Abstract
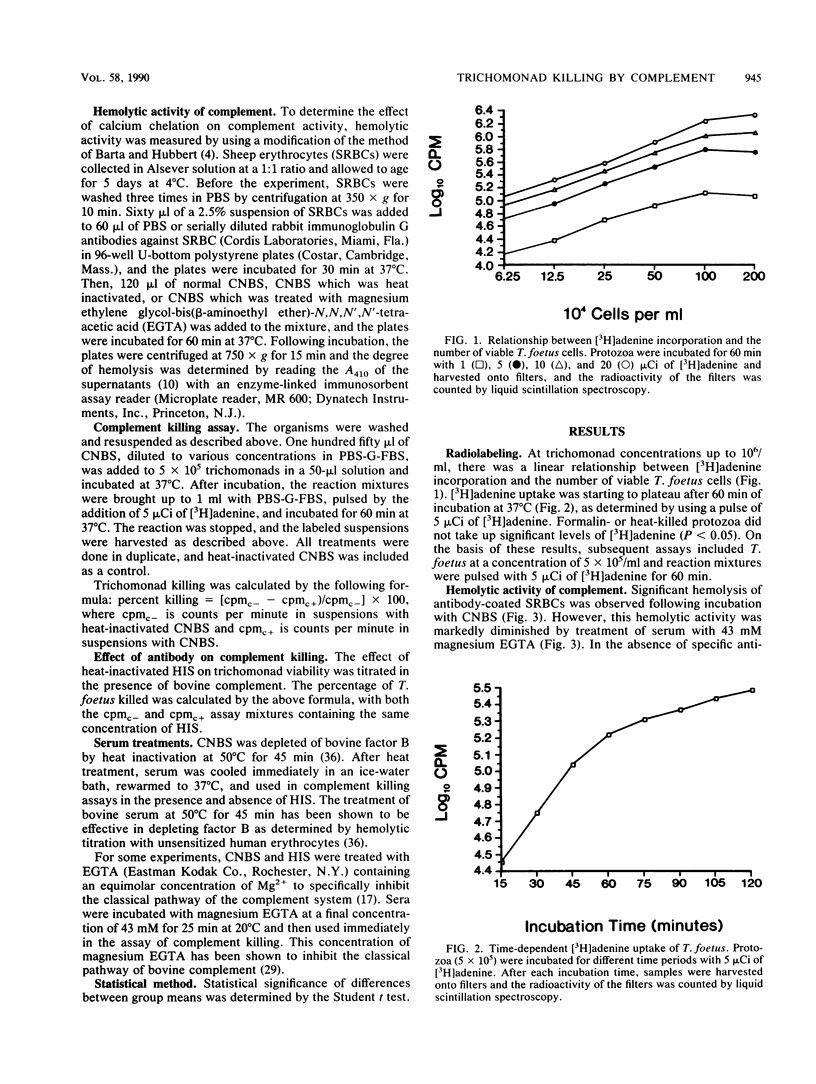
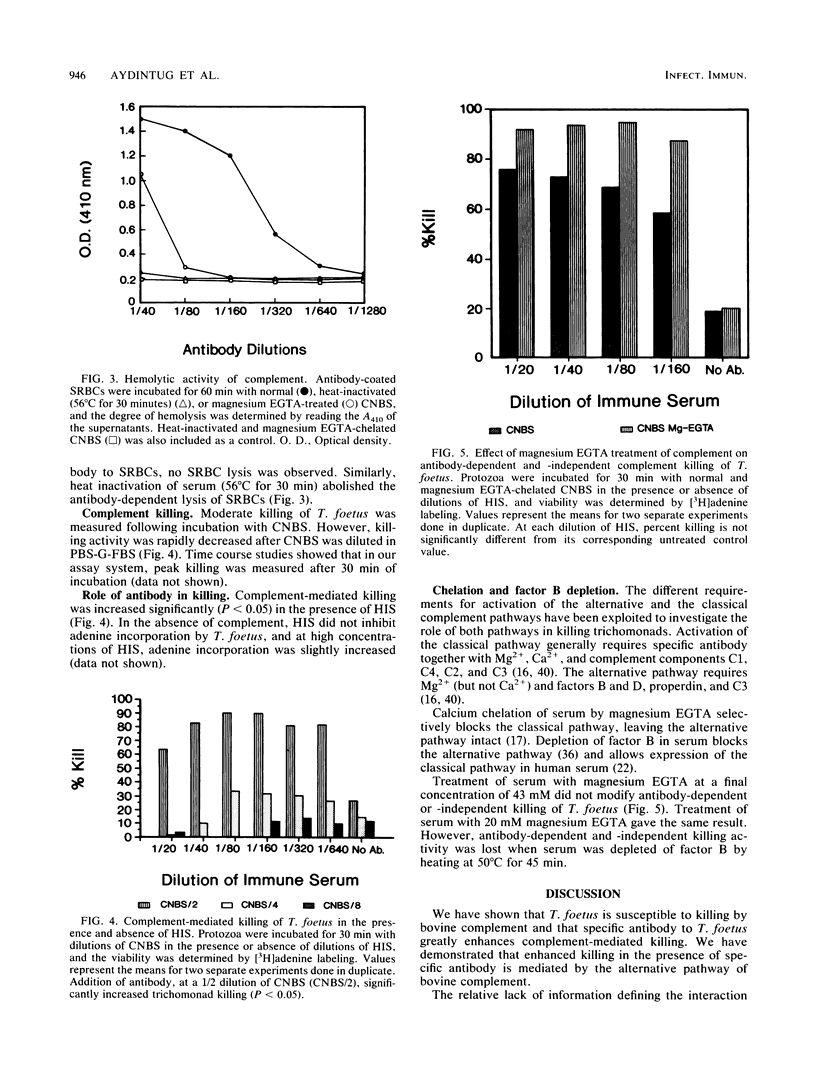
The role of bovine antibody and complement in host defense against Tritrichomonas foetus was measured by using an assay of trichomonad viability based on protozoal uptake of tritiated adenine. Moderate killing was measured in the absence of antibody only with high concentrations of complement-preserved hypogammaglobulinemic bovine serum. However, very low concentrations of hyperimmune serum promoted significant enhancement (P less than 0.05) of killing by complement. Heat inactivation of complement (56 degrees C for 30 min) eliminated antibody-dependent and -independent killing. Similarly, depletion of bovine factor B in serum by heat treatment (50 degrees C for 45 min) abolished antibody-dependent and -independent killing. However, selective inactivation of the classical complement pathway with magnesium ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid did not affect antibody-dependent or -independent killing by complement. These findings demonstrate antibody enhancement of complement-mediated killing of T. foetus by the alternative pathway of bovine complement.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbitt B., Meyerholz G. W. Trichomonas fetus infection of range bulls in South Florida. Vet Med Small Anim Clin. 1979 Sep;74(9):1339–1342. [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M. J., Aikawa M., Nussenzweig R. S. Monoclonal antibodies to Trypanosoma cruzi inhibit motility and nucleic acid synthesis of culture forms. Infect Immun. 1983 Jan;39(1):377–382. doi: 10.1128/iai.39.1.377-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta O., Hubbert N. L. Testing of hemolytic complement components in domestic animals. Am J Vet Res. 1978 Aug;39(8):1303–1308. [PubMed] [Google Scholar]
- Burgess D. E. Tritrichomonas foetus: preparation of monoclonal antibodies with effector function. Exp Parasitol. 1986 Oct;62(2):266–274. doi: 10.1016/0014-4894(86)90031-7. [DOI] [PubMed] [Google Scholar]
- Clark B. L., Dufty J. H., Parsonson I. M. The effect of Tritrichomonas foetus infection on calving rates in beef cattle. Aust Vet J. 1983 Mar;60(3):71–74. doi: 10.1111/j.1751-0813.1983.tb05873.x. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Mayer P., Baggiolini M. Stimulation of phagocytosis in bone marrow-derived mouse macrophages by bacterial lipopolysaccharide: correlation with biochemical and functional parameters. J Immunol. 1984 Aug;133(2):913–922. [PubMed] [Google Scholar]
- Corbeil L. B., Hodgson J. L., Jones D. W., Corbeil R. R., Widders P. R., Stephens L. R. Adherence of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect Immun. 1989 Jul;57(7):2158–2165. doi: 10.1128/iai.57.7.2158-2165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Dennett D. P., Reece R. L., Barasa J. O., Johnson R. H. Observations on the incidence and distribution of serotypes of Tritrichomonas foetus in beef cattle in north-eastern Australia. Aust Vet J. 1974 Oct;50(10):427–431. doi: 10.1111/j.1751-0813.1974.tb06863.x. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Activation of the alternative complement pathway. CRC Crit Rev Immunol. 1979 Nov;1(1):1–32. [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Frank M. M., Joiner K., Hammer C. The function of antibody and complement in the lysis of bacteria. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S537–S545. doi: 10.1093/clinids/9.supplement_5.s537. [DOI] [PubMed] [Google Scholar]
- Fuhrman S. A., Joiner K. A. Toxoplasma gondii: mechanism of resistance to complement-mediated killing. J Immunol. 1989 Feb 1;142(3):940–947. [PubMed] [Google Scholar]
- Gillin F. D., Sher A. Activation of the alternative complement pathway by Trichomonas vaginalis. Infect Immun. 1981 Oct;34(1):268–273. doi: 10.1128/iai.34.1.268-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodger W. J., Skirrow S. Z. Epidemiologic and economic analyses of an unusually long epizootic of trichomoniasis in a large California dairy herd. J Am Vet Med Assoc. 1986 Oct 1;189(7):772–776. [PubMed] [Google Scholar]
- Hicks J. T., Klutch M. J., Albrecht P., Frank M. M. Analysis of complement-dependent antibody-mediated lysis of target cells acutely infected with measles. J Immunol. 1976 Jul;117(1):208–215. [PubMed] [Google Scholar]
- Hill A. W., Shears A. L., Hibbitt K. G. The requirement of specific antibody for the killing of E. coli by the alternate complement pathway in bovine serum. Immunology. 1978 Jan;34(1):131–136. [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R. C., Hammer C. H., Leive L., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. VI. IgG increases the bactericidal efficiency of C5b-9 for E. coli 0111B4 by acting at a step before C5 cleavage. J Immunol. 1983 Nov;131(5):2570–2575. [PubMed] [Google Scholar]
- LEON M. A. Role of cations in conglutination and in formation of properdinzymosan complex from bovine serum. Proc Soc Exp Biol Med. 1957 Oct;96(1):202–204. doi: 10.3181/00379727-96-23432. [DOI] [PubMed] [Google Scholar]
- MacDonald J. T., Maheswaran S. K., Opuda-Asibo J., Townsend E. L., Thies E. S. Susceptibility of Pasteurella haemolytica to the bactericidal effects of serum, nasal secretions and bronchoalveolar washings from cattle. Vet Microbiol. 1983 Nov;8(6):585–599. doi: 10.1016/0378-1135(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Meingassner J. G., Heyworth P. G., Havelec L. Measurement of adenine uptake as an in vitro screen for antitrichomonal agents. Chemotherapy. 1985;31(2):146–150. doi: 10.1159/000238327. [DOI] [PubMed] [Google Scholar]
- Moav N., Draghi E., David A., Gold D. Anti-Trichomonas vaginalis monoclonal antibodies inducing complement-dependent cytotoxicity. Immunology. 1988 Jan;63(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Moore F. D., Jr, Fearon D. T., Austen K. F. IgG on mouse erythrocytes augments activation of the human alternative complement pathway by enhancing deposition of C3b. J Immunol. 1981 May;126(5):1805–1809. [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol. 1986 Apr 1;136(7):2628–2632. [PubMed] [Google Scholar]
- Nelson B., Ruddy S. Enhancing role of IgG in lysis of rabbit erythrocytes by the alternative pathway of human complement. J Immunol. 1979 May;122(5):1994–1999. [PubMed] [Google Scholar]
- Nicholson-Weller A., Daha M. R., Austen K. F. Different functions for specific guinea pig IgG1 and IgG2 in the lysis of sheep erythrocytes by C4-deficient guinea pig serum. J Immunol. 1981 May;126(5):1800–1804. [PubMed] [Google Scholar]
- Pang A. S., Aston W. P. Alternative complement pathway in bovine serum: lysis of human erythrocytes. Am J Vet Res. 1977 Mar;38(3):355–359. [PubMed] [Google Scholar]
- Parsonson I. M., Clark B. L., Dufty J. H. Early pathogenesis and pathology of Tritrichomonas foetus infection in virgin heifers. J Comp Pathol. 1976 Jan;86(1):59–66. doi: 10.1016/0021-9975(76)90028-1. [DOI] [PubMed] [Google Scholar]
- Rein M. F., Sullivan J. A., Mandell G. L. Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J Infect Dis. 1980 Oct;142(4):575–585. doi: 10.1093/infdis/142.4.575. [DOI] [PubMed] [Google Scholar]
- Sandberg A. L., Osler A. G. Dual pathways of complement interaction with guinea pig immunoglobulins. J Immunol. 1971 Nov;107(5):1268–1273. [PubMed] [Google Scholar]
- Schenkein H. A., Ruddy S. The role of immunoglobulins in alternative complement pathway activation by zymosan. I. Human IgG with specificity for Zymosan enhances alternative pathway activation by zymosan. J Immunol. 1981 Jan;126(1):7–10. [PubMed] [Google Scholar]
- Sissons J. G., Cooper N. R., Oldstone M. B. Alternative complement pathway-mediated lysis of measles virus infected cells: induction by IgG antibody bound to individual viral glycoproteins and comparative efficacy of F(ab')2 and Fab' fragments. J Immunol. 1979 Nov;123(5):2144–2149. [PubMed] [Google Scholar]
- Steele N. P., Munson R. S., Jr, Granoff D. M., Cummins J. E., Levine R. P. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984 May;44(2):452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



