Abstract
Controlled clinical trials are unusual in surgery, rare in urology, and almost non‐existent as far as the management of urethral stricture is concerned. What data there are come largely from so called “expert opinion” and the quality of this is variable. None the less, the number of so called experts, past and present, is comparatively small and in broad principle their views more or less coincide. Although this review is therefore inevitably biased, it is unlikely that expert opinion will take issue with most of the general points raised here.
Keywords: urethral stricture
Urethral strictures have always been common. We know something about how the ancient Egyptians treated stricture disease 4000 years ago and other civilisations since and indeed not much has changed until about 50 years ago.
Urethral strictures are still common now. Hospital Episode Statistics in the UK and similar data from the USA suggest that men are affected with an increasing incidence from about 1 in every 10 000 men aged 25 to about 1 in every 1000 men ages 65 or more. Traditionally, strictures in the past were caused by gonococcal urethritis. These days this is rare in the developed world although still common in some societies. In the UK, aetiology is almost equally divided between “inflammation”, trauma, and idiopathic. The inflammation category includes non‐specific urethritis and, increasingly commonly, lichen sclerosus, formerly known as balanitis xerotica obliterans (BXO). Trauma is more usually iatrogenic and attributable to instrumentation these days but also includes the more dramatic fall astride injuries to the bulbar urethra and pelvic fracture related injuries to the membranous urethra and bulbo‐membranous junction. Many idiopathic strictures are “idiopathic” because their cause is lost in the mists of time but many of these occur at the junction of the proximal and middle sections of the bulbar urethra in adolescents and young adults with no previous history and many believe this represents a congenital anomaly. Among the established congenital anomalies of the lower urogenital tract, hypospadias is one of the more common and although this is not itself associated with stricture, a stricture is an occasional consequence of hypospadias surgery in early childhood and an increasingly common cause of strictures in the trauma category.
The pathology of urethral stricture disease is poorly understood. External trauma generally causes partial or complete disruption of an otherwise normal urethra—that is clear. How a stricture develops in other circumstances remains unclear but it would seem that for whatever reason a scar develops as a consequence of changes in the structure and function of the urethral epithelium and the sub‐epithelial spongy tissue causing a fibrotic narrowing of the urethra.1 Secondary changes in the epithelium more proximally develop there afterwards causing a progressive stricturing of an increasing length of the urethra from before backwards. Longstanding urethral obstruction may cause secondary complications in the rest of the urinary tract.2
Clinical assessment and investigation
Trauma aside, most patients present with slow and progressive deterioration of the urinary stream leading to a feeling of incomplete emptying as obstruction progressively develops. More severe cases may be complicated by haematuria or recurrent urinary infection and its sequalae.
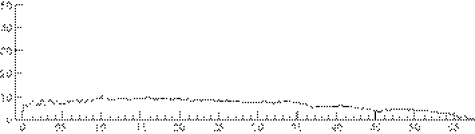
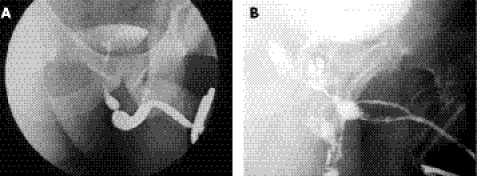
It is usual practice for patients with lower urinary tract symptoms to have their urinary flow rate measured as part of initial investigation. In those who have a urethral stricture the peak flow rate is typically low but the flow pattern is characteristically flat (fig 1). This flow pattern is almost pathognomonic of a urethral stricture; certainly it should lead to a urethrogram as the next step in investigation. If properly performed, this will show the exact site and length of the stricture and most of its potential complications (fig 2). Ultrasound evaluation may show a thickening of the bladder wall associated with longstanding outflow obstruction, and a presence of residual urine. If these two signs are present, an ultrasound scan of the kidneys should be the next step to look for signs of obstructive uropathy and in the presence of hydroureteronephrosis, there should be an estimate of renal function.
Figure 1 Flow pattern of a patient with a urethral stricture.
Figure 2 An uncomplicated (A) and a somewhat more complicated stricture (B).
Treatment
The normal urinary flow rate in young and middle aged men is generally greater than 15 ml/second and the flow pattern a bell shaped curve. Patients with an established diagnosis of urethral stricture but who have a flow rate greater than 10 ml/second and normal bladder thickness, complete bladder emptying and who are untroubled by recurrent urinary infection do not necessarily require any treatment but they should be kept under review. A flow rate less than 10 ml/second is more commonly associated with symptoms and with the secondary effects such as recurrent haematuria or recurrent urinary tract infection and with features of overt bladder obstruction on ultrasonography, but this is not necessarily so. If the stricture is troublesome it should be treated; if not the patient should be kept under review. With a flow rate of less than 5 ml/second, abnormalities such as those listed above are much more likely and the patient is potentially at risk of acute retention, although this is a lot less common than one would expect from the severity of the narrowing of the urethra that is seen in such a situation. In these patients treatment is advisable even if symptoms of voiding difficulty are not troublesome.
The time honoured method of treatment is urethral dilatation—the passage of calibrated instruments—usually made of metal and called “sounds”, Bougies or, more simply, urethral dilators. They are generally straight through most of their length with a hockey stick curve at the end and are calibrated according to the French system, which relates the size of the urethral dilator to the urethral circumference in millimetres. The normal urethral calibre is 24–26F (French) at the external urinary meatus, a little wider in the penile urethra, wider still in the bulbar urethra (about 36F), and then narrower again in the posterior urethra, above the level of the perineal membrane.
Some patients with urethral stricture have the diagnosis made on endoscopic evaluation of the lower urinary tract.
The principle of urethral dilatation is to stretch the urethral stricture up or otherwise, and more commonly, to disrupt it. A graduated series of dilators from smaller to larger size is passed to restore the urethral calibre to normal, or thereabouts. The alternative form of instrumentation, also with a history measured in thousands of years, is urethrotomy. Initially, this was performed blindly by increasingly sophisticated means until visual internal urethrotomy was developed about 35 years ago. Visual control of the process might be expected to give better results but in fact, there is no evidence that this is so. As a general rule however urethral dilatation might best be reserved for more straightforward strictures that can be dilated in an office setting using topical anaesthesia whereas visual internal urethrotomy is probably better for more complicated strictures, performed in a day case operating theatre under regional or general anaesthesia. Both procedures are generally regarded as simple and straightforward but in fact carry a significant risk of complications.3
It is now generally accepted that urethrotomy and dilatation are equally effective and can be expected to cure about 50% of short bulbar urethral strictures when first used. If the procedure has to be repeated, it is rarely curative and it is rarely curative even the first time in strictures other than in the bulbar urethra.4,5,6
When the stricture recurs, it usually does so within weeks or months and almost always within two years. Those that recur comparatively infrequently might be palliated (rather than cured) by repeat instrumentation and, as long as this is agreeable to the patient and is uncomplicated by bleeding or sepsis,3 such palliation might be perfectly acceptable. If instrumentation is required more frequently or is complicated or unacceptable to the patient in the long term, then urethroplasty is the only curative option.
Alternatives have been recommended, such as the use of a laser rather than a cold knife for internal urethrotomy; an indwelling urethral stent to hold the urethra open; or much more commonly, the use of clean intermittent self catheterisation.7 None of these, however, are curative; the laser is expensive as well as ineffective; stents, although popular a decade ago, commonly do more harm than good8,9,10; and only self catheterisation is occasionally helpful: for the patient who is not fit for, or is otherwise unwilling to undergo, a urethroplasty.
General principles of urethroplasty11,12,13
Ideally, one would like to excise a urethral stricture and join the two healthy ends of urethra on either side together again to restore continuity and calibre. Unfortunately, this can only be done with short urethral strictures because of limitations of elasticity and length before erectile function is compromised. Excision and end to end anastomosis, or an anastomotic urethroplasty as it is more commonly called, is however best, even if it can only be used for short strictures of the bulbar urethra and more proximally, in the membranous urethra, because it gives the best and most sustained results in terms of both a low re‐stricture rate and a low complication rate. Whether this high success rate is attributable to the technique itself or because those strictures that are amenable to an anastomotic urethroplasty generally occur in younger patients with a more localised urethral problem (such as trauma) is not clear.
When a stricture is too long for anastomotic urethroplasty the alternative is some form of substitution urethroplasty. Here, there are two alternative approaches. The first is to excise the urethral stricture and perform a circumferential repair of the urethra. Intuitively, this would seem to be a sensible approach but in practice, a circumferential repair needs to be staged to give satisfactory results or, if performed in one stage, tends to give less satisfactory results than the alternative approach of incising the stricture and then putting on a patch to restore urethral calibre at that point (stricturotomy and patch).
Anastomotic urethroplasty
Anastomotic urethroplasty is generally only applicable in two situations. Firstly, for short strictures of the bulbar urethra, no more than 1–2 cm long, which are generally attributable to external trauma or are otherwise idiopathic in origin (possibly congenital). The external trauma is usually a fall astride injury. The second situation is a pelvic fracture related injury of the membranous urethra or bulbo‐membranous junction.
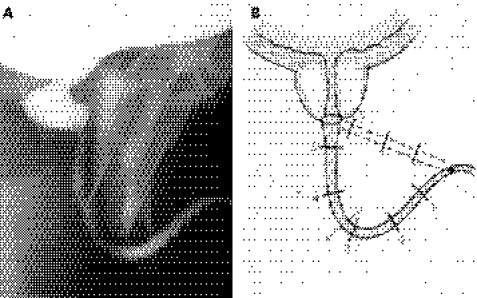
The general principles here are to mobilise the bulbar urethra fully, capitalising on its elasticity to overcome any defect in length as a result of excision of a short stricture or to bridge a gap caused by complete disruption because of external trauma. Should the elasticity of the urethra and mobilisation alone be insufficient to bridge any defect then the second general principle is to straighten out the natural curve of the bulbar urethra by a sequential series of manoeuvres including separation of the crura at the base of the penis, wedge pubectomy of the inferior pubic arch, and, if necessary, re‐routing of the urethra around the shaft of the shaft of the penis until the course of the bulbar urethra from the penoscrotal junction to the apex of the prostate is a straight line rather than the semi‐circle that it normally is (fig 3).14,15,16
Figure 3 Illustrates how straightening out the natural curve of the bulbar urethra allows the surgeon to bridge a considerable defect in urethral length. The numbers are distances in centimetres from the penobulbar junction to the apex of the prostate at usual radiographic magnifications.
Substitution urethroplasty
Unlike anastomotic urethroplasty this can be used anywhere in the urethra. When an anastomotic repair is possible and appropriate this should be used in preference because it gives better and more durable results. Substitution urethroplasty should not therefore be used universally; it should be used selectively for those strictures of the bulbar urethra that are too long for an anastomnotic repair and all strictures of the penile urethra in which an anastomotic repair would cause unacceptable penile deformity on erection.
As a stricturotomy and patch is generally more successful than an excision and circumferential repair then this is the procedure of choice unless a urethral segment positively needs to be excised as after previous surgery for hypospadias when the urethra is irretrievably scarred, in lichen sclerosus when it is irretrievably fibrotic, or in rare situations such as with urethral arteriovenous malformations or tumours.17
What sort of a patch should be used?
It might seem intuitive that a skin flap, which brings with it its own blood supply, would give better results than a free graft, which has to develop its own blood supply having been raised from a remote site. This is not however the case in most circumstances.18,19 Indeed, for most strictures the results are just as good with a graft as with a flap as long as the graft is a thin, full thickness graft with a dense sub‐dermal plexus such as full thickness graft of penile shaft skin or foreskin, a post‐auricular (from behind the ear) full thickness graft, or a buccal mucosal graft (from inside of the cheek). Buccal mucosa is currently the preferred graft material, if only because it has the least donor site morbidity.20,21,22
Occasionally a flap must be used when the local conditions are not favourable for a graft as when there is extensive scarring from previous surgery or active infection or previous radiotherapy or when the stricture is unusually long or trans‐sphincteric. In such cases a flap of local genital skin pedicled on the dartos layer of the penis (which is highly vascular) is preferred.23,24
When a segment of urethra has to be excised, usually because of dense scarring in the penile urethra for whatever reason, then a circumferential repair must be performed. It is possible to do this in one stage, particularly for short strictures in the most distal part of the penile urethra, but in other circumstances a two stage repair is safer and gives the best long term results.7 In such circumstances a graft is interposed as a flat plate between the two ends of the urethra where a segment has been excised (or between the end of the urethra and the external meatus if it is the most distal segment of the urethra that is involved). The flat plate is then rolled in to form a tube and closed in layers at a second stage three to six months later.
Summary of the general principles of urethroplasty
Urethral and visual internal urethrotomy are equally effective
50% of short bulbar strictures are cured by the very first dilatation or urethrotomy
Penile urethral strictures are rarely cured by dilatation or urethrotomy
If a patient develops a recurrent stricture after a previous urethrotomy or dilatation, however long the interval, further instrumentation is never curative
The only curative alternative is urethroplasty
Whenever a urethroplasty is necessary an anastomotic urethroplasty is preferable because it has a lower re‐stricture rate, success is maintained long term and the complication rate is lower
Substitution urethroplasty is necessary for longer bulbar strictures and all strictures of the penile urethra
For most urethral strictures requiring substitution urethroplasty a stricturotomy and patch is preferable to excision and a tube‐graft flap
Grafts and flaps are equally good in most circumstances
In the bulbar urethra grafts are quicker and easier
A dorsal stricturotomy and patch has a higher success rate and a lower complication rate than ventral stricturotomy and patch
Buccal mucosal grafting has advantages for stricturotomy and patch in the bulbar urethra
In the penile urethra a flap is quicker and easier
When a circumferential repair is required a two stage reconstruction is safer and more reliable than a one stage technique
Strictures by site and type
The urethra is typically described as having an anterior and posterior segment. The anterior segment is surrounded by corpus spongiosum and is divided into the penile (or pendulous in American terminology) and bulbar segments. The bulbar segment is the part enclosed by the bulbo‐spongiosus muscle. The posterior urethra is surrounded by the prostate and the urethral sphincter and is divided into the prostatic and membranous parts respectively. There is insufficient space here to discuss any more sophisticated anatomical sub‐division of the posterior urethra; likewise, strictures of the bladder neck and of the prostatic urethra, usually the result of surgical treatment, particularly new technology such as cryotherapy and laser therapy, which are not generally considered as urethral strictures, will not be considered here.
Posterior urethral strictures of the membranous urethra or bulbo‐membranous junction are usually the result of pelvic fracture related urethral injury and are treated by anastomotic urethroplasty in which the full range of manoeuvres referred to above may be used and that may be an extremely difficult surgical undertaking.14,15,16 Membranous urethral strictures are sometimes the result of instrumentation as in transurethral resection of the prostate in which case they are known as “sphincter strictures” because it is fibrosis within the urethral sphincter mechanism that is the primary problem. To reserve sphincter function and therefore avoid incontinence such strictures are best treated by urethral dilatation where possible.
In the anterior urethra, bulbar urethral strictures are more common than penile urethral strictures except in specialist units where penile urethral strictures, because of previous hypospadias surgery or lichen sclerosis,25 are becoming increasingly common. Short bulbar strictures as a result of trauma or otherwise congenital are best treated by anastomotic urethroplasty. If a bulbar stricture is too long for anastomotic urethroplasty, as is commonly the case, then a stricturotomy and patch procedure is performed. When anastomotic urethroplasty is possible this is usually a simple and straightforward procedure when compared with anastomotic urethroplasty of the posterior urethra. A stricturotomy and patch will usually use a buccal mucosal graft as a patch.20,21,22
In the penile urethroplasty a stricturotomy and patch is possible for simple strictures26 but in more complicated circumstances an excision and circumferential repair will be necessary in which buccal mucosa may be used unless preputial or penile shaft skin would be more appropriate. For more complication situations, a staged reconstruction will be used.17
The future of urethral stricture surgery
The results of anastomotic repair are so good and so well sustained that it seems unlikely that this will be superseded at any stage in the foreseeable future by any new development.
Most strictures requiring substitution urethroplasty also do well with modern surgical technique but if the procedure could be performed endoscopically rather than by open surgery then this would clearly be an advantage to the patient. The real problems are the more complicated strictures, which are generally longer and have already undergone previous surgery and have recurred. They are also typically in the penile urethra and associated with lichen sclerosis. Many urologists' hopes have been raised by developments in tissue engineering to provide an alternative source of material for repair of these difficult strictures. I am somewhat sceptical about these developments because I do not think it is the lack of the material that is the problem—I think it is the nature of the underlying disease. Future surgical developments, in my opinion, will be the consequence of a better understanding of the nature of the disease in such patients and from a different approach to treating the diseased but not actually strictured urethra rather than from improvement in technique alone.
Footnotes
Funding: none.
Conflicts of interest: none.
References
- 1.Chambers R, Baitera B. The anatomy of urethral stricture. BJU 197749545–551. [DOI] [PubMed] [Google Scholar]
- 2.Romero Perez P, Mira Linares A. Complications of the lower urinary tract secondary to urethral stenosis. Actas Urol Esp 199620786–793. [PubMed] [Google Scholar]
- 3.Devereux M, Burfield G. Prolonged follow‐up of urethral strictures treated by intermittent dilatation. BJU 197042231–239. [DOI] [PubMed] [Google Scholar]
- 4.Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long‐term follow up. J Urol 199615673–75. [PubMed] [Google Scholar]
- 5.Steenkamp J, Heyns C, De Kock M. Internal urethrotomy versus dilatation as treatment for male urethral strictures: a prospective, randomised comparison. J Urol 199715798–101. [PubMed] [Google Scholar]
- 6.Heyns C, Steenkamp J W, De Kock M L.et al Treatment of male urethral strictures: is repeated dilatation of internal urethrotomy useful? J Urol 1998160356–358. [DOI] [PubMed] [Google Scholar]
- 7.Harriss D, Beckingham I J, Lemberger R J.et al Long‐term results of intermittent low‐friction self‐catheterisation in patients with recurrent urethral strictures. BJU 199474790–792. [DOI] [PubMed] [Google Scholar]
- 8.Milroy E, Chapple C, Wallsten H. A new stent for the treatment of urethral strictures: preliminary report. BJU 198963392–396. [DOI] [PubMed] [Google Scholar]
- 9.Badlani G, Press S M, Defaclo A.et al Urolume endourethral prosthesis for the treatment of urethral strictures disease: long‐term results of the North American multicenter urolume trial. Urology 199545846–856. [DOI] [PubMed] [Google Scholar]
- 10.Milroy E, Allen A. Long‐term results of urolume urethral stent for recurrent urethral strictures. J Urol 1996155904–908. [PubMed] [Google Scholar]
- 11.Mundy A. Results and complications of urethroplasty and its future. BJU 199371322–325. [DOI] [PubMed] [Google Scholar]
- 12.Andrich D E, Mundy A R. Urethral strictures and their surgical treatment. BJU Int 200086571–580. [DOI] [PubMed] [Google Scholar]
- 13.Turner‐Warwick R. Urethral stricture surgery. In: Mundy A, ed. Current operative surgery—urology. London: Bailliere Tindall, 1988160–218.
- 14.Webster G, Ramon J. Repair of pelvic fracture posterior urethral defects using an elaborated perineal approach: experience with 74 cases. J Urol 1991145744–748. [DOI] [PubMed] [Google Scholar]
- 15.Koraitim M. The lessons of 145 post traumatic posterior urethral strictures treated in 17 years. J Urol 199515363–66. [DOI] [PubMed] [Google Scholar]
- 16.Mundy A. Reconstruction of posterior urethral distraction defects. Atlas Urol Clin North Am 19971651492–1495. [Google Scholar]
- 17.Greenwell T, Venn S, Mundy A. Changing practice in anterior urethroplasty. BJU Int 199883631–635. [DOI] [PubMed] [Google Scholar]
- 18.Jordan G, Devine P. Application of tissue transfer techniques to the management of urethral strictures. Semin Urol 19875219–235. [PubMed] [Google Scholar]
- 19.Wessels H, McAninch J. Current controversies in anterior urethral stricture repair: free‐graft versus pedicled skin‐flap reconstruction. World J Urol 199816175–180. [DOI] [PubMed] [Google Scholar]
- 20.Barbagli G, Selli C, di Cello V.et al A one‐stage dorsal free‐graft urethroplasty for bulbar urethral strictures. BJU 199678929–932. [DOI] [PubMed] [Google Scholar]
- 21.Iselin C, Webster G. Dorsal onlay urethroplasty for urethral stricture repair. World J Urol 199816181–185. [DOI] [PubMed] [Google Scholar]
- 22.Andrich D, Mundy A. The Barbagli procedure gives the best results for patch urethroplasty of the bulbar urethra. BJU Int 200188385–389. [DOI] [PubMed] [Google Scholar]
- 23.Mundy A. The long‐term results of skin inlay urethroplasty. J Urol 1972107977–980. [DOI] [PubMed] [Google Scholar]
- 24.Quartey J. One‐stage penile/preputial island flap urethroplasty for urethral stricture. J Urol 1985134474–487. [DOI] [PubMed] [Google Scholar]
- 25.Venn S, Mundy A. Urethroplasty for balanitis xerotica obliterans. BJU 199881735–737. [DOI] [PubMed] [Google Scholar]
- 26.Orandi A. One‐stage urethroplasty. BJU 196840717–719. [DOI] [PubMed] [Google Scholar]