Abstract
Background
In diabetic adults, tight control of risk factors reduces complications.
Objective
To determine whether failure to make visits, monitor risk factors, or intensify therapy affects control of blood pressure, glucose, and lipids.
Design
A non-concurrent, prospective study of data from electronic files and standardized abstraction of hard-copy medical records for the period 1/1/1999–12/31/2001.
Participants
Three hundred eighty-three adults with diabetes managed in an academically affiliated managed care program.
Measurements
Main exposure variable: Intensification of therapy or failure to intensify, reckoned on a quarterly basis. Main outcome measure: Hemoglobin A1c (A1c), systolic blood pressure (SBP), and LDL-cholesterol at the end of the interval.
Results
In this visit-adherent cohort, control of glycemia and lipids showed improvement over 24 months, but many patients did not achieve targets. Only those with the worst blood pressure control (SBP ≥160 mmHg) showed any improvement over 2 years. Failure to intensify treatment in patients who kept visits was the single strongest predictor of sub-optimal control. Compared to their counterparts with no failures of intensification, patients with failures in ≥3 quarters showed markedly worse control of blood glucose (A1c 1.4% higher: 95% CI: 0.7, 2.1); hypertension (SBP 22.2 mmHg higher: 95% CI: 16.6, 27.9) and LDL cholesterol (LDL 43.7 mg/dl higher: 95% CI: 24.1, 63.3). These relationships were strong, graded, and independent of socio-demographic factors, baseline risk factor values, and co-morbidities.
Conclusions
Failure to intensify therapy leads to suboptimal control, even with adequate visits and monitoring. Interventions designed to promote appropriate intensification should enhance diabetes care in primary practice.
KEY WORDS: diabetes mellitus, treatment, outcomes, quality of care, cohort study
Diabetes is a leading cause of morbidity and mortality in the USA.1,2 Randomized controlled trials have demonstrated that tight control of risk factors3,4 and adherence to evidence-based Quality of Care (QOC) guidelines improves processes of care and patient outcomes.5,6 However, most adults with diabetes are still not adequately controlled.7,8 Few studies have examined both the processes and outcomes of care longitudinally in adults with diabetes, and most of those have typically collapsed 1 or more years of panel data for cross-sectional analysis.9
We therefore conducted a non-concurrent, prospective cohort study of 383 adults with type-2 diabetes in a managed care setting to determine the longitudinal relationship of process to outcomes of diabetes care.
METHODS
Objective
To test the hypothesis that (1) sub-optimal control of glucose, blood pressure, and lipids is common and (2) in a patient population that is adherent to visits and monitoring of risk factors, suboptimal control is largely explained by physician failure to intensify therapy.
Participants
The study population was a cohort of federal employees and their dependents with type-2 diabetes enrolled in an academically affiliated managed-care program. The eligible population was identified based on the Health Plan Employer Data Information Set (HEDIS) criteria for diabetes: (1) diagnosis codes (ICD9 codes 250.xx, 357.2, 362.0 or 366.41) and/or pharmacy prescription records for insulin or oral hypoglycemic agents; (2) 18–75 years old on 31 December 2000, with continuous enrollment from 1 January 2000–31 December 2000; and (3) seen for two or more primary care encounters, or one emergency room visit or hospital stay during 1 January 1999–31 December 2000. The total eligible population of 1,120 patients was then systematically sampled with an interval of 2 [1,120/433, i.e., (411 + 5%)]. While random sampling is ideal, systematic sampling is a valid approach when the target population is assembled and selected on an unrelated variable, like alphabetic surname.
Composition of Cohort
Standardized medical chart abstractions were completed for 407/411 patients (99%) based on American Diabetes Association (ADA) clinical practice guidelines.10 Subsequently, patients were excluded due to pregnancy (n = 1), dementia (n = 1), and name and medical records number mismatch (n = 1). Electronic pharmacy data were incomplete for 21 patients. Thus, our final study population was 383 adults (93% of 411).
Data Abstraction
The medical chart provided data on visit frequency (to family physicians and internists), laboratory results, blood pressures, weight, and (in a subset) height (Table 1). Enrollment data provided age, gender, and race. Administrative claims supplemented chart data on co-morbid conditions. Pharmacy records provided drug information and date of refills. These multiple sources of data were merged for the period 1 January 1999–31 December 2001. We generally considered the medical chart as the “gold standard” except for medication use, for which we relied on electronic pharmacy data, since it appeared more complete.
Table 1.
Number and % of Study Population with Values for A1c, SBP, and LDL for Each of the Eight Quarters
| A1c tests | SBP tests | LDL tests | ||||
|---|---|---|---|---|---|---|
| n | % | N | % | n | % | |
| Quarter 1 | 166 | 43.3% | 278 | 72.6% | 127 | 33.2% |
| Quarter 2 | 174 | 45.4% | 299 | 78.1% | 117 | 30.5% |
| Quarter 3 | 173 | 45.2% | 299 | 78.1% | 120 | 31.3% |
| Quarter 4 | 170 | 44.4% | 295 | 77.0% | 115 | 30.0% |
| Quarter 5 | 164 | 42.8% | 273 | 71.3% | 134 | 35.0% |
| Quarter 6 | 168 | 43.9% | 288 | 75.2% | 119 | 31.1% |
| Quarter 7 | 176 | 46.0% | 278 | 72.6% | 115 | 30.0% |
| Quarter 8 | 173 | 45.2% | 291 | 76.0% | 127 | 33.2% |
Exposure Assessment
In accord with the 1999 American Diabetes Association (ADA) guidelines, we considered patients to be in sub-optimal control if the risk factors were “actionable” (drug therapy recommended): Hemoglobin A1c >8%, systolic blood pressure (SBP) ≥140 mmHg, and low density lipoprotein (LDL) cholesterol ≥130 mg/dl. The 2007 ADA guidelines have not changed in this regard.
When there was repeated monitoring in the same quarter, we used the lowest reading in order to capture the short-term impact of therapy. This minimized regression to the mean and built in a conservative bias in favor of the physician. When there was no monitoring during a quarter, we carried forward results from the previous quarter, mimicking ADA recommendations for longer monitoring intervals when risk factors are controlled.
We based our classification scheme for the adequacy of care during the 24 months (or 8 quarters) under review (1 January 2000–31 December 2001, with 1999 as baseline) on the 1999 ADA Clinical Practice Guidelines, designating ≥4 quarters with any visits, as “adequate” visits, and ≥4 quarters with A1c and SBP data, respectively, and ≥2 quarters with LDL data as “adequate” monitoring, still consistent with 2007 ADA guidelines.
For each quarter, we determined that intensification of therapy occurred if the dose of one or more medications was increased (without a corresponding decline in dose of another medication) or if a new medication was started. This is the definition of intensification commonly accepted in the literature.11–13 We identified a “failure to intensify therapy” if during a quarter where risk factor control was deemed “actionable,” (A1c >8%, SBP ≥140 mmHg, or LDL ≥130 mg/dl), but no corresponding intensification occurred.
Outcome Assessment
Blood glucose control was indicated by A1c. Adults were classified as hypertensive if they were taking antihypertensive medications at any time during the study period and/or had visits in two consecutive quarters where the minimum SBP readings were ≥140 mmHg. They were classified as hyperlipidemic if they were taking anti-lipid medications and/or had a minimum LDL cholesterol ≥130 mg/dl at any time during the period under review. Twenty-three patients (6%) had no LDL monitoring in 1999–2001.
First, we assessed visit adherence effects on glycemic (A1c), blood pressure (SBP), and lipid (LDL) control among sub-optimally controlled patients on oral medications who had baseline and final risk factor values (n = 244, 337, and 226, respectively). Next, we assessed the impact of monitoring on risk factors by restricting the analysis to visit-adherent patients (n = 229, 314, and 226, respectively). Lastly, we assessed physician adherence with ADA treatment guidelines by restricting the analysis to visit and monitoring-adherent patients (n = 133, 305 and 183, respectively) (Table 2).
Table 2.
Populations for Visit, Monitoring, and Intensification Analyses
| A1c | SBP | LDL | ||
|---|---|---|---|---|
| Study population overall n | (a) | 383 | 383 | 383 |
| *Used insulin | (b) | 82 | NA | NA |
| No tests 1999–2001 | (c) | 4 | 0 | 23 |
| †No indication for treatment | (d) | 15 | 39 | 40 |
| No baseline and final value for analysis | (e) | 38 | 7 | 94 |
| N for visit analysis (a - [b + c + d + e]) = | (f) | 244 | 337 | 226 |
| ‡Visit quarters <4 | (g) | 15 | 23 | 0 |
| N for monitoring analysis (f-g) = | (h) | 229 | 314 | 226 |
| ‡Monitoring quarters <4 | (i) | 87 | 0 | 12 |
| No drug info for intensification analysis | (j) | 9 | 9 | 31 |
| N for intensification analysis (h-|i + j|) = | (k) | 133 | 305 | 183 |
*Patients using insulin were excluded from the analysis because pharmacy records do not indicate dosage of insulin, so intensification cannot be determined
†Patients without therapy and with all A1c ≤8%, SBP <140 mmHg, or all LDL <130 mg/dl, respectively
‡<2 visit quarters and <2 monitoring quarters for LDL: ADA guidelines recommend monitoring once yearly, so at least two visits and two monitorings should have been done over the 2-year study period
Statistical Analysis
Data from the 24-month period were collapsed into eight quarters of 3 months each, the recommended follow-up frequency for type-2 diabetes, to simplify the analysis, facilitate comparisons, and simulate clinical decision making in the context of visit-to-visit variability. Main outcomes were summarized as proportions or means with standard deviations. To assess potential confounding factors, simple linear regression was used to determine the association between outcome measures (final A1c, SBP, and LDL) and demographic and clinical exposures, including co-morbidities. We did not include clinic site in any of the models since this was not associated with any of the outcomes of interest (p > 0.05). Older age (≥65 years) had higher utilization and better A1c control, but was otherwise similar to the young population.14 We used multiple logistic regression to compare those who were sub-optimally controlled, but not appropriately intensified, with their counterparts who were controlled, after adjusting for age, race, sex, co-morbidity, and baseline A1c, SBP, and LDL, respectively. Including provider in the model did not markedly alter the point estimates or confidence intervals.
To test the hypothesis that there was no change in the control of risk factors (A1c, SBP, and LDL) over the 24-month period, we stratified the baseline values for each risk factor into clinically significant groups. For example, for systolic blood pressure, the strata were <130, 130–139, 140–159, and ≥160 mmHg. Then for each strata, we used generalized estimating equations (GEE)15 to assess whether the trajectory was significantly different from zero.
To determine if failure to intensify treatment predicted end-of-interval (December 2001) values of A1c, SBP, and LDL, we then created ordinal categories of treatment intensification and tested for graded responses. We used multiple linear regression models that included age, sex, race, co-morbidity, and baseline A1c, SBP, and LDL, respectively, as covariates. From claims data, we determined overall burden of co-morbidity, using ICD-9 codes and patient demographics to create resource utilization band (RUB) categories using a formula developed at Johns Hopkins University.16 The higher the RUB, the higher the patient comorbidity. Including provider in the model did not markedly alter the point estimates or confidence intervals. All tests of significance were two-tailed, with an alpha level of 0.05. All statistical analyses were performed using STATA, Intercooled Version 9.0 (Stata Corporation, College Station, TX, 2002).
Patient consent requirement was waived as part of the approvals granted by the Johns Hopkins University IRB, and the Johns Hopkins Community Physicians Research Review Board.
RESULTS
This cohort of 383 federal employees and their dependents with diabetes had a mean age of 63 years; 31% were African-American and 59% male. Mean first A1c was 7.4% (SD 1.6%, range 4.5–14.9%). Hypertension prevalence was 87% (n = 344), including 3% (n = 10) undiagnosed and untreated. Hyperlipidemia prevalence was 84% (n = 320), including 9% (n = 28) undiagnosed and untreated, but excluding 6% (n = 23) who had no LDL test in 1999, 2000, or 2001.
Frequency of Visits and Monitoring
Over 24 months, patients made an average of ten primary care visits (Table 3); women made more visits than men (11 vs. 9; p < 0.001). Ninety percent of the study population (n = 344) met minimal ADA guidelines for visit frequency (≥2 visits/year). Blood pressure monitoring occurred slightly more frequently for women (vs. men) and for blacks (vs. whites) (Table 3).
Table 3.
Selected Patient Characteristics, Visit, and Monitoring Adherence, and Results of A1c, SBP, and LDL Cholesterol in 383 Adults with Diabetes, by Sex and Race
| Males n = 225 | Females n = 158 | Black n = 120 | White n = 225 | Total n = 383 | |
|---|---|---|---|---|---|
| Age (years) | 63.0 (7.9) | 62.7 (8.0) | 64.0 (7.8) | 62.9 (7.8) | 62.8 (7.9) |
| BMI | 30.9 (5.3) | 32.5 (6.0) | 31.6 (5.3) | 32.0 (6.0) | 31.6 (5.6) |
| RUB† | |||||
| Low n (%) | 72 (32.0) | 44 (27.9) | 38 (31.7) | 63 (28.0) | 116 (30.3) |
| Medium n(%) | 77 (34.2) | 52 (32.9) | 40 (33.3) | 75 (33.3) | 129 (33.7) |
| High n (%) | 76 (33.8) | 62 (39.2) | 42 (35.0) | 87 (38.7) | 138 (36.0) |
| N visits | 9.1 (4.0) | 11.4 (5.5)* | 10.7 (5.3) | 9.9 (4.5) | 10.0 (4.8) |
| <2 quarters/year with visits(%) | 11.0 | 7.6 | 5.8 | 9.3 | 9.7 |
| N of quarters monitoring performed: (maximum = 8) | |||||
| A1c | 3.5 (1.7) | 3.7 (1.8) | 3.8 (1.6) | 3.4 (1.7) | 3.6 (1.7) |
| SBP | 5.8 (1.7) | 6.3 (1.7)* | 6.4 (1.6) | 5.9 (1.7)* | 6.0 (1.8) |
| LDL | 2.5 (1.7) | 2.6 (1.7) | 2.6 (1.6) | 2.5 (1.7) | 2.5 (1.7) |
| A1c (%) | |||||
| Baseline (a) | 7.4 (1.6) | 7.7 (1.7) | 8.0 (2.0) | 7.3 (1.4)* | 7.5 (1.7) |
| Last value in 2001 (b) | 7.1 (1.3) | 7.4 (1.5)* | 7.4 (1.5) | 7.1 (1.2) | 7.2 (1.4) |
| Change (b-a) | −0.23 (1.6) | −0.26 (1.3) | −0.57 (1.7) | −0.12 (1.3)* | − 0.24 (1.5) |
| SBP (mmHg) | |||||
| Baseline (a) | 134 (18.7) | 139 (17.8)* | 136 (18.3) | 136 (18.3) | 135.9 (18.5) |
| Last value in 2001 (b) | 133 (17.4) | 138 (20.0)* | 137 (17.6) | 134 (19.0) | 135.0 (18.7) |
| Change (b-a) | − 1.1 (21.0) | − 0.75 (21.4) | +0.55 (20.3) | − 2.2 (21.0) | − 1.0 (21.2) |
| LDL (mg/dl) | |||||
| Baseline (a) | 114 (34.3) | 114 (37.2) | 118 (34.3) | 111 (35.8) | 113.7 (35.5) |
| Last value in 2001 (b) | 101 (28.1) | 106 (35.2) | 106 (33.1) | 101 (28.6) | 103.1 (31.4) |
| Change (b-a) | −13 (33.8) | − 7.1 (34.8) | −12.5 (35.3) | −8.8 (31.4) | −10.7 (34.3) |
*Statistically significant: p < 0.05. Standard deviations and % indicated in parentheses
†RUB comorbidity strata: From claims data, we determined overall burden of co-morbidity, using ICD-9 codes and patient demographics to create resource utilization band (RUB) categories using a formula developed at Johns Hopkins University.16 We then grouped the resultant eight categories into three categories corresponding to low, medium, and high comorbidity
Longitudinal Trends in Control of A1c, SBP, and LDL
Overall, A1c control was fairly good: mean baseline value was 7.5%, declining to 7.2% after 24 months. Compared to whites, African Americans were under poorer control at baseline, but showed greater improvement during follow-up (Table 3). Patients with baseline A1c ≥7% showed steady improvement over 24 months (Fig. 1a). Mean baseline systolic blood pressure was 136 mmHg and showed little change during follow-up (Table 3). In fact, only patients with baseline SBP ≥160 mmHg showed significant improvement; patients with baseline SBP between 140–160 mmHg showed no change over 24 months (Fig. 1b). LDL cholesterol baseline control was good and improved substantially in all demographic groups during follow-up (Table 3). Mean LDL significantly declined by 10.7 mg/dl (Table 3). Patients with baseline LDL ≥130 mg/dl showed the most improvement over the 24-month study period (Fig. 1c).
Figure 1.
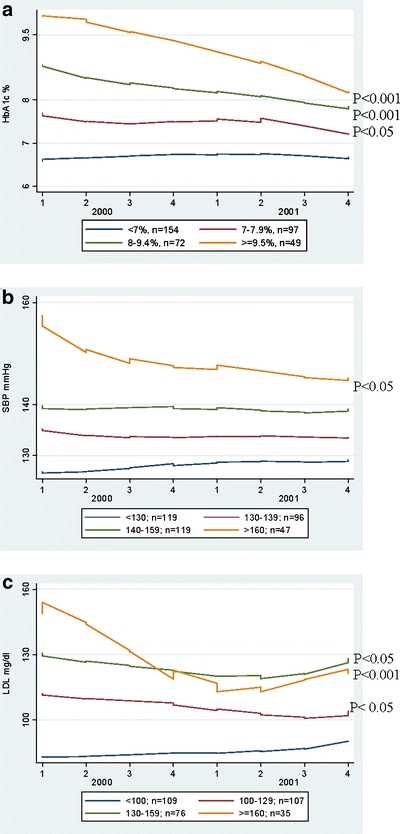
a, b, c: A1c (%), SBP (mmHg), LDL (mg/dl) over time (January 2000–December 2001) by baseline (1999) category. *In 383 adults with type-2 diabetes, we show glycemic, blood pressure, and lipid control over 24 months based on the baseline risk factor category. In the figure, the first value is the lowest value for quarter 1. These values are therefore slightly lower than the baseline 1999 value.
Among patients with sub-optimal baseline control, there was intensification of therapy in approximately one of the eight quarters under review (Table 4), and among those with all three risk factors, two risk factors, or 1 risk factor being “actionable,” 2/25 (8%), 12/65 (18%), and 167/261 (64%), respectively, remained with the same number of sub-optimally controlled risk factors after 2 years. Among the patients with actionable A1c, SBP, and LDL at baseline, 25/125 (20%), 67/158 (42%), and 10/81 (12%), respectively, remained in sub-optimal control after 2 years.
Table 4.
Mean Number of Quarters of Intensification and Number (n) of Study Population Who Met One, Two, and All Three Actionable Criteria
| N actionable criteria at baseline | Mean N quarters of intensification for A1c oral zmedications (n) | Mean N quarters of intensification for BP medications (n) | Mean N quarters of intensificationfor LDL medications (n) |
|---|---|---|---|
| 1 | 0.93 (129) | 1.18 (151) | 0.82 (114) |
| 2 | 1.06 (54) | 1.04 (57) | 0.78 (40) |
| 3 | 1.0 (22) | 1.3 (23) | 0.69 (13) |
Failure to Adhere to Visits or Monitor Risk Factors
Patients who were least visit-adherent had a final mean A1c 0.72% significantly higher, final mean SBP 4.4 mmHg significantly higher, and a final mean LDL 12.3 mg/dl non-significantly higher than their counterparts who were more visit-adherent, even after adjusting for age, race, sex, co-morbidities, and baseline values (Table 5).
Table 5.
Failures to Visit, Monitor, and Intensify Treatment in Relation to A1c, SBP, and LDL Control after 24 Months, Adjusted for Age, Sex, Race, Case Mix, and Baseline Control (All Models Adjusted for Age, Race, Sex, Co-morbidity, and Baseline A1c, Systolic Blood Pressure, or LDL Cholesterol, Respectively)
| Risk factors | *Mean difference in final A1c | *Mean difference in final SBP | *Mean difference in final LDL |
|---|---|---|---|
| FAILURE TO VISIT (n at risk) | 244 | 337 | 226 |
| Made visits in 7 or 8 quarters | (Ref) | (Ref) | (Ref) |
| Missed visits in 2 or 3 of the 8 quarters | 0.33% (−0.00, 0.67) | 4.6 mmHg (0.2, 9.0) | −1.2 mg/dl (−10.1, 7.7) |
| Missed visits in ≥4 of the 8 quarters | 0.72% (0.28, 1.22) | 4.4 mmHg (−1.5, 10.3) | 12.3 mg/dl (−0.4, 24.1) |
| p for trend | =0.002 | 0.05 | 0.15 |
| FAILURE TO MONITOR (n at risk)† | 229 | 314 | 226 |
| Monitored in 7 or 8 quarters | (Ref) | (Ref) | (Ref) |
| Failed to monitor in 2 or 3 of the 8 quarters | 0.50% (0.22, 0.79) | 8.8 mmHg (4.0, 13.6) | 12.2 mg/dl (1.9, 22.6) |
| Failed to monitor in ≥4 of the 8 quarters | 0.83% (0.44, 1.22) | 9.5 mmHg (−1.3, 20.4) | 28.3 mg/dl (17.0, 39.5) |
| p for trend | <0.001 | =0.001 | <0.001 |
| FAILURE TO INTENSIFY THERAPY (n at risk)‡ | 133 | 305 | 183 |
| No failures to intensity therapy | (Ref) | (Ref) | (Ref) |
| Failed to intensify in 1 or 2 quarters with sub-optimal control | 0.8% (0.4, 1.2) | 13.6 mmHg (8.3, 18.9) | 28.9 mg/dl (18.7, 39.0) |
| Failed to intensify in ≥3 quarters with sub-optimal control | 1.4% (0.7, 2.1) | 22.2 mmHg (16.6, 27.9) | 43.7 mg/dl (24.1, 63.3) |
| p for trend | <0.001 | <0.001 | <0.001 |
*Mean difference in final values comparing less adherent to more adherent subjects for each of the three analyses. The last reading in 2001 was used as the final value
†This analysis was limited to patients who were generally visit-adherent (i.e., had ≥4 visit quarters over 2 years for A1c and SBP, and ≥2 visit quarters in 2 years for LDL). This was based on ADA-recommended guidelines
‡This analysis was limited to patients who were generally monitor-adherent (i.e., had ≥4 monitoring quarters over 2 years for A1c and SBP, and ≥2 monitoring quarters in 2 years for LDL). This was based on ADA-recommended guidelines
To isolate the effect of failure to monitor on control at the end of the 24 months, we limited our attention to subsets of patients who were visit-adherent (≥2 visits per year). We found that patients monitored the least had final mean A1c 0.83% higher, final mean SBP 9.5 mmHg higher, and final mean LDL 28.3 mg/dl higher than their counterparts who were adequately monitored, even after adjusting for age, race, sex, co-morbidities, and baseline values. Both failure to visit and monitor appropriately showed graded relationships for final A1c, SBP, and LDL, with lower adherence and lower monitoring associated with higher mean final values (Table 5).
Failure to Intensify Treatment
We next categorized each quarter of the 24-month interval as optimal vs. sub-optimal control, and if sub-optimal, whether therapy was intensified or not. To isolate the effect of failure to intensify therapy on control at the end of the 24 months, we limited our attention to subsets of patients who were visit- and monitoring-adherent. They had prescription refill rates of 79–90% for hypoglycemic and antihypertensive medications. In these sub-sets, failure to intensify showed strong, graded associations with control of glucose, SBP, and LDL cholesterol even after adjusting for age, race, sex, co-morbidities, and baseline risk factor values.
There were 13/133, 113/305, and 8/183 patients with actionable A1c, SBP, and LDL, respectively, who had three or more quarters of failure to intensify therapy. Compared to their counterparts with no failures of intensification, patients with failures in ≥3 quarters showed markedly worse control of blood glucose (A1c 1.4% higher: 95% CI: 0.7, 2.1), hypertension (SBP 22.2 mmHg higher: 95% CI: 16.6, 27.9), and LDL cholesterol (LDL 43.7 mg/dl higher: 95% CI: 24.1, 63.3). These relationships were strong, graded, and independent of socio-demographic factors, baseline risk factor values, and co-morbidities (Table 5).
CONCLUSIONS
Even in this relatively adherent cohort of diabetic adults enrolled in managed care, with ample time for clinical intervention, optimal control of risk factors was often not achieved. Over the 24-month interval, control of glycemia and lipids showed modest improvement, but not to target. Blood pressure control showed the least progress. Only those patients with SBP ≥160 mmHg at baseline showed any improvement over the 24 months. Control of sub-optimal risk factors is associated with significant declines in mortality and morbidity. For example, each 10-mmHg decline is associated with a 15–32% decline in risk of death.17–19
Sub-optimal control was strongly related to three modifiable aspects of primary care: missed visits (lipids showed a non-significant effect), failure to monitor even when visits are kept (lipids especially), and failure to intensify therapy, even when visits are kept and monitoring is completed. These relationships were graded and were independent of socio-demographic factors and co-morbidities. Strengths of the study are: (1) a longitudinal design with detailed information on clinical activity from visit to visit; (2) use of a HEDIS-based sample, the traditional reference population for judging quality of care; (3) data from 14 health center locations throughout the state.
Several studies have evaluated changes in A1c, blood pressure, and lipid control in patients with diabetes.20–22 All found improvement in risk factors during follow-up, but also identified a substantial percentage of patients who failed to reach targets. While there are many studies evaluating the processes of care,23–26 few studies relate these processes of care to outcomes, i.e., relating lack of testing, monitoring, or intensification with ultimate control of risk factors.20,27–29
We were unable to find any studies that evaluated visit adherence in relation to lipid or blood pressure control in diabetic adults. Two studies have evaluated the effects of appointment adherence on glycemic control.28,29 Karter et al.29 found 13% of patients missed >30% of appointments over 1 year, which was associated with a 0.70%–0.79% higher A1c compared to their visit-compliant counterparts. Rhee et al. also showed an increase of 0.12% in A1c for each missed visit.28 These studies are consistent with our findings that 10% of patients were non-adherent to visits, and visit non-adherence was associated with worse glycemic control.
Since 1980, several studies have evaluated the effects of failure to intensify medications on risk factor control, with generally congruent results.20,27,30 Berlowitz et al. found that intensification of therapy was related to better blood pressure control27 and that intensification of diabetes medications improved glycemic control over 2 years.30 Additionally, Brown et al.’s study20 found a peak in glycemic excursion prior to the addition of the metformin and that patients spent numerous months above target A1c before a therapeutic change was made. Straka et al.31 found that 70% of subjects who were above the LDL target of 100 mg/dl were not on lipid medications. The Hypertension Optimal Treatment (HOT) trial and United Kingdom Prospective Diabetes Study (UKPDS) trials demonstrated that a 4- and 10-mmHg difference, respectively, in blood pressure control in patients with diabetes resulted in a 66% and 32% decline in mortality, respectively.5,32
However, our study provides several novel findings. First, while many studies have evaluated the effects of failing to monitor appropriately,33–36 no longitudinal study has directly shown the effects of lack of monitoring on cardiovascular risk factor control after removing patients non-adherent to their visits. As we hypothesized, we found that failure to monitor is associated with poorer glycemic, lipid, and blood pressure control, and further, that lack of adequate monitoring of LDL cholesterol is a significant problem.
Second, our study identified blood pressure control as a special problem in diabetes care. Several explanations are likely. First, blood pressure variability may reduce physician confidence in the reading at any particular visit, encouraging “watchful waiting.” Such variability, whether from biologic variation, measurement error, or transient stimuli like nicotine and anxiety/white coat hypertension, provides the physician with a ‘soft’ rationale to delay treatment.37–42 In contrast, A1c and LDL measurements are much less variable. Second, physicians have underestimated the danger posed by mildly elevated blood pressure. In previous studies, physicians have expressed satisfaction with a target BP of 150 mmHg43,44 and report reluctance to treat diastolic blood pressures between 90–100 mmHg or systolic blood pressures between 140–160 mmHg.45
Several limitations of the study also deserve comment. First, because we used the lowest A1c, SBP, or LDL values in the quarter to represent the entire quarter, we may have underestimated the degree of sub-optimal control. Second, the HEDIS-based sample selection process was not designed for comparing performance between doctors or between facilities.46 However, analyses with clinic site and provider did not affect the results (Table 6). Medication side effects, medication costs, complexity of the regimen, polypharmacy, co-morbidity, patients’ resistance to intensify therapy, visits for acute care, and time constraints in typical primary care office setting may all play a role in failure to intensify therapy. However, the main finding of this study, the selective failure to intensity SBP therapy, is notable.
Table 6.
Failures to Visit, Monitor, and Intensify Treatment in Relation to A1c, SBP, and LDL Control after 24 Months, Adjusted for Age, Sex, Race, Case Mix, and Baseline Control (All Models Adjusted for Age, Race, Sex, Co-morbidity, Physician Effects, and Baseline A1c, Systolic Blood Pressure, or LDL Cholesterol, Respectively)
| Risk factors | *Mean difference in final A1c | *Mean difference in final SBP | *Mean difference in final LDL |
|---|---|---|---|
| FAILURE TO VISIT (n at risk) | 244 | 337 | 226 |
| Made visits in 7 or 8 quarters | (Ref) | (Ref) | (Ref) |
| Missed visits in 2 or 3 of the 8 quarters | 0.34% (0.01, 0.67) | 4.6 mmHg (0.2, 8.9) | –2.1 mg/dl (–10.7, 6.6) |
| Missed visits in ≥4 of the 8 quarters | 0.76% (0.30, 1.21) | 4.4 mmHg (–1.4, 10.1) | 11.3 mg/dl (–0.2, 22.8) |
| p for trend | 0.002 | 0.05 | 0.15 |
| FAILURE TO MONITOR (n at risk)† | 229 | 314 | 226 |
| Monitored in 7 or 8 quarters | (Ref) | (Ref) | (Ref) |
| Failed to monitor in 2 or 3 of the 8 quarters | 0.50% (0.21, 0.79) | 8.8 mmHg (4.1, 13.5) | 13.1 mg/dl (3.2, 23.0) |
| Failed to monitor in ≥4 of the 8 quarters | 0.84% (0.44, 1.23) | 9.5 mmHg (–1.1, 20.2) | 27.7 mg/dl (16.8, 38.7) |
| p for trend | <0.001 | =0.001 | <0.001 |
| FAILURE TO INTENSIFY THERAPY (n at risk)‡ | 133 | 305 | 183 |
| No failures to intensity therapy | (Ref) | (Ref) | (Ref) |
| Failed to intensify in 1 or 2 quarters with sub-optimal control | 0.8% (0.4, 1.2) | 13.6 mmHg (8.4, 18.8) | 28.3 mg/dl (18.4, 38.2) |
| Failed to intensify in ≥3 quarters with sub-optimal control | 1.4% (0.7, 2.0) | 22.2 mmHg (16.7, 27.8) | 41.4 mg/dl (22.5, 60.2) |
| P for trend | <0.001 | <0.001 | <0.001 |
*Mean difference in final values comparing less adherent to more adherent subjects for each of the three analyses. The last reading in 2001 was used as the final value
†This analysis was limited to patients who were generally visit-adherent (i.e., had ≥4 visit quarters over 2 years for A1c and SBP, and ≥2 visit quarters in 2 years for LDL). This was based on ADA-recommended guidelines
‡This analysis was limited to patients who were generally monitor adherent (i.e., had ≥4 monitoring quarters over 2 years for A1c and SBP, and ≥2 monitoring quarters in 2 years for LDL). This was based on ADA-recommended guidelines
Third, we cannot exclude the possibility of missing data on visits, monitoring, and medication from outside of the Managed Care Organization network, but this is likely to be small in a staff model Health Maintenance Organization,47 and in the visit and monitoring compliant population used for the intensification analysis, drug refill compliance was high.
Fourth, our data were collected from 1999–2001 and may not be fully generalizable to the present day. Systems changes, including electronic medical record availability and increased awareness of clinical inertia, may have caused an increase in intensification rates by providers since the time of this study. However, a few more recent studies had similar rates of intensification.42–46 Further, there has been no change in ADA guidelines up to 2007 with regards to “actionable” values of risk factors. Finally, because we limited our sample to generally adherent patients under the care of academically affiliated physicians, our findings may not be fully generalizable.
In summary, failure to appropriately intensify therapy for glucose, lipids, and especially blood pressure is common and leads to poor risk factor control in diabetic adults, even with adherent patients.
Future research evaluating barriers and promoters to intensification, and evaluation of interventions to improve treatment intensification, especially of blood pressure, patient visit adherence, and LDL-cholesterol testing, are urgently required.
Acknowledgements
The authors thank:
The Johns Hopkins Community Physicians staff
Elizabeth Lasner, RN, and Grace Ford, RN, for medical chart abstractions.
Dr. T. Alafia Samuels was supported by a NIH Training Grant (HD 08487) and NIH/NHLBI Cardiovascular Epidemiology Training Grant (T32HL07024)
Dr. F. Brancati was supported by a Mid-Career Award for Patient-Oriented Research in Diabetes from the NIDDK, Bethesda, MD (1K24-DK6222-01)
Funding was received from AHRQ Dissertation Grant (1R03HS011946-01) and from the Johns Hopkins Department of Epidemiology, Summer Epidemiology Program Fund
Conflict of Interest None disclosed.
References
- 1.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21:1138–45. [DOI] [PubMed]
- 2.Saydah SH, Eberhardt MS, Loria CM, Brancati FL. Age and the burden of death attributable to diabetes in the United States. Am J Epidemiol. 2002;156:714–9. [DOI] [PubMed]
- 3.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. [DOI] [PMC free article] [PubMed]
- 4.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9. [DOI] [PMC free article] [PubMed]
- 5.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed]
- 6.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed]
- 7.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. [DOI] [PubMed]
- 8.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–74. [DOI] [PubMed]
- 9.Beaton SJ, Nag SS, Gunter MJ, Gleeson JM, Sajjan SS, Alexander CM. Adequacy of glycemic, lipid, and blood pressure management for patients with diabetes in a managed care setting. Diabetes Care. 27:694–8. [DOI] [PubMed]
- 10.American Diabetes Association: clinical practice recommendations 1999. Diabetes Care. 1999;22:S1–114. [PubMed]
- 11.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23:588–94. [DOI] [PMC free article] [PubMed]
- 12.Chaudhry SI, Berlowitz DR, Concato J. Do age and comorbidity affect intensity of pharmacological therapy for poorly controlled diabetes mellitus? J Am Geriatr Soc. 2005;53:1214–6. [DOI] [PubMed]
- 13.Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168:271–6. [DOI] [PubMed]
- 14.Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996 Dec 18;276:1886–92. [DOI] [PubMed]
- 15.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–39. [DOI] [PubMed]
- 16.Weiner J, Abrams C, Kaplowitz C.http://www.acg.jhsph.edu/ACGDocuments/newsletter_f2002.pdf. 2002. Accessed 1-9-2007.
- 17.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9. [DOI] [PMC free article] [PubMed]
- 18.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. [DOI] [PMC free article] [PubMed]
- 19.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614–20. [DOI] [PubMed]
- 20.Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213–7. [PubMed]
- 21.Gudbjornsdottir S, Cederholm J, Nilsson PM, Eliasson B. The National Diabetes Register in Sweden: an implementation of the St. Vincent Declaration for Quality Improvement in Diabetes Care. Diabetes Care. 2003;26:1270–6. [DOI] [PubMed]
- 22.Jorgensen LG, Petersen PH, Christensen C, Eriksen EW, Brandslund I. Improvement in glycemic control over 11 years in patients monitored for diabetes in one county. Clin Chem Lab Med. 2006;44:92–8. [DOI] [PubMed]
- 23.Chin MH, Su AW, Jin L, Nerney MP. Variations in the care of elderly persons with diabetes among endocrinologists, general internists, and geriatricians. J Gerontol A Biol Sci Med Sci. 2000;55:M601–6. [DOI] [PubMed]
- 24.Beaton SJ, Nag SS, Gunter MJ, Gleeson JM, Sajjan SS, Alexander CM. Adequacy of glycemic, lipid, and blood pressure management for patients with diabetes in a managed care setting. Diabetes Care. 2004;27:694–8. [DOI] [PubMed]
- 25.Grant RW, Cagliero E, Murphy-Sheehy P, Singer DE, Nathan DM, Meigs JB. Comparison of hyperglycemia, hypertension, and hypercholesterolemia management in patients with type 2 diabetes. Am J Med. 2002;112:603–9. [DOI] [PubMed]
- 26.Peters AL, Legorreta AP, Ossorio RC, Davidson MB. Quality of outpatient care provided to diabetic patients. A health maintenance organization experience. Diabetes Care. 1996;19:601–6. [DOI] [PubMed]
- 27.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–63. [DOI] [PubMed]
- 28.Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31:240–50. [DOI] [PubMed]
- 29.Karter AJ, Parker MM, Moffet HH, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care. 2004;42:110–5. [DOI] [PubMed]
- 30.Berlowitz DR, Ash AS, Glickman M, et al. Developing a quality measure for clinical inertia in diabetes care. Health Serv Res. 2005;40:1836–53. [DOI] [PMC free article] [PubMed]
- 31.Straka RJ, Taheri R, Cooper SL, Tan AW, Smith AC. Assessment of hypercholesterolemia control in a managed care organization. Pharmacotherapy. 2001;21:818–27. [DOI] [PubMed]
- 32.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. [DOI] [PubMed]
- 33.Beaton SJ, Nag SS, Gunter MJ, Gleeson JM, Sajjan SS, Alexander CM. Adequacy of glycemic, lipid, and blood pressure management for patients with diabetes in a managed care setting. Diabetes Care. 2004;27:694–8. [DOI] [PubMed]
- 34.Chin MH, Auerbach SB, Cook S, et al. Quality of diabetes care in community health centers. Am J Public Health. 2000;90:431–4. [DOI] [PMC free article] [PubMed]
- 35.Peters AL, Legorreta AP, Ossorio RC, Davidson MB. Quality of outpatient care provided to diabetic patients. A health maintenance organization experience. Diabetes Care. 1996;19:601–6. [DOI] [PubMed]
- 36.McClain MR, Wennberg DE, Sherwin RW, Steinmann WC, Rice JC. Trends in the diabetes quality improvement project measures in Maine from 1994 to 1999. Diabetes Care. 2003;26:597–601. [DOI] [PubMed]
- 37.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41:183–7. [DOI] [PubMed]
- 38.Matsui Y, Kario K, Ishikawa J, Hoshide S, Eguchi K, Shimada K. Smoking and antihypertensive medication: interaction between blood pressure reduction and arterial stiffness. Hypertens Res. 2005;28:631–8. [DOI] [PubMed]
- 39.Parati G, Ulian L, Santucciu C, Omboni S, Mancia G. Difference between clinic and daytime blood pressure is not a measure of the white coat effect. Hypertension 1998;31:1185–9. [DOI] [PubMed]
- 40.Jhalani J, Goyal T, Clemow L, Schwartz JE, Pickering TG, Gerin W. Anxiety and outcome expectations predict the white-coat effect. Blood Press Monit. 2005;10:317–9. [DOI] [PubMed]
- 41.Glen SK, Elliott HL, Curzio JL, Lees KR, Reid JL. White-coat hypertension as a cause of cardiovascular dysfunction. Lancet. 1996;348:654–7. [DOI] [PubMed]
- 42.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–34. [DOI] [PubMed]
- 43.Oliveria SA, Lapuerta P, McCarthy BD, L’Italien GJ, Berlowitz DR, Asch SM. Physician-related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–20. [DOI] [PubMed]
- 44.Grant RW, Devita NG, Singer DE, Meigs JB. Improving adherence and reducing medication discrepancies in patients with diabetes. Ann Pharmacother. 2003;37:962–9. [DOI] [PubMed]
- 45.Hyman DJ, Pavlik VN, Vallbona C. Physician Role in Lack of Awareness and Control of Hypertension. J Clin Hypertens (Greenwich.). 2000;2:324–30. [PubMed]
- 46.Krein SL, Hofer TP, Kerr EA, Hayward RA. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Serv Res. 2002;37:1159–80. [DOI] [PMC free article] [PubMed]
- 47.Kerr EA, Smith DM, Hogan MM, et al. Comparing clinical automated, medical record, and hybrid data sources for diabetes quality measures. Jt Comm J Qual Improv. 2002;28:555–65. [DOI] [PubMed]


