis and a 74-year-old cancer patient, who showed no neuropathological abnormalities. γH2AX was not present in the neurons in any regions including the hippocampal area (Fig. 1a,c).
Fig. 1a–d.
Expression of γH2AX in astrocytes in Alzheimer disease (AD). Virtually no nuclear H2AX is detected in the CA4 region of the hippocampus in control cases (a), even though a large number of astrocytes are present, as seen in an adjacent serial section immunostained with anti-GFAP (b). However, specific nuclear immunoreactivity is seen in many astroglial cells in the hippocampus of a 91-year-old AD case (c), while the number of GFAP-positive astrocytes in an adjacent serial section (d) is similar to that in the control case. Individual cells are visualized by counterstaining with hematoxylin. The pyramidal neurons remain unlabeled for H2AX (arrows in a and c). Asterisks Landmark vessels. Bar 100 μm
Double-immunostaining of γH2AX with GFAP, AT8, and TAL1B5
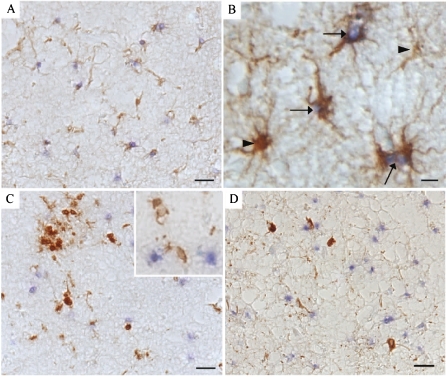
To confirm the specific cell types containing increased γH2AX, we performed double immunocytochemistry with specific cell markers. All the immunopositive nuclei were proven to be those of astrocytes following double immunostaining for γH2AX and GFAP, a specific marker for astrocytes (Fig. 2a,b). All cases, AD and control, displayed many GFAP-positive astrocytes, yet only AD cases, and a minority of controls, revealed concomitant γH2AX. In fact, in many of the AD cases, most of the GFAP-positive astrocytes also contained γH2AX. In contrast, double immunostaining of γH2AX with a microglia marker, TAL1B5 (class II MHC, an activated microglia indicator), showed no colocalization of γH2AX-positive nuclei with microglia (Fig. 2c). Likewise, neurofibrillary-tangle-bearing neurons (identified with AT8 antibody to phosphorylated tau) also never contained γH2AX (Fig. 2d). These data suggest that the expression of γH2AX is limited to astrocytes in AD.
Fig. 2a–d.
In an 82-year-old AD case, double-labeling immunocytochemistry confirms the localization of H2AX in astrocytes. a, b H2AX-positive nuclei (blue, arrows) display complete overlap with astrocytes detected with antisera to GFAP (brown). Only a small percentage of astrocytes do not display H2AX (b, arrowhead). c (and inset) Microglia are stained brown and never contain H2AX positive nuclei (blue). d Neurofibrillary tangles, stained brown, also do not contain H2AX (blue). Barsa, c, d 50 μm; b 10 μm
Discussion
In this study, we found evidence for unique and selective DNA damage in the astrocytes of AD brains, as evidenced by the astrocytic nuclear accumulation of γH2AX.
DNA damage to astrocytes has been reported in a variety of neuropathological conditions, including Pick’s disease (Gleckman et al. 1999), FTD (Su et al. 2000), amyotrophic lateral sclerosis (ALS) (Migheli et al. 1997) as well as AD (Lucassen et al. 1997; Petito and Roberts 1995). In these latter studies, DNA damage was found in the reactive (Parkinson’s disease, FTD, ALS) or non-reactive (ALS) astrocytes and had the features of fragmentation or degeneration, which seemed to appear prior to or unrelated to apoptotic cell death.
The role of degenerated astrocytes in the pathogenesis of AD is not clear although several lines of evidence indicate that astrogliosis in chronic neurodegenerative disorders (AD, Parkinson’s disease, ALS, and Huntington’s disease) may play a secondary compensatory or protective role. Ablation of normal proliferating astrocytes exacerbates loss of neurons and tissue after brain or spinal cord injury (Bush et al. 1999; Faulkner et al. 2004; Myer et al. 2006). In addition, large numbers of proliferating astrocytes in the cortex after mild contusion injury are associated with a very normal appearing cortex, in which there is very little loss of neurons or tissue in mice. Therefore, DNA damage in astrocytes might affect the vitality of astrocytes or directly alter gene expression essential to the supportive functions of astrocytes.
Our results show that γH2AX-immunopositive nuclei were significantly increased in the astrocytes of AD in comparison to those of control cases. Also, γH2AX was selectively found in the astrocytic nuclei and not in the nuclei of vulnerable neurons or microglia, as demonstrated using double immunocytochemistry for GFAP or phosphorylated tau. Our results suggest that AD patients have selectively increased DNA damage in astrocytes, whereas no significant DNA damage is found in the astrocytes of age-matched controls. Interestingly, γH2AX expression was consistently found in the hippocampal areas, a region known to be vulnerable in AD, thus suggesting a correlation with the clinical symptoms of AD.
In proliferating cells, DNA damage may be repairable, or non-repairable resulting in apoptosis. Without a DNA repair system, cells could not live because DNA damage occurs constantly: about 10,000 DNA damaging events every day in each cell. DNA damage, if not repaired, interferes with DNA replication and transcription. The DNA damage response is a complex event comprised of many different enzymes and factors, including γH2AX, which works as an early marker for DNA double-strand breaks to activate other factors and enzymes responsible for DNA repair, apoptotic signaling, or cell cycle arrest. Of note, in AD, the activation of a number of cell cycle molecules in neurons followed by arrest is common (Bowser and Smith 2002; Harris et al. 2000; McShea et al. 1997, 1999; Ogawa et al. 2003a; Zhu et al. 2004). Presumably, if the DNA damage is too severe to be repaired, a cell activates the apoptotic program, a part of the DNA damage response that serves to eliminate such cells. Once a DNA double-strand break is repaired, γH2AX becomes dephosphorylated and is no longer recognizable by the γH2AX antibody used here (Bartek and Lukas 2007; Kruman 2004; Rios-Doria et al. 2006; Tanaka et al. 2007).
Technically, it is difficult to determine both apoptosis and DNA damage in chronic diseases such as AD compared to acute damage such as ischemia (Perry et al. 1998; Raina et al. 2001; Zhu et al. 2006). In proliferating cells, the repair machinery is much more powerful than in post-mitotic cells. For example, the same concentrations of DNA damaging agents that kill neurons do not kill astrocytes, i.e., the threshold is higher in proliferating cells. Double-strand breaks are the most dangerous type of DNA damage, and the occurrence of this type of damage is much lower than 8-oxoguanine modification or single-strand breaks; 8-oxoguanine in neurons is associated with hereditary chronic disorders in animal models (Bogdanov et al. 2000, 2001; Kruman et al. 2002). While cell culture models provide clear evidence that cells will either undergo apoptosis if they cannot repair DNA after DNA damage or display dephosphorylated γH2AX following repair, the actual situation in vivo, especially in a chronic disease condition, is likely different than in a cell culture model system. If cells are under acute cytotoxic stress causing severe DNA damage in vivo, such as under ischemic conditions, γH2AX would be increased and the cells would progress down apoptotic pathways resulting in massive cell death. If under chronic, subcytotoxic, or intermittent cytotoxic stress, cells may keep repairing the damage until the damage level becomes intolerable; in this scenario, γH2AX would continuously disappear and reappear in such cells. Since the massive level of acute cell death as shown in either ischemia models or in cell culture models is not evident in AD, the latter mechanism more reasonably explains the pathogenesis of AD, where minimal levels of apoptosis in neurons and astrocytes are observed (Perry et al. 1998; Raina et al. 2001; Zhu et al. 2006). Astrocytes and neurons likely have different endurable levels of DNA damage and would express γH2AX at different levels.
While the exact effect of the astrocytic DNA damage on the functions and vitality of astrocytes is unclear, the presence of DNA damage alone may not signify apoptosis but more likely identifies a subset of vulnerable astrocytes in which mechanisms of both cell death and repair have been activated (Gleckman et al. 1999).
In conclusion, our results demonstrate that AD brains contain DNA damage, as evidenced by the selective presence of γH2AX in astrocytes, and that the damaged astrocytes were diffusely distributed in the cortex, white matter and hippocampal region. These data suggest that astrocytic DNA damage might be involved in the pathogenesis of AD in a significant way such as to compromise the neuronal support functions of astrocytes.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health, the Alzheimer’s Association, and by Philip Morris USA Inc. and Philip Morris International.
Contributor Information
Mark A. Smith, Phone: +1-216-3683670, FAX: +1-216-3688964, Email: mark.smith@case.edu
Hyoung-gon Lee, Phone: +1-216-3686708, FAX: +1-216-3688964, Email: hyoung-gon.lee@case.edu.
References
- Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19:238–245 [DOI] [PubMed]
- Bogdanov M, Brown RH, Matson W, Smart R, Hayden D, O’Donnell H, Flint Beal M, Cudkowicz M (2000) Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med 29:652–658 [DOI] [PubMed]
- Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF (2001) Increased oxidative damage to DNA in a transgenic mouse model of Huntington’s disease. J Neurochem 79:1246–1249 [DOI] [PubMed]
- Bowser R, Smith MA (2002) Cell cycle proteins in Alzheimer’s disease: plenty of wheels but no cycle. J Alzheimers Dis 4:249–254 [DOI] [PubMed]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276:42462–42467 [DOI] [PubMed]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308 [DOI] [PubMed]
- Cheung P, Allis CD, Sassone-Corsi P (2000) Signaling to chromatin through histone modifications. Cell 103:263–271 [DOI] [PubMed]
- Enomoto R, Tatsuoka H, Komai T, Sugahara C, Takemura K, Yamauchi A, Nishimura M, Naito S, Matsuda T, Lee E (2004) Involvement of histone phosphorylation in apoptosis of human astrocytes after exposure to saline solution. Neurochem Int 44:459–467 [DOI] [PubMed]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155 [DOI] [PMC free article] [PubMed]
- Gleckman AM, Jiang Z, Liu Y, Smith TW (1999) Neuronal and glial DNA fragmentation in Pick’s disease. Acta Neuropathol (Berl) 98:55–61 [DOI] [PubMed]
- Harris PL, Zhu X, Pamies C, Rottkamp CA, Ghanbari HA, McShea A, Feng Y, Ferris DK, Smith MA (2000) Neuronal polo-like kinase in Alzheimer disease indicates cell cycle changes. Neurobiol Aging 21:837–841 [DOI] [PubMed]
- Iida T, Furuta A, Nakabeppu Y, Iwaki T (2004) Defense mechanism to oxidative DNA damage in glial cells. Neuropathology 24:125–130 [DOI] [PubMed]
- Ikegami Y, Goodenough S, Inoue Y, Dodd PR, Wilce PA, Matsumoto I (2003) Increased TUNEL positive cells in human alcoholic brains. Neurosci Lett 349:201–205 [DOI] [PubMed]
- Khachaturian ZS (1985) Diagnosis of Alzheimer’s disease. Arch Neurol 42:1097–1105 [DOI] [PubMed]
- Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y (2002) Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathol Appl Neurobiol 28:238–251 [DOI] [PubMed]
- Kruman II (2004) Why do neurons enter the cell cycle? Cell Cycle 3:769–773 [PubMed]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP (2002) Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci 22:1752–1762 [DOI] [PMC free article] [PubMed]
- Lucassen PJ, Chung WC, Kamphorst W, Swaab DF (1997) DNA damage distribution in the human brain as shown by in situ end labeling; area-specific differences in aging and Alzheimer disease in the absence of apoptotic morphology. J Neuropathol Exp Neurol 56:887–900 [DOI] [PubMed]
- Martin JA, Craft DK, Su JH, Kim RC, Cotman CW (2001) Astrocytes degenerate in frontotemporal dementia: possible relation to hypoperfusion. Neurobiol Aging 22:195–207 [DOI] [PubMed]
- McShea A, Harris PL, Webster KR, Wahl AF, Smith MA (1997) Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am J Pathol 150:1933–1939 [PMC free article] [PubMed]
- McShea A, Zelasko DA, Gerst JL, Smith MA (1999) Signal transduction abnormalities in Alzheimer’s disease: evidence of a pathogenic stimuli. Brain Res 815:237–242 [DOI] [PubMed]
- Migheli A, Piva R, Atzori C, Troost D, Schiffer D (1997) c-Jun, JNK/SAPK kinases and transcription factor NF-kappa B are selectively activated in astrocytes, but not motor neurons, in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 56:1314–1322 [DOI] [PubMed]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486 [DOI] [PubMed]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV (2006) Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761–2772 [DOI] [PubMed]
- Ogawa O, Lee HG, Zhu X, Raina A, Harris PL, Castellani RJ, Perry G, Smith MA (2003a) Increased p27, an essential component of cell cycle control, in Alzheimer’s disease. Aging Cell 2:105–110 [DOI] [PubMed]
- Ogawa O, Zhu X, Lee HG, Raina A, Obrenovich ME, Bowser R, Ghanbari HA, Castellani RJ, Perry G, Smith MA (2003b) Ectopic localization of phosphorylated histone H3 in Alzheimer’s disease: a mitotic catastrophe? Acta Neuropathol 105:524–528 [DOI] [PubMed]
- Perry G, Nunomura A, Lucassen P, Lassmann H, Smith MA (1998) Apoptosis and Alzheimer’s disease. Science 282:1268–1269 [DOI] [PubMed]
- Petito CK, Roberts B (1995) Effect of postmortem interval on in situ end-labeling of DNA oligonucleosomes. J Neuropathol Exp Neurol 54:761–765 [DOI] [PubMed]
- Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, Siedlak SL, Boux H, Castellani RJ, Perry G, Smith MA (2001) Abortive apoptosis in Alzheimer’s disease. Acta Neuropathol 101:305–310 [DOI] [PubMed]
- Rios-Doria J, Fay A, Velkova A, Monteiro AN (2006) DNA damage response: determining the fate of phosphorylated histone H2AX. Cancer Biol Ther 5:142–144 [DOI] [PubMed]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868 [DOI] [PubMed]
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM (2000) Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 275:9390–9395 [DOI] [PubMed]
- Sedelnikova OA, Pilch DR, Redon C, Bonner WM (2003) Histone H2AX in DNA damage and repair. Cancer Biol Ther 2:233–235 [DOI] [PubMed]
- Sternberger LA (1986) Immunocytochemistry, edn 3. Wiley, New York
- Su JH, Nichol KE, Sitch T, Sheu P, Chubb C, Miller BL, Tomaselli KJ, Kim RC, Cotman CW (2000) DNA damage and activated caspase-3 expression in neurons and astrocytes: evidence for apoptosis in frontotemporal dementia. Exp Neurol 163:9–19 [DOI] [PubMed]
- Tanaka T, Huang X, Halicka HD, Zhao H, Traganos F, Albino AP, Dai W, Darzynkiewicz Z (2007) Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A 71:648–661 [DOI] [PMC free article] [PubMed]
- Zhu X, McShea A, Harris PL, Raina AK, Castellani RJ, Funk JO, Shah S, Atwood C, Bowen R, Bowser R, Morelli L, Perry G, Smith MA (2004) Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer’s disease. J Neurosci Res 75:698–703 [DOI] [PubMed]
- Zhu X, Raina AK, Perry G, Smith MA (2006) Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res 3:393–396 [DOI] [PubMed]