Abstract
Motor function declines with increasing adult age. Proper regulation of the balance between dopamine (DA) and acetylcholine (ACh) in the striatum has been shown to be fundamentally important for motor control. Although other factors can also contribute to this age-associated decline, a decrease in the concentration and binding potential of the DA D2 receptor subtype in the striatum, especially in the cholinergic interneurons, are involved in the mechanism. Our studies have shown that gene transfer of the DA D2 receptor subtype with adenoiviral vectors is effective in ameliorating age-associated functional decline of the striatal cholinergic interneurons. These achievements confirm that an age-associated decrease of D2R contributes functional alteration of the interaction of DA and ACh in the striatum and demonstrate that these age-associated changes indeed are modifiable.
Keywords: Dopamine receptor D2, Viral vector, Acetylcholine, Interneuron
Introduction
Motor function declines with increasing adult age (Potvin et al. 1980; Kolb et al. 1998). The mechanisms of this age-associated decline of motor function are complex and multifactorial. They include changes in musculoskeletal architecture (Lexell 1997; Dutta et al. 1997) and both peripheral and central nerve conduction (Dorfman and Bosley 1979; Delbono 2003).
The nigrostriatal system has also been implicated in the decline of motor function in senescence. Pharmacological interference of dopamine (DA) synthesis (Rech et al. 1966), blockade of receptor binding of DA (Carp and Anderson 1979), or destruction of dopaminergic neurons (Kozlowski and Marshall 1981) have been demonstrated to induce motor functional impairment similar to that observed in aged animals. Age-related structural alterations in the nigrostriatum system with aging have also been established. Although the issue of an age-related decrease in the number of nigral cells remains controversial (Stark and Pakkenberg 2004), age-related decline in striatal DA levels (Carlsson and Winblad 1976) and DA receptors, specifically D2R subtype (Morgan and Finch 1988; Joseph et al. 1990), have been repeatedly reported. Moreover, almost 30 years ago, Marshall and Berrios demonstrated that DA injections improved swimming performance in aged rats (Marshall and Berrios 1979), and pharmacological interventions to increase the DA receptor density have been shown to enhance motor function in senescent rats (Joseph and Roth 1988a). Taken altogether, the alteration of dopaminergic systems may contribute the age-associated motor functional decline.
Structure of the striatum
The striatum is one of the main structures of the basal ganglia, which receives various inputs from several brain regions, including the cortex, substantia nigra, and thalamus. Principal projecting neurons in the striatum are medium-sized cells (10–20 µm diameter) with spiny dendrites [medium spiny (MS) cells]. MS cells occupy 95% of the striatal neuronal population. The other neuronal population consists of interneurons, and several types of interneurons have been identified. There are three types of GABAergic interneurons in the striatum, distinguished by neurochemical characteristics, and cholinergic interneurons also have been identified (Tepper and Bolam 2004).
Cholinergic interneurons are the largest aspiny neurons (20–50 µm diameter, 300–2,000 µm2), accounting for less than 2% of total neuronal population in the striatum (Pisani et al. 2000, 2001). The axons of interneurons extend well into the striatum and also possess widespread dendritic trees (Graybiel 1990). In terms of localization, these cells form pairs with MS cells and provide modulatory interactions on output signals from the striatum (Mensah 1980; Pickel and Chan 1991). Based on these features, these interneurons appear able to integrate synaptic inputs and project over large regions within the striatum.
Compared with other brain regions, the striatum possesses one of the highest contents of ACh despite the relatively small occupancy of the cholinergic interneurons in striatal neuronal populations. Several studies demonstrated that cholinergic interneurons receive dopaminergic inputs from the substantia nigra and glutamatergic inputs from the neocortex (Dimova et al. 1993; Chang 1988; Kubota et al. 1987; Bolam and Bennett 1995; Lapper and Bolam 1992). However, not all the reports have confirmed dopaminergic terminals at synapses of cholinergic interneurons (Pickel and Chan 1990). Because tyrosine hydroxylase (TH) and choline acetyl transferase (ChAT) immunoreactive terminals are closely positioned, possibly to allow simple diffusion of neurotransmitters as they are released, the possibility that DA is regulating the activity of cholinergic cells in a non-synaptic way also exists (Stoof et al. 1992) as well as in a synaptic way. Reciprocally, ACh also transmits the inputs to DA neurons by both synaptic and non-synaptic mechanisms (Contant et al. 1996).
Functional interactions between dopaminergic and cholinergic neurons in the striatum
Proper regulation of the balance between DA and ACh in the striatum has been known to be fundamentally important for motor control (Di Chiara et al. 1994; Calabresi et al. 2000). The apomorphine-induced rotational model has been well established to evaluate asymmetrical D2R distributions in the striata, typically following a unilateral injection of 6-hydroxydopamine (6OHDA) into the substantia nigra. (Pycock 1980). The unilateral dopaminergic innervation to the striatum is markedly reduced by the toxic effects of 6OHDA, and upregulation of striatal D2R in the lesioned side is observed. With this alteration in the nigrostriatal system, following a systemic injection of the DA agonist, apomorphine, 6OHDA-lesioned rats display a stereotypic rotational behavior in the direction contralateral to the lesioned side where the D2R upregulation occurs. Kaneko and co-workers demonstrated that the neurochemical ablation of unilateral cholinergic neurons in the striatum could induce ipsilateral rotational behavior following systemic apomorphine (DA agonist) injection, suggesting cholinergic dysfunction could also change the dopaminergic neuronal functions (Kaneko et al. 2000). These experiments clearly demonstrated that ACh-DA interaction was regulated to coordinate straital synaptic integration.
Regarding clinical issues, patients with Parkinson’s disease, who have reduced DA binding to the striatal receptors because of dopaminergic neuronal loss in the substantia nigra, show impaired extrapyramidal motor function (Fahn 2003). Some of the symptoms of this disease can also be alleviated by inhibiting ACh activities (Berg et al. 1987). These findings also suggest that ACh-DA interactions in the striatum are important in regulating motor function.
Yan et al. (1997) reported that all the cholinergic interneurons possess the DA D2 subtype of receptor, and 90% of the cells coexpressed D1 type. Dopaminergic regulation of straital ACh is reciprocal, i.e., DA D2 receptor is inhibitory, while the D1 receptor is stimulatory (DeBoer et al. 1996; Di Chiara et al. 1994). Activation of D2R in cholinergic interneurons reduces the N-type calcium currents through the protein kinase C pathway to modulate the excitability of the cell and reduce ACh release (Yan et al. 1997; Pisani et al. 2000; Maurice et al. 2004; Stoof et al. 1992)
Age-assocaited changes of DA and ACh interactions in the striatum
An age-associated decrease in the concentration or binding potential of D2R in the striatum has been documented in a variety of species ranging from rodents to humans. The hypothesized mechanisms of these decrements are both molecular and cellular (Roth and Joseph 1994). At the molecular level, the biosynthesis of the D2R has been shown to be reduced in the aged striatum (Henry and Roth 1984; Henry et al. 1987; Norman et al. 1987), and the concentration of mRNA for this receptor also decreases with advancing age (Della Vedova et al 1992; Weiss et al. 1992). At the cellular level, approximately 20% of the striatal neurons are lost (Han et al. 1989). In effect, in the mammalian brain there appears to be about a 30–50% loss of D2R in the aged striata.
No direct evidence has indicated the age-associated decrease of D2R on the interneuron. However, Zhang and co-workers evaluated D2R mRNA levels in the striatum neurons according to cellular size. They concluded that the significant decrease in D2R mRNA appeared in positive large neurons (over 210 µm2), which were probably were interneurons (Zhang et al. 1995).
Age-associated changes in the striatal cholinergic system have also been reported. The baseline release of ACh in the striatum was decreased in aged rats compared with young rats (Wu et al. 1988; Wang et al. 2007). A decline of cholinergic activity in the striatum was suggested by several studies (Sherman and Friedman 1990; Ogawa et al. 1994; Zambrzycka et al. 2002). The activity of cholinesterase, which degrades ACh, was also decreased by age (Das et al. 2001). Using a striatal slice preparation, Joseph and Roth (1988b) reported reduced inhibition of ACh release by apomorphine in aged rats compared with young and middle-aged rats.
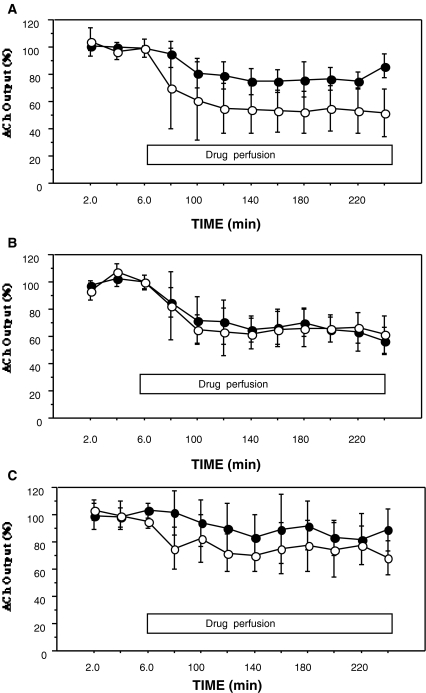
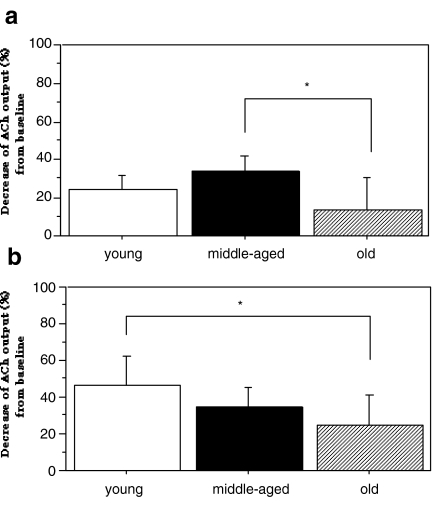
We also investigated the age-asociated changes of DA and Ach interactions in the striatum by in-vivo microdialysis. The effects of different concentrations of a D2R agonist, quinpirole, on the striatal ACh release in three groups of rats at different ages (6-, 15-, 25-months old) were examined. The infusion of quinpirole into the striatum decreased ACh levels in all three age group as shown in Fig. 1. Significant differences between the effects of the two doses of quinpirole were found in the young and old rats (P < 0.05) (Fig. 1a,c), but not in the middle-aged rats (Fig. 1b). In the mean levels of ACh release (120–220 min) suppressed by quinpirole, there were significant differences between the middle-aged and old rats (P < 0.05) at the dose of 0.1 μM quinpirole (Fig. 2a), and between the young and old rats (P < 0.05) at the dose of 1 μM quinpirole (Fig. 2b). These data suggested that the striatal DA-ACh interaction was affected in aged animals (Kurotani et al. 2003). The reason why there were no dose-dependent effects in the middle-aged rats was unclear; however, we speculate that supersensitivity of D2R induced by a slight decrease of D2R numbers in middle-aged rats may exert an almost full response by a lower dose of D2R agonist.
Fig. 1.
Effects of quinpirole on striatal ACh release. Time courses of ACh release in young (a, n = 6), middle-aged (b, n = 7), and old (c, n = 7) rats are shown. The results are expressed as a percentage of the basal levels (calculated as the mean of the three samples before drug perfusion). Each point represents the mean ± SD. Quinpirole at the concentration of 0.1 μM (●) or 1 μM (○) was perfused through the microdialysis probe from 60 to 240 min after the experiment was begun. Repeated-measures ANOVA revealed a significant effect of dose in young (a) (P = 0.011), and in old rats (c) (P = 0.043), but no significant difference (P = 0.71) in middle-aged rats (b)
Fig. 2.
Attenuation of striatal D2R-mediated ACh output with age. Percent decrease of ACh output from the baseline is presented as the mean ± SD. In 0.1 μM quinpirole (a), statistical significance was revealed between the middle-aged and old rats (P = 0.024). In 1 μM quinpirole (b), statistical significance was revealed between the young and old-aged rats (P = 0.046). *P < 0.05
After considering the results discussed above, we can offer the following hypothesis: the age-related decrease in D2R concentration could lead to a “cascade” of intrastriatal effects that initially change the reciprocal inhibitory control between ACh and DA, leading to diminutions in motor performance. We could propose that one way of testing this hypothesis would be to determine if restorations of age-associated decreased D2R signaling in the striatal interneurons could ameliorate the functional impairments of ACh-DA interactions.
Interventions to ameloriate the decline in age-associated DA-ACH interactions in the striatum
There are several ways of supplementing dopaminergic input in aged nigrostrial systems. One of these ways is by introducing intrastriatal nigral grafts. Such grafts have been shown to improve movement coordination in aged rats (Gage et al. 1983), which suggested that age-associated motor functional impairment was at least partly due to reduced strialtal dopminergic input, and the supplement of dopaminergic input could ameriolate these impairments. Another important suggestion which these graft studies gave us was that intrastriatal nigral grafts restored cholinergic interneuronal function via actions at DA receptors located on interneurons (Herman et al. 1988; Jackisch et al. 1991). The increased input of DA receptors on interneurons is possibly needed to restore the age-associated functional decline in DA-ACh interactions and could ameliorate motor function deficits (Puschban et al. 2000).
Another strategy to manipulate the D2R density in the striatum is gene transfer. This intervention would provide a more specific manipulation of DA function than offered by neural tissue grafting. To this end, we developed methods using adenoviral vectors for intracerebral D2R gene transfer.
Vector construction
Details of the vector construction are documented elsewhere (Ikari et al. 1995). In brief, the vector was constructed by deletion of the majority of E1 and a portion of E3 region, and insertion of an expression cassette containing the rat D2R cDNA along with the cytomegalovirus immediate early promoter and enhancer (AdCMV.DopD2R).
In-vitro expression
By using HeLa cell lines, which normally do not express D2R, the viability of the viral vector, AdCMV.DopD2R was confirmed in vitro. In membrane preparations extracted from HeLa cells infected with AdCMV.DopD2R, specific binding of [3H] spiperone, a D2R ligand, was very evident showing the protein expression of D2R in cellular membrane along with abundant mRNA production.
In-vivo expression and functional assessment
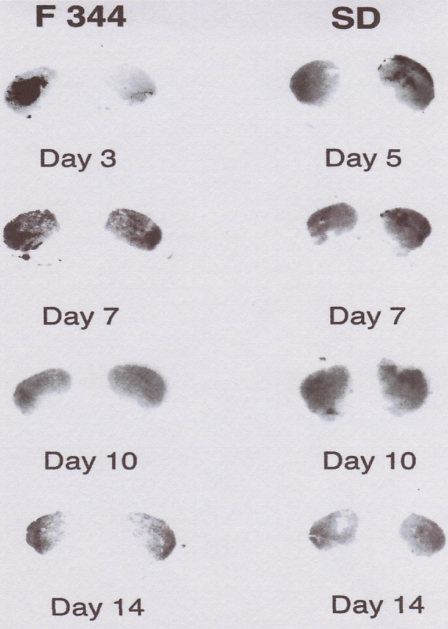
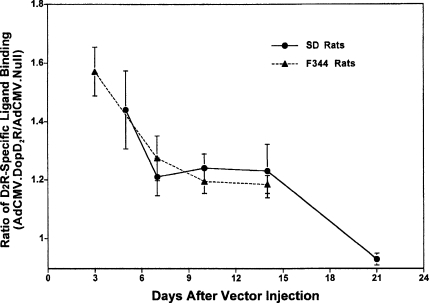
Autoradiographic studies demonstrated that this vector system accomplished similar D2R overexpression in the brains of Sprague-Dawley and Fischer-344 rats (Fig. 3) (Umegaki et al. 1997; Ingram et al. 1998). However, the overexpression of D2R was transient. The maximum expression was observed as early as 2 or 3 days after vector injection, and expression had declined to almost baseline levels by 3 weeks post-injection (Fig. 4).
Fig. 3.
Increased expression of D2R in rat striatum. Representative autoradiographic images of [125I]iodosulpride binding in striatal slices are shown for Sprague-Dawley (SD) and Fischer-344 (F344) rats. The striatal slices (10-μm thickness) were incubated with 0.125 nM [125I]iodosulpride for 30 min. Note the greater binding (darker images) near sites of AdCMV.DopD2R striatal injection (left side for F344 rats; right side for SD rats on this figure) compared with contralateral AdCMV.Null striatal injection
Fig. 4.
Time-course of increased D2R expression. At each time-point, the ratio (AdCMV.DopD2R-injected striatum/AdCMV.Null-injected striatum) of the mean density (± SEM) of grains in the autoradiographic images using [125I]iodosulpride as a ligand is shown. Two representative slices, which exhibited the highest levels of expression, were taken from four rats of each strain for each time-point
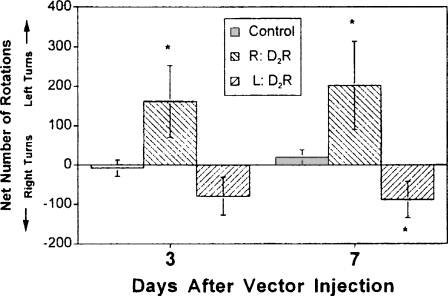
To determine if the expressed D2R by AdCMV.DopD2R was functional, we selected to use the apomorphine-induced rotational model described earlier (Pycock 1980). The unilateral striatal injection of the adenoviral vectors would be designed to induce upregulation of D2R similar to what occurs in the injected side to 6OHDA lesioned rats. We would then expect to observe rotational behavior toward the ipsolateral side of the injection. By using this model, we demonstrated that striatal D2R induced by the gene transfer with the AdCMV.DopD2R was functional (Fig. 5) despite the non-selective character of the gene transfer; i.e., many different types of neurons and glia appeared to be expressing D2R following the vector treatment.
Fig. 5.
Apomorphine-induced rotational behavior reflects the functionality of increased D2R expression. Represented are the group means (± SEM) for the net number of rotations (number of turns ipsilateral to the AdCMV.DopD2R-injected striatum subtracted from the number of turns contralateral to the AdCMV.Dop D2R-injected striatum) induced by apomorphine (1 mg/ kg). Experimental groups (n = 10) received unilateral striatal injection of AdCMV.Dop D2R and AdCMV.Null contralateral striatum. R: D2R refers to AdCMV.Dop D2R injected into right striatum and AdCMV.Null injected into left striatum, L: D2R refers to AdCMV.Dop D2R injected into left striatum and AdCMV.Null injected into right striatum, Control refers to AdCMV.Null injected bilaterally (n = 11). * P < 0.05 from control
Expression in aged rats
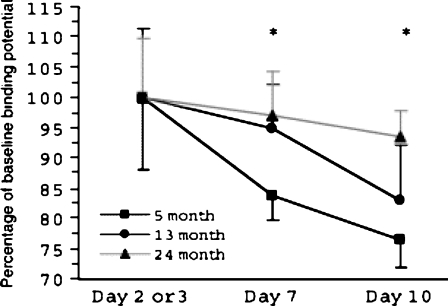
By taking advantage of PET technology designed for small animals (Ogawa et al. 2000; Umegaki et al. 2002), we showed that AdCMV.DopD2R-mediated gene transfer of D2R was feasible in aged rats (Figs. 6, 7). We also found that the overexpression period of D2R induced by gene transfer was longer in aged rats than in young rats (Fig. 8) (Umegaki et al. 2003).
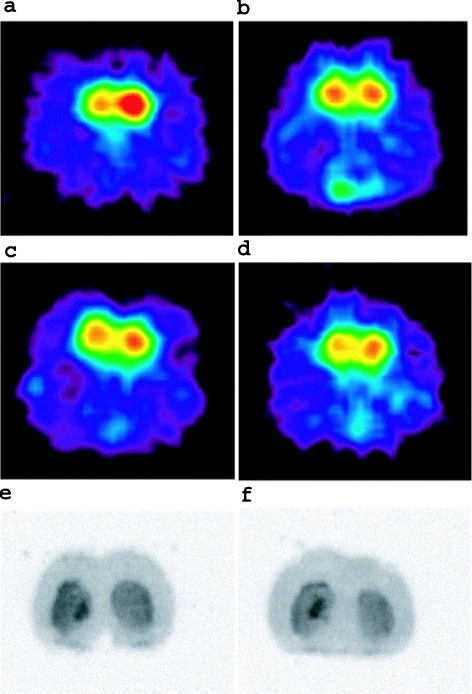
Fig. 6.
Brain PET image of the adenoviral vectors-injected rat brain scanned with [11C]raclopride by coronal section at bregma (a–d). The images of a, b and c, d are taken from the same individuals, respectively. Right striatum was injected with AdCMV.DopD2R. a Young rat at day 3 after vector injection. b Young rat at day 10 after vector injection. c Old rat at day 3 after vector injection. d Old rat at day 10 after vector injection. The PET images were acquired for 20 min, starting 20 min after the tracer injection. e, f Ex-vivo autoradiography images of the adenoviral vectors-injected rat brain with [11C]raclopride at day 3 after vector injection. The AP coordinate was –0.5 mm from the bregma. e Young rat; f old rat
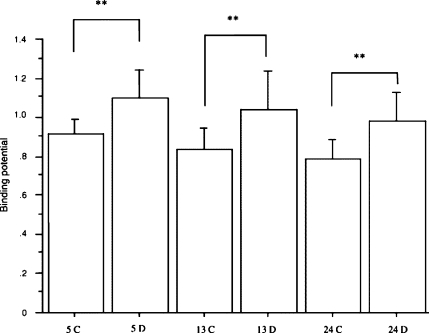
Fig. 7.
Binding potential for [11C]raclopride in both Ad.CMV.DopD2R- and Ad.CMV.LacZ-injected striata of the three age groups (5, 13, 24 months) of rats 2 or 3 days after the vector injection (5C, 13C, and 24C stand for the Ad.CMV.LacZ-injected striata in 5-, 13-, and 24-month-old rats, respectively, and 5D, 13D, and 24D stand for the Ad.CMV.DopD2R-injected striata in 5-, 13-, and 24-month-old rats, respectively). All data were expressed mean ± SD (n = 12–25). The paired t-test between Ad.CMV.DopD2R-injected side and Ad.CMV.LacZ-injected side in each age group showed significant increase of binding potential in Ad.CMV.DopD2R -injected side (P < 0.01 in each age group). **P < 0.01
Fig. 8.
Time-course of binding potential from day 2 or 3 to day 10 after vector injection in three age groups. Each rat of the three groups underwent three PET scans: n = 8 for 5-months old, n = 5 for 13-months old, and n = 9 for 24-months old. Data at day 7 and day 10 were expressed as percentages of the AdCMV.DopD2R-injected side to AdCMV.LacZ-injected side ratio of binding potential at day 2 or day 3; i.e., the largest AdCMV.DopD2R-injected side to AdCMV.LacZ-injected side ratio observed at day 2 or day 3 in each group was normalized as 100. Data are expressed as mean ± SD. Repeated ANOVA showed significant difference among three groups (P = 0.0112), and Scheffe’s post-hoc analysis showed the significant difference between the 5- and 24-month-old groups at days 7 and day 10 (P = 0.0007, and <0.0001, respectively)
Application of gene transfer to age-associated alterations in DA-ACh interactions
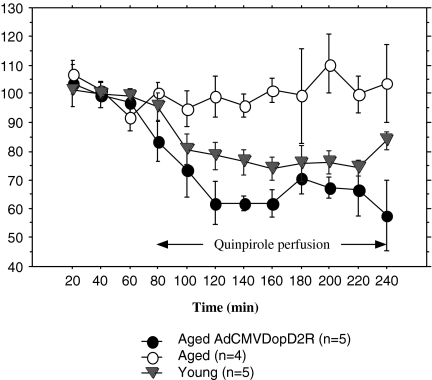
To test the hypothesis that D2R augmentation by adenoviral vector-mediated gene transfer could ameliorate the control of the ACh release by DA, we performed microdialysis studies of vector-injected rats (Umegaki et al. 2006). Quinpirole, a specific D2R agonist (Sigma, St. Louis, Mo., USA) was infused at a concentration of 0.1 μM through the microdialysis probe and ACh levels were then analyzed using a high-performance liquid chromatographic assay. Figure 9 shows the striatal release of ACh when 0.1 μM quinpirole was infused in control-vector-injected or AdCMV.DopD2R-injected old rats. The old rats exhibited significantly less ACh decrease than the young controls (P = 0.014). The AdCMV.DopD2R injection significantly restored the release of ACh of old rats (P = 0.006). We documented a significant difference among the three groups (P < 0.001) and also showed statistically significant differences between AdCMV.DopD2R and AdCMV.LacZ-injected old rats (P = 0.006), and between AdCMV.LacZ-injected young and old rats (P = 0.014).
Fig. 9.
Time-courses of ACh release in AdCMV.DopD2R old (●) and AdCMV.LacZ injected old (○) and young (▾) rats are shown. The results are expressed as a percentage of the basal levels (calculated as the mean of the three samples before drug perfusion). Each point represents the mean ± SD. Quinpirole at the concentration of 0.1 μM was perfused through the microdialysis probe from 60 to 240 min after the experiment was begun. Repeated-measures ANOVA revealed a significant difference among three groups (P < 0.001), and Scheffe’s post-hoc analysis showed a statistically significant difference between AdCMV.DopD2R and AdCMV.LacZ-injected old rats (P = 0.006), and between AdCMV.LacZ-injected young and old rats (P = 0.014)
The response of cholinergic neurons in aged striatum was compatible with the results of our previous study when 0.1 μM of quinpirole was infused (Kurotani et al. 2003). The D2R gene-transferred rats showed significantly stronger responses of striatal cholinergic neurons to the D2R agonist infusion compared with control-vector-treated rats.
The successful restoration of age-associated functional decline of DA-ACh interactions in the striatum stimulated several suggestions. First, the age-associated decrease of D2R appears at least partly involved in the mechanism affected by the age-associated deterioration of ACh control of dopaminergic input. Second, age-associated functional decline in the brain could be improved by several modalities including gene transfer. Third, the prevention of age-associated functional decline would be the best strategy; however, it also appeared that inverventions were possible after age-associated functional decline manifested. The challenge now is find interventions that can act in a long-term and safe fashion to maintain striatal function.
Summary
With age both functional and anatomical alterations occur in the nigrostriatum system. Of these alterations, decline of D2R numbers in the striatum is one of the most established features of mammalian brain aging. Functional interactions of DA and ACh in the striatum are also changed; specifically, inhibition of ACh release by D2R stimulation is reduced in aged striatum, possibly due to age-associated decrease of D2R in the cholinergic interneurons. By taking advantage of gene transfer technology, we demonstrated that D2R augmentation ameliorated the control of the ACh release by DA. Our studies suggested that age-associated decrease of D2R contributes to functional alteration of the interaction of DA and ACh in the striatum and these age-associated changes indeed are modifiable. Future studies are warranted to see if the functional restoration of the nigrostriatum DA system could lead to motor functional improvement in aged animals and by extension in aged humans.
References
- Berg MJ, Ebert B, Willis DK, Host T, Fincham RW, Schottelius DD (1987) Parkinsonism-drug treatment. Part I. Drug Intell Clin Pharm 21:10–21 [DOI] [PubMed]
- Bolam JP, Bennett BD (1995) Microcircuitry of the neostriatum. In: Ariano MA, Surmeier DJ (eds) Molecular and cellular mechanisms of neostriatal function. Landes, Austin
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G (2000) Acetylcholine-mediated modulation of striatal function. Trends Neurosci 23:120–126 [DOI] [PubMed]
- Carlsson A, Winblad B (1976) Influence of age and time interval between death and autopsy on dopamine and 3-methoxytyramine levels in human basal ganglia. J Neural Transm 38:271–276 [DOI] [PubMed]
- Carp JS, Anderson RJ (1979) Sensorimotor deficits produced by phenytoin and chlorpromazine in unanesthetized cats. Pharmacol Biochem Behav 10:513–520 [DOI] [PubMed]
- Chang HT (1988) Dopamine-acetylcholine interaction in the rat striatum: a dual-labeling immunocytochemical study. Brain Res Bull 21:295–230 [DOI] [PubMed]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L (1996) Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience 71:937–944 [DOI] [PubMed]
- Das A, Dikshit M, Nath C (2001) Profile of acetylcholinesterase in brain areas of male and female rats of adult and old age. Life Sci 68:1545–1555 [DOI] [PubMed]
- DeBoer P, Heeringa MJ, Abercrombie ED (1996) Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol 317:257–262 [DOI] [PubMed]
- Delbono O (2003) Neural control of aging skeletal muscle. Aging Cell 2:21–29 [DOI] [PubMed]
- Della Vedova F, Fumagalli F, Sacchetti G, Racagni G, Brunello N (1992) Age-related variations in relative abundance of alternative spliced D2 receptor mRNAs in brain areas of two rat strains. Brain Res Mol Brain Res 12:357–359 [DOI] [PubMed]
- Di Chiara G, Morelli M, Consolo S (1994) Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci 17:228–233 [DOI] [PubMed]
- Dimova R, Vuillet J, Nieoullon A, Kerkerian-Le Goff L (1993) Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience 53:1059–1071 [DOI] [PubMed]
- Dorfman LJ, Bosley TM (1979) Age-related changes in peripheral and central nerve conduction in man. Neurology 29:38–44 [DOI] [PubMed]
- Dutta C, Hadley EC, Lexell J (1997) Sarcopenia and physical performance in old age: overview. Muscle Nerve 5:S5–S9 [DOI] [PubMed]
- Fahn S (2003) Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci 991:1–14 [DOI] [PubMed]
- Gage NH, Dunnett SB, Stenevi U, Bjorklund A (1983) Aged rats: recovery of motor impairments by intrastriatal nigral grafts. Science 221:966–969 [DOI] [PubMed]
- Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13:244–254 [DOI] [PubMed]
- Han Z, Kuyatt BL, Kochman KA, DeSouza EB, Roth GS (1989) Effect of aging on concentrations of D2-receptor-containing neurons in the rat striatum. Brain Res 498:299–307 [DOI] [PubMed]
- Henry JM, Roth GS (1984) Effect of aging on recovery of striatal dopamine receptors following N-ethoxycarbonyl-2-ethoxy-1, 2-dihydroquino- line (EEDQ) blockade. Life Sci 35:899–904 [DOI] [PubMed]
- Henry JM, Joseph JA, Kochman K, Roth GS (1987) Effect of aging on striatal dopamine receptor subtype recovery following N-ethoxycarbonyl -2-ethoxy-1,2-dihydroquinoline blockade and relation to motor function in Wistar rats. Brain Res 418:334–342 [DOI] [PubMed]
- Herman JP, Lupp A, Abrous N, Le Moal M, Hertting G, Jackisch R (1988) Intrastriatal dopaminergic grafts restore inhibitory control over striatal cholinergic neurons. Exp Brain Res 73:236–248 [DOI] [PubMed]
- Ikari H, Zhang L, Chernak JM, Mastrangeli A, Kato S, Kuo H, Crystal RG, Ingram DK, Roth GS (1995) Adenovirus-mediated gene transfer of dopamine D2 receptor cDNA into rat striatum. Brain Res Mol Brain Res 34:315–320 [DOI] [PubMed]
- Ingram DK, Ikari H, Umegaki H, Chernak JM, Roth GS (1998) Application of gene therapy to treat age-related loss of dopamine D2 receptor. Exp Gerontol 33:793–804 [DOI] [PubMed]
- Jackisch R, Duschek M, Neufang B, Rensing H, Hertting G, Herman JP (1991) Long-term survival of intrastriatal dopaminergic grafts: modulation of acetylcholine release by graft-derived dopamine. J Neurochem 57:267–276 [DOI] [PubMed]
- Joseph JA, Roth GS (1988a) Upregulation of striatal dopamine receptors and improvement of motor performance in senescence. Ann N Y Acad Sci 515:355–356 [DOI] [PubMed]
- Joseph JA, Roth GS (1988b) Altered striatal dopaminergic and cholinergic reciprocal inhibitory control and motor behavioral decrements in senescence. Ann N Y Acad Sci 521:110–122 [DOI] [PubMed]
- Joseph JA, Roth GS, Strong R (1990) The striatum, a microcosm for the examination of age-related alterations in the CNS. Review of biological research in aging. Liss, New York
- Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S (2000) Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science 289:633–637 [DOI] [PubMed]
- Kolb B, Forgie M, Gibb R, Gorny G, Rowntree S (1998) Age, experience and the changing brain. Neurosci Biobehav Rev 22:143–159 [DOI] [PubMed]
- Kozlowski MR, Marshall JF (1981) Plasticity of neostriatal metabolic activity and behavioral recovery from nigrostriatal injury. Exp Neurol 74:318–323 [DOI] [PubMed]
- Kubota Y, Inagaki S, Shimada S, Kito S, Eckenstein F, Tohyama M (1987) Neostriatal cholinergic neurons receive direct synaptic inputs from dopaminergic axons. Brain Res 413:179–184 [DOI] [PubMed]
- Kurotani S, Umegaki H, Ishiwata K, Suzuki Y, Iguchi A (2003) The age-associated changes of dopamine-acetylcholine interaction in the striatum. Exp Gerontol 38:1009–1013 [DOI] [PubMed]
- Lapper SR, Bolam JP (1992) Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51:533–545 [DOI] [PubMed]
- Lexell J (1997) Evidence for nervous system degeneration with advancing age. J Nutr 127:1011S–1013S [DOI] [PubMed]
- Marshall JF, Berrios N (1979) Movement disorders of aged rats: reversal by dopamine receptor stimulation. Science 206:477–479 [DOI] [PubMed]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ (2004) D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci 24:10289–10301 [DOI] [PMC free article] [PubMed]
- Mensah PL (1980) Distribution of the largest neuron in mouse caudate-putamen nucleus: its position in large-cell–medium-cell clusters. Exp Brain Res 38:267–271 [DOI] [PubMed]
- Morgan DG, Finch CE (1988) Dopaminergic changes in the basal ganglia. A generalized phenomenon of aging in mammals. Ann N Y Acad Sci 515:145–160 [DOI] [PubMed]
- Norman AB, Battaglia G, Creese I (1987) Differential recovery rates of rat D2 dopamine receptors as a function of aging and chronic reserpine treatment following irreversible modification: a key to receptor regulatory mechanisms. J Neurosci 7:1484–1491 [DOI] [PMC free article] [PubMed]
- Ogawa N, Asanuma M, Kondo Y, Nishibayashi S, Mori A (1994) Reduced choline acetyltransferase activity and muscarinic M1 receptor levels in aged Fisher 344 rat brains did not parallel their respective mRNA levels. Brain Res 658:87–92 [DOI] [PubMed]
- Ogawa O, Umegaki H, Ishiwata K, Asai Y, Ikari H, Oda K, Toyama H, Ingram DK, Roth GS, Iguchi A, Senda M (2000) In vivo imaging of adenovirus-mediated over-expression of dopamine D2 receptors in rat striatum by positron emission tomography. Neuroreport 11:743–748 [DOI] [PubMed]
- Pickel VM, Chan J (1990) Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. J Neurosci Res 25:263–280 [DOI] [PubMed]
- Pickel VM, Chan J (1991) Plasmalemmal appositions between cholinergic and non-cholinergic neurons in rat caudate-putamen nuclei. Neuroscience 41:459–472 [DOI] [PubMed]
- Pisani A, Bonsi P, Centonze D, Calabresi P, Bernardi G (2000) Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J Neurosci 20:RC69 [DOI] [PMC free article] [PubMed]
- Pisani A, Bonsi P, Picconi B, Tolu M, Giacomini P, Scarnati E (2001) Role of tonically-active neurons in the control of striatal function: cellular mechanisms and behavioral correlates. Prog Neuropsychopharmacol Biol Psychiatry 25:211–213 [DOI] [PubMed]
- Potvin AR, Syndulko K, Tourtellotte WW, Lemmon JA, Potvin JH (1980) Human neurologic function and the aging process. J Am Geriatr Soc 28:1–9 [DOI] [PubMed]
- Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, Poewe W, Wenning GK (2000) Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience 95:377–388 [DOI] [PubMed]
- Pycock CJ (1980) Turning behaviour in animals. Neuroscience 5:461–514 [DOI] [PubMed]
- Rech RH, Borys HK, Moore KE (1966) Alterations in behavior and brain catecholamine levels in rats treated with alpha-methyltyrosine. J Pharmacol Exp Ther 153:412–499 [PubMed]
- Roth GS, Joseph JA (1994) Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann N Y Acad Sci 719:129–135 [DOI] [PubMed]
- Sherman KA, Friedman E (1990) Pre- and post-synaptic cholinergic dysfunction in aged rodent brain regions: new findings and an interpretative review. Int J Dev Neurosci 8:689–708 [DOI] [PubMed]
- Stoof JC, Drukarch B, de Boer P, Westerink BH, Groenewegen HJ (1992) Regulation of the activity of striatal cholinergic neurons by dopamine. Neuroscience 47:755–770 [DOI] [PubMed]
- Stark AK, Pakkenberg B (2004) Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res 318:81–92 [DOI] [PubMed]
- Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–689 [DOI] [PubMed]
- Umegaki H, Chernak JM, Ikari H, Roth GS, Ingram DK (1997) Rotational behavior produced by adenovirus-mediated gene transfer of dopamine D2 receptor into rat striatum. Neuroreport 8:3553–3558 [DOI] [PubMed]
- Umegaki H, Ishiwata K, Ogawa O, Ingram DK, Roth GS, Yoshimura J, Oda K, Matsui-Hirai H, Ikari H, Iguchi A, Senda M (2002) In vivo assessment of adenoviral vector-mediated gene expression of dopamine D(2) receptors in the rat striatum by positron emission tomography. Synapse 43:195–200 [DOI] [PubMed]
- Umegaki H, Ishiwata K, Ogawa O, Ingram DK, Roth GS, Oda K, Kurotani S, Kawamura K, Wang WF, Ikari H, Senda M, Iguchi A (2003) Longitudinal follow-up study of adenoviral vector-mediated gene transfer of dopamine D2 receptors in the striatum in young, middle-aged, and aged rats: a positron emission tomography study. Neuroscience 121:479–486 [DOI] [PubMed]
- Umegaki H, Yamaguchi Y, Ishiwata K, Ingram DK, Roth GS, Iguchi A (2006) Functional recovery of the striatal cholinergic system in aged rats by adenoviral vector-mediated gene transfer of dopamine D2 receptor. Mech Ageing Dev 127:813–815 [DOI] [PubMed]
- Wang L, Albrecht MA, Wurtman RJ (2007) Dietary supplementation with uridine-5′-monophosphate (UMP), a membrane phosphatide precursor, increases acetylcholine level and release in striatum of aged rat. Brain Res 1133:42–48 [DOI] [PMC free article] [PubMed]
- Weiss B, Chen JF, Zhang S, Zhou LW (1992) Developmental and age-related changes in the D2 dopamine receptor mRNA subtypes in rat brain. Neurochem Int 20:49S–58S [DOI] [PubMed]
- Wu CF, Bertorelli R, Sacconi M, Pepeu G, Consolo S (1988) Decrease of brain acetylcholine release in aging freely-moving rats detected by microdialysis. Neurobiol Aging 9:357–361 [DOI] [PubMed]
- Yan Z, Song WJ, Surmeier J (1997) D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol 77:1003–1015 [DOI] [PubMed]
- Zambrzycka A, Alberghina M, Strosznajder JB (2002) Effects of aging and amyloid-beta peptides on choline acetyltransferase activity in rat brain. Neurochem Res 27:277–281 [DOI] [PubMed]
- Zhang L, Ravipati A, Joseph J, Roth GS (1995) Aging-related changes in rat striatal D2 receptor mRNA-containing neurons: a quantitative nonradioactive in situ hybridization study. J Neurosci 15:1735–1740 [DOI] [PMC free article] [PubMed]