Abstract
Objective
To test the hypothesis that an acute increase in plasma homocysteine produced by methionine is associated with an acute increase in pulse wave velocity.
Design
A double blind, cross over, placebo controlled design was used and pulse wave velocity, plasma homocysteine, total cholesterol: high density lipoprotein ratio, plasma triglyceride, oxidised low density lipoprotein cholesterol concentrations, apolipoproteins A1 and B, and C reactive protein were measured between 12.5 and 20 hours after methionine loading or placebo.
Results
Between 12.5 and 20 hours after exposure to a methionine loading test, arterial pulse wave velocity showed no significant difference compared with placebo. At 12 hours after exposure to the methionine loading test, in the presence of a controlled diet, triglyceride concentration significantly increased by 32.6% (p<0.02), cholesterol: high density lipoprotein ratio increased significantly by 22.5% (p<0.05) compared with placebo. Simultaneously, systolic blood pressure increased significantly by 4.9% (p<0.02).
Conclusion
In elderly volunteers, acute hyperhomocysteinaemia induced by methionine loading resulted in no overall significant delayed reduction in peripheral arterial distensibility. A significant deterioration in the lipid profile and increased blood pressure was seen during acute hyperhomocysteinaemia.
Keywords: blood pressure, cardiovascular risk, cholesterol:HDL ratio, lipid profile, methionine
The significant association of homocysteine with cardiovascular morbidity and mortality in middle age has been known for many years.1 Hyperhomocysteinaemia is also a risk factor for elderly patients2 among whom it is prevalent.3 Cardiovascular morbidity increases with mildly increased homocysteine regardless of age and there may be no lower threshold defining risk.2 Numerous underlying fundamental pathophysiological processes associating increased homocysteine concentrations with vascular dysfunction and increased cardiovascular risk have been proposed. These interlink the aetiologies of thrombosis, atherosclerosis, and hypertension.1,4 In particular, physiological increments in plasma homocysteine induce endothelial dysfunction in healthy young volunteers5,6 and promote vascular smooth muscle hypertrophy through generation of free radicals and decreased nitric oxide synthesis.7,8
Plasma homocysteine concentration temporarily increases during the hours after oral methionine loading.9 Methionine is an essential amino acid and is the only dietary source of homocysteine, to which it is metabolised. An acute reduction in arterial compliance and distensibility after methionine loading has been found in young adults9 although the response of elderly vessels is as yet unknown. Age related vascular processes may determine the mechanisms of homocysteine related pathophysiology and hence potential age specific interventions. Thus, understanding how transient hyperhomocysteinaemia causes arterial dysfunction may lead to new approaches to reducing cardiovascular risk in elderly people in whom hyperhomocysteinaemia is not only prevalent, but can be easily corrected.
The aim of this study was to test the hypothesis that acute increases in plasma homocysteine produced by methionine are associated with an acute decrease in arterial wall compliance resulting in increased pulse wave velocity (PWV) in healthy elderly volunteers.
Method
Healthy volunteers over the age of 60 years were selected from an elderly volunteer database. Having obtained approval of the local ethics committee and written informed consent from volunteers, cardiovascular risk was assessed by questionnaire, examination of medical records, physical examination and investigations including an electrocardiogram and baseline blood tests: creatinine, homocysteine, methionine, total cholesterol, total cholesterol:high density lipoprotein (HDL) ratio, folate, B12, full blood count, and the methylenetetrahydrofolate reductase (MTHFR) genotype. The use of antioxidant vitamin supplements (containing vitamins that included A, C, E, B12, or folate) was recorded. None of the volunteers was taking prescribed drugs.
Exclusion criteria included the presence of common cardiovascular risk factors: hypertension, diabetes mellitus, current smoking or history of smoking for more than 10 years, hypercholesterolaemia (total cholesterol>6.5 mmol/l), history of stroke, myocardial infarction, or ischaemic heart disease. Other exclusion criteria were factors that might influence homocysteine concentration or change its cardiovascular effects including: renal impairment (plasma creatinine>110 μmol/l), B12 less than 150 μg/l, or folate less than 2.1 μg/l.
Volunteers were then randomised into two groups: treatment and placebo. One group received the first methionine loading test dose 12.5 hours before (PWV measurement and then the second dose eight hours later (4.5 hours before the first PWV measurement). The standard oral methionine loading test doses were used (0.1 g/kg body weight) provided as a ready mixed flavoured drink making placebo and treatment difficult to distinguish. The other group received a placebo at similar times.
All times of measurements described are relative to the time of the first methionine loading test. Twelve and a half hours after the first methionine loading dose (or placebo), peripheral arterial PWV was recorded. Non‐invasive tonometers were placed over the left common carotid and the left radial arteries simultaneously to measure the timing of the arterial pulse wave from which PWV was calculated using a Complier. This technique for measuring PWV has been validated.10 Measurements were repeated at 1.5 hour intervals from 12.5 until 20 hours after the first methionine dose (or placebo) had been taken as shown in table 1.
Table 1 Protocol.
| Time/hours | 0 | 8 | 12.5 | 14 | 15.5 | 17 | 18.5 | 20 |
|---|---|---|---|---|---|---|---|---|
| Methionine 1 OR Placebo | Methionine 2 OR placebo | PWV, plasma homocysteine, oxidised LDL | PWV | PWV | PWV | PWV | PWV, plasma homocysteine, oxidised LDL |
At 12.5 and 20 hours after the first methionine dose, plasma homocysteine, C reactive protein (CRP), total cholesterol, HDL, low density lipoprotein (LDL), triglyceride, and apolipoprotein (Apo) A1 and B concentrations were also measured. Total cholesterol: HDL ratios were calculated. All subjects were asked to avoid a protein or meat rich diet from 12 hours before PWV measurements until measurements were complete, to standardise methionine intake. After an interval of two weeks, volunteers received the other treatment.
Changes in PWV, homocysteine concentration, oxidised LDL, lipoprotein fractions, CRP before and after each treatment were tested for statistical significance using Student's paired t test.
Results
Twenty volunteers completed the study. The mean (SD) age of volunteers was 74.0 (7.1) years. All patients had a normal plasma creatinine. The mean (SD) Framingham 10 year risk point score was 16.0 (2.3)% (range 14 to 20). The mean (SD) total cholesterol: HDL ratio was 4.38 (0.89) (range 2.9 to 5.9). Five volunteers were allowed to continue taking multivitamin supplement tablets that contained antioxidant vitamins. The accuracy and reproducibility of PWV measurement was assessed by calculations of coefficient of variability (3.7%) and intraclass correlation coefficient (0.95).
Pulse wave velocity
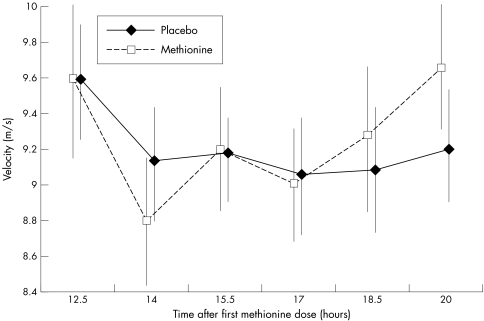
Over the whole period from 12.5 to 20 hours after exposure to the first methionine loading test, arterial PWV showed no significant differences compared with placebo.
PWV was significantly faster, by 7.2%, after 20 hours of exposure to increased homocysteine compared with 17 hours (mean PWV 9.67 (1.57) m/s v 9.02 (1.44) m/s, p<0.02). Mean PWV at 20 hours was faster after methionine than placebo (fig 1). At 12 hours after methionine, systolic and mean arterial blood pressures were significantly increased compared with placebo (155.4 v 148.2 mm Hg, p<0.02 and 117.9 v 112.8, p<0.02 respectively).
Figure 1 Arterial pulse wave velocity after methionine loading at 12.5 and 20 hours in 20 healthy volunteers over 60 years.
Sixteen subjects were analysed for MTHFR genotype; three were TT homozygous for the common T allele mutation, five CT heterozygotes, and eight CC homozygotes. There were no significant associations between genotype and either homocysteine concentrations or PWV.
Homocysteine and methionine
Twelve and a half hours after the first dose (4.5 hours after a second) standard oral methionine loading dose there was a significant, fourfold increase in mean plasma homocysteine compared with placebo (means (SD) 48.6 (11.8) μmol/l v 11.7 (4.4) μmol/l, p<0.001). Mean plasma homocysteine was still significantly increased at 20 hours after the first methionine dose (50.3 μmol/l v 12.0 (4.2) μmol/l p<0.001). There was a significant 35‐fold increase in methionine at 12.5 hours after oral methionine compared with placebo (mean 1111 (340) μmol/l v 31.5 (10.5) μmol/l, p<0.001). Mean plasma methionine still showed a 20‐fold increase at 20 hours after oral methionine compared with placebo (740 (353) μmol/l v 36.5 (10.5) μmol/l, p<0.001).
Lipid profile
At 12.5 hours after methionine loading, the total cholesterol: HDL ratio increased significantly by 22.5% (4.69 (1.12) v 3.83 (0.83), p<0.05) compared with placebo. Triglyceride concentration increased significantly by 32.6% (p<0.02), 12.5 hours after methionine in comparison with placebo (1.18 (0.39) v 0.89 (0.19) mmol/l). Neither Apo A1 nor B showed any significant change compared with placebo at either 12.5 or 20 hours after methionine.
Inflammation and oxidation
CRP showed no significant differences at 12.5 or 20 hours after methionine compared with placebo (4.57 (9.60) v 4.6 (9.56) and 4.74 (11.0) v 4.95 (10.85) respectively).
Oxidised LDL showed no statistically significant changes in response to methionine loading compared with placebo.
Discussion
Hyperhomocysteinaemia induced by methionine loading caused no acute change in peripheral arterial compliance measured by PWV in elderly volunteers. Despite a double methionine loading dose and maintainance of hyperhomocysteinaemia throughout, effects were not seen in elderly volunteers 12.5 to 20 hours after the first methionine dose (4.5 to 12 hours after the second dose). Therefore, it is possible to conclude that acute hyperhomocysteinaemia does not decrease arterial distensiblity during this observation period.
There are no directly comparable studies as previous investigations have either assessed young healthy volunteers during different time intervals or have used forearm blood flow to detect changes in arterial reactivity. One PWV study described a significant response in young volunteers (at four to five hours),9 while another reported no significant change in PWV.11 Attenuation of the response in elderly subjects that had been reported in young volunteers9 may have been attributable to atherosclerosis (or some other processes related to aging) that is likely to have been present even though none of the elderly subjects showed any clinical atherosclerotic manifestation.
In contrast with those measuring PWV, the overwhelming majority of previous studies that measured dynamic arterial reactivity by forearm blood flow provided evidence for endothelial dysfunction after methionine loading,6,12,13,14,15 although crucially, only one of these studied the elderly.6 The latter showed significant acute endothelial dysfunction in response to acute hyperhomocysteinaemia.
There may be a number of explanations for the lack of significant response to acute hyperhomocysteinaemia. Firstly, the peak increase in PWV may have occurred later as homocysteine concentrations were still increasing at 20 hours after methionine; a time when some of the late vascular in vivo responses to acute hyperhomocysteinaemia have been seen.5,15 Secondly, five volunteers were taking daily vitamin supplements that may have protected against homocysteine induced endothelial dysfunction.1,5,13,15 However, when those taking vitamins were excluded, there was still no significant response to hyperhomocysteinaemia. Conversely, the mean increase in PWV was very slightly but non‐significantly greater after methionine in those subjects taking multivitamins compared with those taking no vitamin supplements. However, as there were only five volunteers taking multivitamins, the power of the comparison did not permit any conclusion about the effects of daily multivitamins to be made.
Key points box
In older volunteers, acute hyperhomocysteinaemia changed vascular risk profile by significantly increasing cholesterol:HDL ratio, triglyceride concentration, and systolic blood pressure
Acute hyperhomocysteinaemia resulted in no overall significant delayed reduction in peripheral arterial distensibility in elderly volunteers
There was a significantly greater increase in PWV between 14 and 20 hours compared with placebo and a significantly faster PWV at 20 compared with 14 hours after methionine. However, this was not considered sufficient evidence to conclude that there was a trend of increasing effect of methionine loading between 14 and 20 hours after administration. These findings may be related to the inexplicable decreases in PWV at 14 hours compared not only with 20 hours but also compared with 12 hours.
A possible explanation for the absence of an early response in this study, in contrast with the early acute response reported by others9 was that measurements were begun immediately after the arrival of volunteers. The cardiovascular systems of volunteers may have been stimulated by initial anxiety or exercise on arrival reflected in the increased initial PWV in both placebo and methionine groups. Indeed, any physiological factor resulting from the study method should have affected both placebo and post‐methionine groups equally. Thus the significantly greater increase in PWV between 14 and 20 hours after methionine compared with placebo may reflect a possible late decrease in arterial distensibility.
Acute hyperhomocysteinaema after methionine loading was associated with significant changes in other determinants of cardiovascular risk. In particular, acute increases in both triglyceride concentrations and total cholesterol:HDL ratio were seen. Hypertriglyceridaemia17 and hyperhomocysteinaemia both cause oxidative stress and endothelial dysfunction; a potential common pathway for promotion of atherosclerosis. The significant acute increases in systolic blood pressure in the elderly were consistent with the findings of previous studies in young volunteers.15
In conclusion, in elderly volunteers, certain vascular risk factors, the presence of which over time have previously been shown to be associated with an increase in cardiovascular event rate are modified in a negative fashion during the first 20 hours of exposure to hyperhomocysteinaemia. However, there was no significant direct change in arterial distensiblity.
Acknowledgements
We wish to thank Dr Roy Sherwood, Dr A Wierzbicki, A Austin, and E Ouldred for their assistance.
Abbreviations
PWV - pulse wave velocity
HDL - high density lipoprotein
LDL - low density lipoprotein
CRP - C reactive protein
Apo - apolipoprotein
Footnotes
Funding: this project was supported by a single grant (a British Geriatric Society Startup Grant).
Conflicts of interest: none.
References
- 1.Haynes W G. Homocysteine and atherosclerosis: potential mechanisms and clinical implications. Proc R Coll Physicians Edinb 200030114–122. [Google Scholar]
- 2.Still R, McDowell I. Clinical implications of homocysteine measurement in cardiovascular disease. J Clin Pathol 199851183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart S R, Iversen A. Plasma homocysteine: Implications for setting a reference range Age and Aging Supplement 20013031 [Google Scholar]
- 4.Mangoni A, Jackson S. Homocysteine and cardiovascular disease: current evidence and future prospects. Am J Med 2002112556–565. [DOI] [PubMed] [Google Scholar]
- 5.Chambers J, Obeid O, Kooner J. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arterioscler Thromb Vasc Biol 199919)2922–2927. [DOI] [PubMed] [Google Scholar]
- 6.Takawol A, Omland T, Gerhard M.et al Hyperhomocysteinaemia is associated with impaired endothelium dependent vasodilatation in humans. Circulation 1997951119–1121. [DOI] [PubMed] [Google Scholar]
- 7.Tsai X, Perrella M A, Yoshizumi M.et al Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci U S A 1994916369–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 1996985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcaro G, Fava R, Dagradi P.et al Acute hyperhomocysteinaemia induces a reduction in arterial distensibility and compliance. J Hypertens 200018198. [DOI] [PubMed] [Google Scholar]
- 10.Asmar R, Benetos A, Topouchian J.et al Assessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studies. Hypertension 199526485–490. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson I B, Megson I L, MacCallum H.et al Acute methionine loading does not alter arterial stiffness in humans. J Cardiovasc Pharmacol 2001371–5. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy M F, McDowell I F, Ramsey M W.et al Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation 1998981848–1852. [DOI] [PubMed] [Google Scholar]
- 13.Chambers J C, McGregor A, Jean‐Marie J.et al Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation 1999991156–1160. [DOI] [PubMed] [Google Scholar]
- 14.Kanani P M, Sinkey C A, Browning R L.et al Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 19991001161–1168. [DOI] [PubMed] [Google Scholar]
- 15.Nappo F, De Rosa N, Marfella R.et al Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA 19992812113–2118. [DOI] [PubMed] [Google Scholar]
- 16.Kanani P M, Sinkey C A, Browning R L.et al Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 19991001161–1168. [DOI] [PubMed] [Google Scholar]
- 17.Ceriello A, Taboga C, Tonutti L.et al Evidence for an independent and cumulative effect of postprandial hypertriglyceridaemia on endothelial dysfunction and oxidative stress generation. Circulation 20021061211. [DOI] [PubMed] [Google Scholar]