Abstract
The high-growth (HG) phenotype in mice is characterized by a 30–50% postweaning overgrowth with a substantial increase in plasma insulin-like growth factor I (IGF1) levels, which is directly related to a deletion (hg) on chromosome 10 that includes the suppressor of cytokine signaling 2 (Socs2) gene. Reduced plasma IGF1 levels have been associated with extended lifespan in mice, although the aging-related effects of abnormally high IGF1 levels without elevated growth hormone levels have never been assessed in mammals. Within this context, the hg deletion was introgressed into C57BL/6J (B6) and FVB backgrounds, and a survival analysis was performed on the longevity records of 200 B6 (91 wild-type and 109 homozygous hg mutants) and 69 FVB (32 wild-type and 37 hg mutants) mice. Longevity was examined using a piecewise Weibull proportional hazards model solved through a Bayesian perspective and Markov chain Monte Carlo sampling. Lifespan was significantly reduced in both strains in homozygous hg mice, with a death risk between 3.689 (B6) and 4.347 (FVB) times higher than in wild-type mice (non-overlapped highest posterior density regions at 95%). These results highlight the effects of the Socs2 gene on aging regulation, likely related with variations described in plasma IGF1 levels. This result is consistent with previous research in dwarf mutant mice and other species, and characterizes the HG mutant mice as a unique and interesting animal model for accelerated aging research.
Keywords: Cytokine signaling 2, Growth hormone, Insulin-like growth factor I, Lifespan, Mice, Survival analysis
Introduction
The high-growth (HG) phenotype in mice is characterized by a 30–50% postweaning overgrowth without increasing adiposity (Bradford and Famula 1984; Corva and Medrano 2000) and is due to a 500 kb deletion on chromosome 10 that includes the suppressor of cytokine signaling 2 (Socs2) gene (Horvat and Medrano 2001; Wong et al. 2002). This mutation deregulates the growth hormone (GH)/Insulin-like growth factor I (IGF1) system (Medrano et al. 1991), a biological pathway shown to be related to lifespan in mice (Coschigano et al. 2000; Bartke 2005; de Magalhães et al. 2005). Although GH/IGF1 overexpression has been related with shortened lifespan in mice (Cecim et al. 1994), the increase of plasma IGF1 with reduced plasma and pituitary GH levels of the HG mice (Medrano et al. 1991) highlights HG mice as a unique and relevant animal model for lifespan analyses. However, the effect of the HG mutation on lifespan has not yet been evaluated.
Materials and methods
The HG mutation was introgressed into C57BL/6J (B6+/+) and FVB (FVB+/+) genetic backgrounds by nine backcrosses to create the congenic strains B6hg/hg and FVBhg/hg, respectively. For this study, 91 B6+/+ (12 week weight: males, 38.1 ± 0.8 g; females, 34.6 ± 1.1 g; P < 0.10), 109 B6hg/hg (males, 48.8 ± 0.8 g; females, 46.7 ± 0.8 g; P > 0.10), 32 FVB+/+ (males, 46.0 ± 1.9 g; females, 39.0 ± 1.7 g; P < 0.05) and 37 FVBhg/hg (males, 59.5 ± 4.3 g; females, 60.8 ± 1.6 g; P > 0.10) mice were randomly picked after the breeding period (ages between 100 and 300 days) and kept under standard management and specific pathogen-free conditions to register their lifespan (Table 1). Although death causes were not determined for all mice, a histopathological study was done in a random sample of 16 B6+/+ and B6hg/hg 2.5-year-old males. There were no differences between genotypes in histopathology (results not shown) or tumor incidence (40% and 43%, respectively; P > 0.10). A survival analysis was performed with a piecewise Weibull proportional hazards model (Casellas 2007), where the effect of the mouse strain (B6 and FVB), genotype (+/+ and hg/hg), sex/parturitions (male, female with zero, one or more than one parturitions) and their interactions were evaluated. The proportional hazard hypothesis was checked with the log test of Kalbfleisch and Prentice (1980). After comparing alternative models by the deviance information criterion (Spiegelhalter et al. 2002), the operational model included sex/parturitions (SPi) effect and strain × genotype interaction (Sj × Gk) as follows, 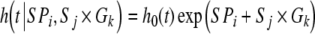 , where
, where  was the hazard function at time t and h0(t) was the piecewise Weibull baseline hazard function (Cox 1972). The Bayesian analysis was performed by launching a single Monte Carlo Markov chain with 100,000 elements, after discarding the first 25,000 as burn-in (Raftery and Lewis 1992).
was the hazard function at time t and h0(t) was the piecewise Weibull baseline hazard function (Cox 1972). The Bayesian analysis was performed by launching a single Monte Carlo Markov chain with 100,000 elements, after discarding the first 25,000 as burn-in (Raftery and Lewis 1992).
Table 1.
Descriptive statistics of the lifespan data set
| n | Censoring (%) | Complete records | ||
|---|---|---|---|---|
| Mean ± SE | Maxa | |||
| Genotype | ||||
| +/+ | 123 | 45.53 | 693.54 ± 27.31 | 1,103 |
| hg/hg | 146 | 10.27 | 448.31 ± 18.39 | 953 |
| Strain | ||||
| B6 | 200 | 30.50 | 556.62 ± 21.05 | 1,103 |
| FVB | 59 | 16.95 | 471.63 ± 24.48 | 894 |
| Sex/parturitions | ||||
| Male | 163 | 28.22 | 564.67 ± 25.09 | 1,103 |
| Female, 0 parturitions | 65 | 12.31 | 470.42 ± 42.92 | 894 |
| Female, 1 parturition | 23 | 56.52 | 494.50 ± 290.25 | 822 |
| Female, >1 parturitions | 18 | 22.22 | 526.50 ± 194.59 | 783 |
aMaximum lifespan (days)
Results and discussion
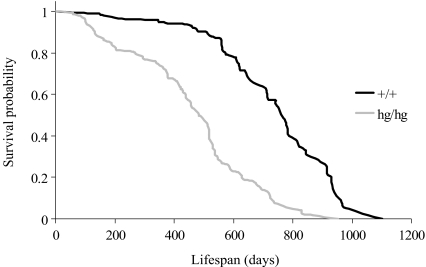
Non-parametric Kaplan-Meier survival curves (Kaplan and Meier 1958) between wild-type and HG mice, and between B6 and FVB strains showed significant differences (P < 0.001 and P < 0.01, respectively) when compared by log-rank test (Fig. 1), whereas there were no significant differences (P > 0.05) when comparisons were made across sex/parturitions levels (see below for details about this effect). As is suggested in Fig. 1, mutant mice began dying at a young age (e.g., first 10% of deaths occurred before 132 days and 360 days of age in B6hg/hg and B6+/+ mice, respectively, or before 105 days and 349 days of age in FVBhg/hg and FVB+/+ mice, respectively), and survival curves showed a similar trend, with survival differences persisting throughout the lifespan of wild type and mutant mice. Note that our dataset does not allow evaluation of mortality patterns at very early stages because mice were picked after the first breeding period.
Fig. 1.
Kaplan-Meier survival curves for wild-type (+/+) and high growth mutant mice (hg/hg) averaged across sexes and genetic backgrounds (FVB and B6)
The hazard ratio (HR) determines the quotient between the death probability intrinsic to two different levels of a given effect or interaction, and can be easily calculated as the exponential of the difference between the regression coefficient of both levels (Allison 1995). The death probability increased with adult mouse size as suggested by HR (Table 2). Death risk in hg/hg mice was 3.689 (B6) and 4.347 (FVB) times higher than in their +/+ counterparts (Fig. 1). Similarly, the FVB strain reached a heavier adult weight and exhibited a death risk between 1.74 (+/+) and 2.05 (hg/hg) times greater than B6 mice (Table 2). Note that differences between all Lj × Gk levels were statistically significant because all the highest posterior density regions at 95% did not overlap (Table 2). These HR implied large differences in predicted lifespan, as shown by modal estimates for males, where FVBhg/hg and B6hg/hg mice had a shorter lifespan (416.8 days and 525.3 days, respectively) in contrast to wild-type individuals (668.2 days and 804.9 days, respectively). Within this context, smaller adult body sizes appear to benefit lifespan (Roberts 1961; Eklund and Bradford 1977; Miller et al. 2002).
Table 2.
Regression coefficients and hazard ratios for strain × genotype and sex/parturitions effects on mice survival probability
| Parameter | Regression coefficientsf | Hazard ratiog | |
|---|---|---|---|
| Mode | Highest posterior density region at 95% | ||
| Strain × Genotype | |||
| B6 × +/+ | −2.023 a | −2.294 to −1.760 | 0.132 |
| B6 × hg/hg | −0.719 b | −0.963 to −0.485 | 0.487 |
| FVB × +/+ | −1.470 c | −1.700 to −1.247 | 0.230 |
| FVB × hg/hg | 0 d | 1.000 | |
| Sex/parturitions | |||
| Male | 0 a | 1.000 | |
| Female, 0 parturitions | 0.412 b | 0.174 to 0.648 | 1.510 |
| Female, 1 parturition | 0.326 b | 0.109 to 0.544 | 1.385 |
| Female, >1 parturitions | 0.158 a,b | −0.149 to 0.454 | 1.171 |
fWithin a Bayesian context, the posterior distribution of each regression coefficient (β) was characterized with its modal estimate and the higher posterior density region at 95%. Modal estimates followed by the same letter did not differ substantially (overlapped highest posterior density regions at 95%)
gThe hazard ratio is calculated as exp(β), and describes the ratio between the death probability of each level and the level of reference (“FVB × hg/hg” and “Female, >1 parturitions”, respectively)
SOCS2 is a protein closely related to growth in mammals through the GH/IGF1 axis, an important physiological mechanism modulating aging from yeast to humans (for a detailed review, see Barbieri et al. 2003). The SOCS2 protein interacts with IGF1 and GH receptors (Dey et al. 1998) as a negative regulator of GH signaling (Favre et al. 1999; Greenhalgh et al. 2005). The lack of SOCS2 expression in the HG mouse increases plasma IGF1, and reduces plasma and pituitary GH levels (Medrano et al. 1991). These higher IGF1 plasma levels, combined with the reduced lifespan of the hg/hg mouse, suggests a key role for IGF1 on mice aging as recently suggested by several authors (Coschigano et al. 2000; Flurkey et al. 2001; Holzenberger et al. 2003). Although the putative IGF1-related mechanism modulating lifespan remains unclear, it seems associated with insulin release and sensitivity (Barbieri et al. 2003), and low IGF1 levels probably involving a greater resistance to oxidative stress (Holzenberger et al. 2003). Nevertheless, additional SOCS2-related mechanisms could also modulate mice lifespan (Rico-Bautista et al. 2006).
As reported in previous studies (Yunis et al. 1984), males showed the greatest survivability, whereas females did not reveal significant differences (Table 2). As the number of parturitions increased, the survival probability of females had a suggested increase. The relation between aging and obesity is well established (Barzilai and Gupta 1999) and the differential fat deposition between male and females (Prasetyo and Elsen 1989) could account for survival differences shown in Table 2, although further studies are required to confirm this hypothesis.
Although there are some mutant mouse strains, such as the Snell and Ames Dwarf mice (Brown-Borg et al. 1996; Flurkey et al. 2001), with increased lifespan attributable to GH/IGF1 deficiency, the HG mutant is the only mouse model on the opposite end of the spectrum, where a deletion of the Socs2 gene that abnormally increases IGF1 without elevated GH levels increases adult body weight and significantly reduces lifespan. Controversy exists around mutations decreasing lifespan and their usefulness in aging research (Hasty and Vijg 2004; Miller 2004). The decreased lifespan of HG mice provides an interesting model of premature senescence given that the GH/IGF1 pathway has an established role in longevity determination and aging. Nevertheless, its potential contribution to the understanding of normal aging processes remains unclear. Accelerated aging models offer unique opportunities to study specific aging phenotypes and the development of interventions to postpone or prevent them (Hasty and Vijg 2004).
Acknowledgments
We are appreciative of the excellent efforts of Vince De Vera in mouse husbandry and with the phenotypic data collection. We thank Jim Carey and Roger McDonald for their useful suggestions related to this manuscript. This research was supported by the National Research Initiative grant number 2005-35205-15453 from the USDA Cooperative State Research, Education, and Extension Service and by the California Agricultural Experimental Station. The research contract of J. C. was partially financed by Spain’s Ministerio de Educación y Ciencia (Juan de la Cierva program).
References
- Allison PD (1995) Survival analysis using the SAS® system. A practical guide. SAS, Cary, NC
- Barbieri M, Bonafè M, Franceschi A, Paolisso G (2003) Insulin/IGF-I-signaling pathway: an evolutionary conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab 285:E1064–E1071 [DOI] [PubMed]
- Bartke A (2005) Minireview: role of the growth hormone/insuline-like growth factor system in mammalian aging. Endocrinology 146:3718–3723 [DOI] [PubMed]
- Barzilai N, Gupta G (1999) Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol Biol Sci 54A:B89–B96 [DOI] [PubMed]
- Bradford GE, Famula TR (1984) Evidence for a major gene for rapid postweaning growth in mice. Genet Res 44:293–308 [DOI] [PubMed]
- Brown-Borg HM, Borg KF, Meliska CJ, Bartke A (1996) Dwarf mice and the ageing process. Nature 384:33 [DOI] [PubMed]
- Casellas J (2007) Bayesian inference in a piecewise Weibull proportional hazards model with unknown change points. J Anim Breed Genet 124:176–184 [DOI] [PubMed]
- Cecim M, Bartke A, Yun JS, Wagner TE (1994) Expression of human, but not bovine, growth hormone genes promotes development of mammary tumors in transgenic mice. Transgenics 1:431–437
- Corva PM, Medrano JF (2000) Diet effects on weight gain and body composition in high growth (hg/hg) mice. Physiol Genomics 3:17–23 [DOI] [PubMed]
- Coschigano KT, Clemmons D, Bellushi LL, Kopchick JJ (2000) Assessment of growth parameters and life span of GHF/BP gene-disrupted mice. Endocrinology 141:2608–2613 [DOI] [PubMed]
- Cox DR (1972) Regression models and life tables (with discussion). J R Stat Soc B 34:187–220
- de Magalhães JP, Cabral JAS, Magalhães D (2005) The influence of genes on the aging process of mice: a statistical assessment of the genetics of aging. Genetics 169:265–274 [DOI] [PMC free article] [PubMed]
- Dey BR, Spence SL, Nissley P, Furlanetto RW (1998) Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insuline-like growth factor-I receptor. J Biol Chem 37:24095–24101 [DOI] [PubMed]
- Eklund J, Bradford GE (1977) Longevity and lifetime body weight in mice selected for rapid growth. Nature 265:48–49 [DOI] [PubMed]
- Favre H, Benhamou A, Finidori J, Kelly PA, Edery M (1999) Dual effects of suppressor of cytokine signaling (SOCS-2) on growth hormone signal transduction. FEBS Lett 453:63–66 [DOI] [PubMed]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE (2001) Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA 98:6736–6741 [DOI] [PMC free article] [PubMed]
- Greenhalgh CJ, Rico-Bautista E, Lorentzon M, Thaus AL, Morgan PO, Willson TA, Zervoudakis P, Metcalf D, Street I, Nicola NA, Nash AD, Fabri LJ, Norstedt G, Ohlsson C, Flores-Morales A, Alexander WS, Hilton DJ (2005) SOCS2 negatively regulates growth hormone action in vitro and in vivo. J Clin Invest 115:397–406 [DOI] [PMC free article] [PubMed]
- Hasty P, Vijg J (2004) Rebuttal to Miller: ‘Accelerated aging’: a primrose path to insight? Aging Cell 3:67–69 [DOI] [PubMed]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187 [DOI] [PubMed]
- Horvat S, Medrano JF (2001) Lack of Socs2 expression causes the high-growth phenotype in mice. Genomics 72:209–212 [DOI] [PubMed]
- Kalbfleisch JD, Prentice RL (1980) The statistical analysis of failure time data. Wiley, New York
- Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 [DOI]
- Medrano JF, Pomp D, Sharrow L, Bradford GE, Downs TR, Frohman LA (1991) Growth hormone and insulin-like growth factor-I measurements in high growth (hg) mice. Genet Res 58:67–74 [DOI] [PubMed]
- Miller RA (2004) ‘Accelerated aging’: a primrose path to insight? Aging Cell 3:47–51 [DOI] [PubMed]
- Miller RA, Harper JM, Galecki A, Burke DT (2002) Big mice die young: early body weight predicts longevity in genetially heterogeneous mice. Aging Cell 1:22–29 [DOI] [PubMed]
- Prasetyo H, Elsen EJ (1989) Correlated responses in development and distribution of fat depots in mice selected for body composition traits. Theor Appl Genet 78:217–223 [DOI] [PubMed]
- Raftery AE, Lewis SM (1992) How many iterations in the Gibbs sampler? In: Bernardo JM, Berger JO, David AP, Smith AFM (eds) Bayesian Statistics IV. Oxford University Press, Oxford, UK
- Rico-Bautista E, Flores-Morales A, Fernández-Pérez L (2006) Supresor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factors Rev 17:431–439 [DOI] [PubMed]
- Roberts RC (1961) The lifetime growth and reproduction of selected strains of mice. Heredity 16:369–381 [DOI]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc B 64:583–639 [DOI]
- Yunis EJ, Watson ALM, Gelman RS, Sylvia SJ, Bronson R, Dorf ME (1984) Traits that influence longevity in mice. Genetics 108:999–1011 [DOI] [PMC free article] [PubMed]
- Wong MJ, Islas-Trejo AD, Medrano JF (2002) Structural characterization of the mouse High Growth deletion and discovery of a novel fusion transcript between suppressor of cytokine signaling-2 (Socs-2) and viral encoded semaphoring receptor (Plexin C1). Gene 299:153–163 [DOI] [PubMed]