Abstract
Background
Physicians are increasingly asked to improve the delivery of clinical services and patient experiences of care.
Objective
We evaluated the association between clinical performance and patient experiences in a statewide sample of physician practice sites and a sample of physicians within a large physician group.
Design, Setting, Participants
We separately identified 373 practice sites and 119 individual primary care physicians in Massachusetts.
Measurements
Using Health Plan Employer Data and Information Set data, we produced two composites addressing processes of care (prevention, disease management) and one composite addressing outcomes. Using Ambulatory Care Experiences Survey data, we produced seven composite measures summarizing the quality of clinical interactions and organizational features of care. For each sample (practice site and individual physician), we calculated adjusted Spearman correlation coefficients to assess the relationship between the composites summarizing patient experiences of care and those summarizing clinical performance.
Results
Among 42 possible correlations (21 correlations involving practice sites and 21 involving individual physicians), the majority were positive in site level (71%) and physician level (67%) analyses. For the 28 possible correlations involving patient experiences and clinical process composites, 8 (29%) were significant and positive, and only 2 (7%) were significant and negative. The magnitude of the significant positive correlations ranged from 0.13 to 0.19 at the site level and from 0.28 to 0.51 at the physician level. There were no significant correlations between patient experiences and the clinical outcome composite.
Conclusions
The modest correlations suggest that clinical quality and patient experience are distinct, but related domains that may require separate measurement and improvement initiatives.
KEY WORDS: quality of care, patient centered care, primary care, quality measurement, quality improvement
INTRODUCTION
Quality measurement and reporting programs are now widespread.1–4 Clinical performance measures such as those defined in the Health Plan Employer Data and Information Set (HEDIS) are used increasingly for public reporting5–7 and pay for performance programs.8–10 These widely recognized clinical measures of quality include both processes of care (e.g., rates of screening hemoglobin A1c exams) and outcomes of care (e.g., proportion of patients achieving specified levels of hemoglobin A1c control).
Valid measures of the patient’s experience of care have also appeared as an integral part of quality measurement and improvement initiatives by provider organizations, large payers’ pay for performance programs,8 and public reporting efforts.5 The Ambulatory Care Experiences Survey (ACES) is one such measurement tool developed specifically for use in the assessment of patient experiences of primary care,11 providing an assessment of both organizational features of care and the quality of clinical interactions.12
As physician groups and individual physicians strive to deliver care that is both clinically effective and patient-centered,13,14 it is increasingly important to improve our understanding of the relationship between measures of clinical performance and patient experience. Physicians may express concern that conflicts arise as systems are implemented to standardize care across all patients to assure outstanding clinical performance, while the delivery of patient-centered care may ultimately rely on customizing care to suit the needs of a particular patient.15 At the same time, consumers of performance data, including purchasers, insurers, and patients, would benefit from knowledge of whether different methods of performance assessment provide equivalent information or whether multiple independent tools are required to obtain a complete picture of health-care delivery.
We used statewide data in Massachusetts to evaluate the association between clinical measures of quality and measures of patient experiences at the level of the physician practice site and the individual physician.
METHODS
Overview
To evaluate the correlations between clinical performance measures and patient experience survey measures, we separately analyzed data from (1) a statewide cohort of 373 practice sites and (2) a cohort of 118 individual physicians that are part of one large physician organization in Eastern Massachusetts.
Practice Site Data
Data on practice site performance were obtained through the Massachusetts Health Quality Partners (MHQP), a broad-based coalition of physicians, health plans, and purchasers.6 MHQP derives its patient samples from the enrollment databases of five participating health plans, which together cover over 4 million Massachusetts residents, or 85% of managed care enrollees in Massachusetts. As part of the MHQP initiative, primary care physicians were linked to distinct practice sites for public reporting purposes. Practice sites were defined as a physical location where a physician confirmed he or she practiced as of December 31, 2004. To be included in the sample, practice sites must have had at least 3 eligible physicians with a panel size of at least 50 eligible patients across the five health plans. The eligible practice sites together represented 92% of licensed primary care physicians in the state.
MHQP collects clinical performance measures according to standard HEDIS specifications (Table 1) using either claims data alone (process measures) or a hybrid method of claims data and medical record review (outcomes measures). To calculate HEDIS performance scores for each physician, the denominator is equal to the number of opportunities an individual physician has to satisfy a given HEDIS measure, and the numerator is equal to the number of times that HEDIS measure is satisfied. Because the numerators for the process measures are derived from claims data alone, they are multiplied by a claims adjustment factor, which is derived by comparing the difference between the health plan’s HEDIS performance rate based on administrative data alone with the health plan’s rate derived from the hybrid method (combination of claims and medical record review). There is no claims adjustment factor applied to clinical outcomes measures as they are derived solely from medical record review.16
Table 1.
Measures of Clinical Quality
| Composite measure | Individual item content |
|---|---|
| Processes of care: Prevention (αST = 0.93, αMD = 0.97) | Cervical cancer screening |
| Denominator: Women 21–64 years old | |
| Numerator: Pap smear within the prior 3 years | |
| Breast cancer screening | |
| Denominator: Women 52–69 years old | |
| Numerator: Mammogram within the prior 2 years | |
| Colorectal cancer screening | |
| Denominator: Adults 51–80 years old | |
| Numerator: (1) fecal occult blood test within 1 year; or (2) flexible sigmoidoscopy within the prior 5 years; or (3) double contrast barium enema within the prior 5 years,* or (4) colonoscopy within the prior 10 years | |
| Chlamydia screening | |
| Denominator: Sexually active women 16–25 years old | |
| Numerator: Chlamydia screening test within 1 year | |
| Processes of care: Disease management (αST = 0.88, αMD = 0.94) | Cholesterol screening for patients with cardiovascular conditions |
| Denominator: Adults 18–75 years old discharged alive for acute myocardial infarction, coronary artery bypass graft or percutaneous transluminal coronary angioplasty, or who had a diagnosis of ischemic vascular disease | |
| Numerator: Low density lipoprotein (LDL) cholesterol test within 1 year | |
| Appropriate asthma medications† | |
| Denominator: Adults 18–75 years old diagnosed with persistent asthma | |
| Numerator: Provided at least one prescription for inhaled steroid, nedocromil, cromolyn sodium, leukotriene modifier, or methylxanthine during the measurement year | |
| Diabetes care: | |
| Eye exam | |
| HbA1c testing | |
| LDL cholesterol testing | |
| Nephropathy screening | |
| Denominator: Adults 18–75 years with diabetes diagnosis | |
| Numerators: Dilated eye exam within the prior year | |
| HbA1c exam within the prior year | |
| LDL cholesterol exam within the prior year | |
| Urine microalbumin exam within prior year | |
| Outcomes of care (αST = 0.86, αMD = 0.93) | Cholesterol management for patients with cardiovascular conditions |
| Denominator: Adults 18–75 years old meeting criteria for cardiovascular disease and having an LDL cholesterol test within 1 year | |
| Numerator: LDL cholesterol result <130 mg/dL | |
| Diabetes care: | |
| LDL cholesterol control diabetes care – poor HbA1c control | |
| Denominator: Adults 18–75 years old with diabetes and laboratory test within 1 year | |
| Numerators: LDL cholesterol result <130 mg/dl HbA1c >9% | |
| Controlling high blood pressure | |
| Denominator: Adults 46–85 years old with diagnosis of hypertension | |
| Numerator: Blood pressure ≤140/90 | |
*Barium enema criteria not applied to the physician-level data
†This measure was only available for the site-level analyses
αST = Cronbach’s alpha derived from site-level data, αMD = Cronbach’s alpha derived from adult physician-level data
Patient experience data were derived from MHQP’s administration of the ACES instrument, which produces summary measures covering quality of clinical interactions and organizational features of care (Table 2).12 The survey sample consisted of adults 18 to 64 years of age with at least 1 year of continuous enrollment in one of five participating health plans, and at least one visit with the primary care physician in the last 12 months. The survey was fielded between July and September 2005 using a three-stage mail protocol,17 beginning with a mailing containing a cover letter on their health plan stationery and a copy of the survey, followed by a reminder post card (2 weeks later) and a second survey packet to non-respondents (1 week later). The cover letter provided the option of completing the survey online and afforded a web address and unique respondent log-in information. The overall response rate was 39% (n = 61,683), producing patient experience data for 373 (75%) practice sites statewide. Prior analyses of this survey process have confirmed that survey non-response does not pose a threat to the validity of the findings, with no differences across physician practices in individuals’ propensity to respond to the survey according to age, race, education, and socioeconomic status.12 We limited our analyses to practice sites with data available on both patient experiences and HEDIS measures (n = 334).
Table 2.
Measures of Patients’ Experience
| Composite measures | Individual item content |
|---|---|
| Clinical interactions Doctor/patient communication (αST = 0.81, αMD = 0.68) | |
| How often did your personal doctor explain things in a way that was easy to understand? | |
| How often did your personal doctor listen carefully to you? | |
| How often did your personal doctor give you clear instructions about what to do to take care of the health problems or symptoms that were bothering you? | |
| How often did your personal doctor give you clear instructions about what to do if your symptoms got worse or came back? | |
| How often did your personal doctor show respect for what you had to say? | |
| How often did your personal doctor spend enough time with you? | |
| How often did your personal doctor seem to know all the important information about your medical history? | |
| How would you rate your personal doctor’s knowledge of you as a person, including values and beliefs that are important to you? | |
| Clinical team interactions (αST = 0.67, αMD = 0.25) | How often did other doctors and nurses at your personal doctor’s office explain things in a way that was easy to understand? |
| How often did you feel that other doctors and nurses at your personal doctor’s office had all the information they needed to correctly diagnose and treat your health problems? | |
| How often did other doctors and nurses at your personal doctor’s office spend enough time with you? | |
| Overall, how would you rate the care you got from other doctors and nurses at your personal doctor’s office? | |
| Health promotion support (αST = N/A, αMD = N/A) | Did your personal doctor give you the help you needed to make changes in your habits or lifestyle that would improve your health or prevent illness? |
| Organizational features | |
| Integration of care (αST = 0.78, αMD = 0.49) | How often did your personal doctor seem informed and up-to-date about the care you received from specialist doctors? |
| When your personal doctor sent you for a blood test, x-ray or other test, how often did someone from your doctor’s office follow up to give you the test results? | |
| Office staff (αST = 0.84, αMD = 0.24) | How often were office staff at your personal doctor’s office as helpful as you thought they should be? |
| How often did office staff at your personal doctor’s office treat you with courtesy and respect? | |
| Visit-based continuity (αST = 0.90, αMD = 0.36) | When you had an appointment at your personal doctor’s office, how often did you see your personal doctor? |
| Organizational access (αST = 0.89, αMD = 0.39) | When you called your personal doctor’s office to get an appointment for care you needed right away, how often did you get an appointment as soon as you needed it? |
| When you scheduled an appointment for a check-up or routine care at your personal doctor’s office, how often did you get an appointment as soon as you needed it? | |
| When you called your personal doctor’s office with a medical question during regular office hours, how often did you get an answer to your question that same day? | |
| When you called your personal doctor’s office after regular office hours, how often did you get the help or advice you needed? | |
| Once you were in the exam room, how often did the person you were scheduled to see come in within 15 minutes? |
αST = Cronbach’s alpha derived from site-level data
αMD = Cronbach’s alpha derived from adult physician-level
Individual Physician Data
Data on individual physician performance were obtained from Harvard Vanguard Medical Associates, an integrated group practice consisting of 14 ambulatory health centers in eastern Massachusetts, with approximately 120 primary care physicians caring for approximately 300,000 adult patients. HVMA has used a fully functional electronic medical record system, EpicCare Ambulatory (www.epicsystems.com) since 1997, which allows for the electronic capture of all aspects of outpatient encounters, including clinician notes, diagnostic codes, procedure codes, and all laboratory results. The HVMA electronic record system assigns a unique primary care physician to each patient, and data from this medical record system have been used extensively in formal analyses of quality of care.18–22 We supplemented electronic medical record system data with claims data to assess colorectal cancer screening and diabetic eye exams, as these services are sometimes received outside of the HVMA network. We applied HEDIS criteria to identify appropriate denominator and numerator populations using diagnostic and billing codes within the electronic medical record during 2005.23 As HVMA does not maintain membership enrollment similar to health plan enrollment databases, we restricted the inclusion of patients in all denominator populations to those with a primary care physician visit within the past 36 months.
HVMA administered the ACES instrument to patients of all adult primary care physicians on a quarterly basis with sample sizes defined to achieve a 0.70 Spearman Brown-derived reliability at the individual physician level. All primary care physicians with at least a 50% full-time equivalent practice are included in the survey process. Surveys were administered to patients who had an office visit within the past 12 months with the primary care physician and used the same three-stage data collection protocol as used by MHQP. This study used survey data obtained between January 2005 and December 2005, and had a 40% (n = 9,120) response rate. Patient experience data were available for all 119 (100%) primary care physicians maintaining at least a 50% full-time equivalent practice at HVMA.
Composite Measure Creation
We created three composite measures of clinical quality and seven composite measures of patient experiences (Tables 1 and 2). Composite measures of clinical quality were developed by first separating process of care measures from clinical outcomes, as these represent distinct concepts of quality.24 Finally, we categorized process measures based on whether they focused on preventive health or chronic disease management, as these activities likely represent different care processes and may be targeted by independent quality improvement strategies.
The patient experience composites include three measures of the quality of clinical interactions (doctor/ patient communication, clinical team interactions, and health promotion support) and four measures of organizational features of care (integration of care, office staff, visit-based continuity, and organizational access).12
Numeric composite scores for both patient experience and clinical quality measures were calculated using the adjusted half-scale rule to produce ratings on a scale from 0 to 100, with higher scores representing either better patient experiences or superior clinical quality. This method has been described previously,25 and involves first transforming the individual item to a 0 to 100 scale based on the number of responses. HEDIS scores are already ranked as such, while patient survey responses require conversion (e.g., a six-point Likert response item would generate a score of 0, 20, 40, 60, 80, or 100). Scores were adjusted by adding or subtracting a constant for each item so that all items have the same mean (the average of the overall mean of all items in the scale), satisfying a key assumption of the half-scale.
Correlation Analyses
We calculated Spearman correlation coefficients between each clinical quality composite measure and each patient experience composite measure at the practice site and individual physician level. We further defined the convergence estimates for each of these correlations to account for the limitations in the reliability of the composite scores. These convergence estimates, or adjusted correlation coefficients, represent the true correlation that would be observed between the two composite scores in the absence of measurement error associated with creating the composites. The convergence estimates were calculated using the Spearman-Brown reliability coefficients for the patient experience composite (α1) and the clinical quality composite (α2), along with the Spearman correlation coefficient (ρ) in the following manner: 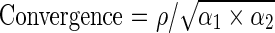 .
.
This study was approved by the Human Studies Committees at Harvard Vanguard Medical Associates, Tufts-New England Medical Center, and Harvard Medical School. All analyses were carried out using the STATA statistical package, version 9.0.
RESULTS
Clinical performance for the process of care prevention measures was generally comparable for the practice group and individual physician level data, with the exception of colorectal cancer screening and the diabetes care process measures (Table 3). The aggregate patient experience ratings were comparable for the practice group and individual physician level data, with the exceptions of health promotion support and office staff (Table 4).
Table 3.
Clinical Quality Composite Measures
| Practice site level | Individual physician level | |||||
|---|---|---|---|---|---|---|
| HEDIS indicator | Number of patients, n | Number of sites, n | Average rate | Number of patients, n | Number of doctors, n | Average rate |
| Process of care: Prevention | ||||||
| Cervical cancer screening | 322, 436 | 334 | 0.88 | 89, 197 | 118 | 0.83 |
| Breast cancer screening | 128, 600 | 330 | 0.83 | 23, 591 | 118 | 0.88 |
| Colorectal cancer screening | 275, 048 | 335 | 0.66 | 59, 138 | 118 | 0.73 |
| Chlamydia screening | 41, 434 | 326 | 0.43 | 11, 979 | 109 | 0.40 |
| Process of care: Disease management | ||||||
| Cholesterol screening (cardiac) | 560 | 37 | 0.87 | 8, 747 | 111 | 0.91 |
| Eye exams (diabetes) | 45,507 | 334 | 0.71 | 11, 311 | 117 | 0.71 |
| HbA1c testing (diabetes) | 45,532 | 334 | 0.92 | 11, 311 | 117 | 0.77 |
| LDL cholesterol testing (diabetes) | 45,547 | 334 | 0.94 | 11, 311 | 117 | 0.71 |
| Nephropathy screening (diabetes) | 45,508 | 334 | 0.67 | 11, 311 | 117 | 0.54 |
| Clinical outcomes | ||||||
| Cholesterol management (cardiac) | 147 | 11 | 0.77 | 8, 747 | 111 | 0.79 |
| Poor HbA1c control (diabetes) | 205 | 11 | 0.20 | 8, 901 | 116 | 0.15 |
| LDL cholesterol control (diabetes) | 349 | 21 | 0.77 | 8, 091 | 115 | 0.83 |
| Controlling high blood pressure | 249 | 19 | 0.76 | 18, 642 | 117 | 0.72 |
Table 4.
Patient Experiences of Care Composite Scores
| Practice site level (n = 373 groups) | Individual physician level (n = 119 physicians) | |||
|---|---|---|---|---|
| HEDIS indicator | Mean score | Standard deviation | Mean score | Standard deviation |
| Clinical interactions | ||||
| Doctor/patient communication | 88.2 | 3.8 | 85.4 | 7.2 |
| Clinical team interactions | 83.5 | 4.7 | 80.9 | 5.0 |
| Health promotion support | 92.6 | 6.1 | 78.5 | 12.0 |
| Organizational features of care | ||||
| Integration of care | 84.4 | 4.9 | 83.7 | 7.2 |
| Office staff | 86.3 | 4.8 | 95.0 | 5.6 |
| Visit-based continuity | 88.5 | 6.7 | 86.1 | 5.6 |
| Organizational access | 81.9 | 5.7 | 81.7 | 5.7 |
Among 42 possible HEDIS-ACES composite correlations (21 involving practice sites and 21 involving individual physicians), the majority were positive in both site level (71%) and physician level (67%) analyses (Table 5). There were no significant correlations between composite measures of patient experiences and clinical outcomes at either the practice site or individual physician level.
Table 5.
Adjusted Correlations Between Patient Experiences of Care and Clinical Quality Composites*
| Practice site level | ||||
|---|---|---|---|---|
| Process of care: Prevention | Process of care: Disease management | Outcomes of care | ||
| α2 | 0.93 | 0.88 | 0.86 | |
| α1 | ||||
| Clinical interactions | ||||
| Doctor/patient communication | 0.81 | 0.001 (0.99) | -0.044 (0.52) | 0.141 (0.18) |
| Clinical team interactions | 0.67 | 0.153 (0.02) | 0.193 (0.01) | 0.104 (0.37) |
| Health promotion support | 0.35 | 0.013 (0.89) | -0.082 (0.43) | 0.011 (0.95) |
| Organizational features | ||||
| Integration of care | 0.78 | 0.126 (0.04) | 0.151 (0.03) | 0.148 (0.17) |
| Office staff | 0.84 | 0.002 (0.98) | 0.045 (0.51) | 0.053 (0.61) |
| Visit-based continuity | 0.90 | -0.122 (0.03) | -0.032 (0.62) | -0.006 (0.95) |
| Organizational access | 0.89 | -0.042 (0.48) | 0.057 (0.39) | 0.005 (0.96) |
| Individual physician level | ||||
| α2 | 0.97 | 0.94 | 0.93 | |
| α1 | ||||
| Clinical interactions | ||||
| Doctor/patient communication | 0.68 | 0.284 (0.02) | 0.005 (0.01) | 0.084 (0.49) |
| Clinical team interactions | 0.25 | 0.512 (0.01) | -0.468 (0.02) | 0.277 (0.17) |
| Health promotion support | 0.49 | 0.257 (0.06) | 0.025 (0.86) | 0.095 (0.51) |
| Organizational features | ||||
| Integration of care | 0.49 | 0.254 (0.07) | -0.149 (0.31) | 0.111 (0.43) |
| Office staff | 0.24 | 0.373 (0.06) | -0.052 (0.80) | 0.040 (0.85) |
| Visit-based continuity | 0.36 | -0.082 (0.61) | -0.055 (0.75) | 0.036 (0.83) |
| Organizational access | 0.39 | 0.390 (0.01) | -0.071 (0.66) | -0.077 (0.63) |
*Values represent convergence estimates (adjusted Spearman correlation coefficients) between clinical quality composite measure and patient experience composite measure. Numbers in parentheses indicate p value; statistically significant correlations indicated in boldface
α1 = Spearman Brown reliability coefficient for group level or physician level patient experience composite scores
α2 = Spearman Brown reliability coefficient for clinical composite score
Within the practice site cohort, the HEDIS process-health prevention composite was positively correlated with one ACES measure of clinical interactions (clinical team interactions,adjusted ρ = 0.15, p = 0.02) and one ACES measure of organizational features of care (integration of care, adjusted ρ = 0.13, p = 0.04). The HEDIS process-disease management composite was positively correlated with the same ACES measures of clinical team interactions (adjusted ρ = 0.13, p = 0.01) and integration of care (adjusted ρ = 0.15, p = 0.03). The HEDIS process-health prevention composite was also negatively correlated with with one ACES measure of organizational features of care (visit-based continuity, adjusted ρ = -0.12, p = 0.03).
Within the individual physician cohort, the HEDIS process-health prevention composite was positively correlated with two ACES measures of clinical interaction quality (doctor/patient communication, adjusted ρ = 0.284, p = 0.02; and clinical team interactions, adjusted ρ = 0.512, p = 0.01), and one ACES measure of organizational features of care (organizational access, adjusted ρ = 0.390, p = 0.01). Three additional correlations between the HEDIS health prevention composite and ACES composite measures were of borderline statistical significance (health promotion support, adjusted ρ = 0.257, p = 0.06; integration of care, adjusted ρ = 0.254, p = 0.07; and office staff, adjusted ρ = 0.373, p = 0.06). The HEDIS process-disease management composite was negatively correlated with one ACES measure of organizational features of care (clinical team interactions, adjusted ρ = -0.468, p = 0.02).
DISCUSSION
Enhancing the quality of care in the ambulatory setting includes assuring the delivery of clinically effective care in a patient-centered manner.26,27 In this statewide evaluation, we found that measures of patient experiences were positively, but modestly correlated with process measures of clinical quality at both the practice site and individual physician level, with only one-third of these correlations achieving statistical significance. There were no significant correlations between patient experiences of care and clinical outcomes among practice sites and individual physicians.
Our study is consistent with prior research on the association of clinical performance and patient experience measures at the level of health plans, which demonstrated that better performance on some, but not all, patient reports of care experiences were associated with higher scores on clinical HEDIS measures.28 The magnitude of the correlations we observed were generally modest and similar in magnitude to previously reported correlations at the health plan level, which ranged from 0.24 to 0.38.28 The absence of overwhelmingly strong correlations in our study suggests that clinical care delivery and patient experience represent sufficiently distinct activities that ongoing quality measurement programs should include independent measurement of both domains in order to obtain a comprehensive evaluation of care. Patients using such data to select a primary care physician may need to make trade-offs between technical performance and interpersonal performance.29 Similarly, pay for performance programs will likely need to incorporate both aspects of health-care delivery to achieve broad-based improvements in care.
The modest correlations we observed suggest that improvements in clinical quality will not automatically produce improvements in patient experiences and vice versa.4,30 Monitoring both patient experience and clinical quality can ensure that efforts to improve patient experiences of care do not come at the expense of assuring the highest possible clinical performance.15 Importantly, the majority of all of the correlations examined were positive, with no evidence to support the notion that delivering high quality clinical processes is somehow in conflict with positive patient experiences in the primary care setting.
We found no association between patient experiences of care and clinical outcomes. Earlier research suggests that better patient experiences may improve patient outcomes through improved adherence.31–35 Our null findings may reflect aspects of our study design, such as the cross-sectional nature or the limited sample sizes for the outcomes measures. However, the absence of correlations between patient experiences of care and clinical outcomes parallels findings from recent studies demonstrating the lack of a consistent relationship between improvements in clinical process measures and clinical outcomes.3,21,36 Rather than reflecting a limitation of measures of patient experiences, our findings reinforce the inherent difficulty of linking process to outcome and that existing measures of process, including patient experiences of care, may not represent the full spectrum of factors that contribute to improved outcomes.
Our findings are strengthened by several aspects of the study design. Our data focused on care delivered by doctor’s offices and individual clinicians, including a statewide sample of practice sites and a large number of individual physicians across eastern Massachusetts. We were also able to include a wide range of measures of clinical quality and outcomes and patient experiences that were similar at both the practice site and individual physician level. However, our findings should be interpreted in the context of study design limitations as well. The availability of a large number of quality measures necessitated multiple testing, increasing the probability of detecting significant correlations by chance alone. However, our study goal was focused more on overall patterns rather than the importance of any single correlation.
While we included practice sites throughout Massachusetts, our sample was dependent on enrollment in one of five major commercial health plans and so may not generalize to other populations, particularly those lacking health insurance or the elderly. At the individual physician level, we were only able to include data from a single multispecialty practice group, though it is important to note that this is the largest ambulatory practice group in the state, providing care for over 300,000 patients and employing over 100 primary care physicians across 14 health centers in a mix of urban and distant suburban settings. In addition, while actual performance on HEDIS and ACES measures may not generalize across different health settings, there is no evidence to suggest that the relationship between these two domains of care differs across settings.
We are unable to draw conclusions regarding the mediators of the correlations we identified. In particular, we do not have information regarding structural characteristics of the practices, as well as individual characteristics of the physicians and patients in our study. It is very likely that specific attributes of a practice or physician mediate the strength of correlations between patient experiences of care and clinical measures of quality, and future research will be needed to explore this important question.
The definition and collection of patient experience measures were identical at the practice site and individual physician level; however, the collection of the clinical HEDIS measures differed between the two levels. At the practice site level, we relied mainly on health plan administrative data supplemented in some cases by chart review, while at the individual physician level we used mainly electronic medical record data. This resulted in a limited number of practice sites available for analysis of clinical outcomes, which are reliant on medical chart review. However, since our analyses were focused on determining correlations within these two levels (practice site and individual physician) and not between these two levels, the analyses are internally consistent at each level and are not substantially affected by differences in data collection for the clinical HEDIS measures. Finally, we were unable to correlate HEDIS chronic disease measures to patient experience measures for patients with those specific conditions, a technique that may have identified stronger associations.
In conclusion, we found that patient experiences of care were modestly correlated with clinical process measures and not correlated with clinical outcomes. Improving both patient experiences of care and clinical process measures should remain high priorities for health-care providers; however, the limited strength of the associations implies that distinct efforts in these areas are required to both monitor and improve these two domains of quality.
Acknowledgements
We would like to thank Massachusetts Health Quality Partners for allowing us use of statewide data for these analyses; Jo-Anne Foley at HVMA for helping to obtain physician-level data; and Angela Li, Angie Rodday, and Hong Chang at Tufts-New England Medical Center for analytic support. This study was funded by the Commonwealth Fund and the William Randolph Hearst Foundation. Dr. Sequist has served as a consultant on the Aetna External Advisory Committee for Racial and Ethnic Equality. Dr. Sequist had full access to all the data in the study and takes responsibility for the integrity and the accuracy of the data analysis.
Conflict of Interest None disclosed.
Footnotes
Acknowledgements: This study was funded by the Commonwealth Fund and the William Randolph Hearst Foundation. Dr. Sequist had full access to all the data in the study and takes responsibility for the integrity and the accuracy of the data analysis. This work was presented in abstract form at the 2007 Society for General Internal Medicine Annual Conference. We would like to thank Massachusetts Health Quality Partners for allowing us use of statewide data for these analyses; Jo-Anne Foley at HVMA for helping to obtain physician-level data; and Angela Li, Angie Rodday, and Hong Chang at Tufts-New England Medical Center for analytic support. Dr. Sequist has served as a consultant on the Aetna External Advisory Committee for Racial and Ethnic Equality.
REFERENCES
- 1.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. [DOI] [PubMed]
- 2.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348:2218–27. [DOI] [PubMed]
- 3.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 2005;353:692–700. [DOI] [PubMed]
- 4.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998–1999 to 2000–2001. JAMA. 2003;289:305–12. [DOI] [PubMed]
- 5.Marshall MN, Shekelle PG, Leatherman S, Brook RH. The public release of performance data: what do we expect to gain? A review of the evidence. Jama. 2000;283:1866–74. [DOI] [PubMed]
- 6.Massachusetts Health Quality Partners. Quality Reports. Available at: http://www.mhqp.org/quality/whatisquality.asp?nav = 030000. Accessed August 5, 2008.
- 7.Fung CH, Lim YW, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148:111–23. [DOI] [PubMed]
- 8.Rosenthal MB, Landon BE, Normand SL, Frank RG, Epstein AM. Pay for performance in commercial HMOs. N Engl J Med. 2006;355:1895–902. [DOI] [PubMed]
- 9.Doran T, Fullwood C, Gravelle H, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med. 2006;355:375–84. [DOI] [PubMed]
- 10.Rosenthal MB, Frank RG, Li Z, Epstein AM. Early experience with pay-for-performance: from concept to practice. Jama. 2005;294:1788–93. [DOI] [PubMed]
- 11.Institute of Medicine. Primary Care: America’s Health in a New Era. Washington, DC: National Academy Press; 1996.
- 12.Safran DG, Karp M, Coltin K, et al. Measuring patients’ experiences with individual primary care physicians. Results of a statewide demonstration project. J Gen Intern Med. 2006;21:13–21. [DOI] [PMC free article] [PubMed]
- 13.Audet AM, Davis K, Schoenbaum SC. Adoption of patient-centered care practices by physicians: results from a national survey. Arch Intern Med. 2006;166:754–9. [DOI] [PubMed]
- 14.Bergeson SC, Dean JD. A systems approach to patient-centered care. Jama. 2006;296:2848–51. [DOI] [PubMed]
- 15.Hasnain-Wynia R. Is evidence-based medicine patient-centered and is patient-centered care evidence-based? Health Serv Res. 2006;41:1–8. [DOI] [PMC free article] [PubMed]
- 16..Friedberg MW, Coltin KL, Pearson SD, et al. Does Affiliation of Physician Groups with One Another Produce Higher Quality Primary Care? J Gen Intern Med. 2007. [DOI] [PMC free article] [PubMed]
- 17.Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York: John Wiley; 1978.
- 18.Barton MB, Dayhoff DA, Soumerai SB, Rosenbach ML, Fletcher RH. Measuring access to effective care among elderly medicare enrollees in managed and Fee-for-Service care: a retrospective cohort study. BMC Health Serv Res. 2001;1:11. [DOI] [PMC free article] [PubMed]
- 19.Pereira AG, Kleinman KP, Pearson SD. Leaving the practice: effects of primary care physician departure on patient care. Arch Intern Med. 2003;163:2733–6. [DOI] [PubMed]
- 20.Sequist TD, Marshall R, Lampert S, Buechler EJ, Lee TH. Missed opportunities in the primary care management of early acute ischemic heart disease. Arch Intern Med. 2006;166:2237–43. [DOI] [PubMed]
- 21.Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med. 2006;166:675–81. [DOI] [PubMed]
- 22.Sequist TD, Fitzmaurice GM, Marshall R, Shaykevich S, Safran DG, Ayanian JZ. Physician performance and racial disparities in diabetes mellitus care. Arch Intern Med. 2008;168:1145–51. [DOI] [PubMed]
- 23.National Center for Quality Assurance. HEDIS 2005 Volume 1: Narrative - What’s in it and why it matters.; 2004.
- 24.Donabedian A. The definition of quality and approaches to its assessment. Ann Arbor: Health Administration Press; 1980.
- 25.Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw Hilll; 1994.
- 26.Davis K, Schoenbaum SC, Audet AM. A 2020 vision of patient-centered primary care. J Gen Intern Med. 2005;20:953–7. [DOI] [PMC free article] [PubMed]
- 27.Safran DG. Defining the future of primary care: what can we learn from patients? Ann Intern Med. 2003;138:248–55. [DOI] [PubMed]
- 28.Schneider EC, Zaslavsky AM, Landon BE, Lied TR, Sheingold S, Cleary PD. National quality monitoring of Medicare health plans: the relationship between enrollees’ reports and the quality of clinical care. Med Care. 2001;39:1313–25. [DOI] [PubMed]
- 29.Fung CH, Elliott MN, Hays RD, et al. Patients’ preferences for technical versus interpersonal quality when selecting a primary care physician. Health Serv Res. 2005;40:957–77. [DOI] [PMC free article] [PubMed]
- 30.Montgomery JE, Irish JT, Wilson IB, et al. Primary care experiences of medicare beneficiaries, 1998 to 2000. J Gen Intern Med. 2004;19:991–8. [DOI] [PMC free article] [PubMed]
- 31.Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431–9. [DOI] [PubMed]
- 32.Kahn KL, Tisnado DM, Adams JL, et al. Does ambulatory process of care predict health-related quality of life outcomes for patients with chronic disease? Health Serv Res. 2007;42:63–83. [DOI] [PMC free article] [PubMed]
- 33.Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47:213–20. [PubMed]
- 34.DiMatteo MR. Enhancing patient adherence to medical recommendations. Jama. 1994;271:79–83. [DOI] [PubMed]
- 35.DiMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: results from the Medical Outcomes Study. Health Psychol. 1993;12:93–102. [DOI] [PubMed]
- 36.Werner RM, Bradlow ET. Relationship between Medicare’s hospital compare performance measures and mortality rates. Jama. 2006;296:2694–702. [DOI] [PubMed]


