Abstract
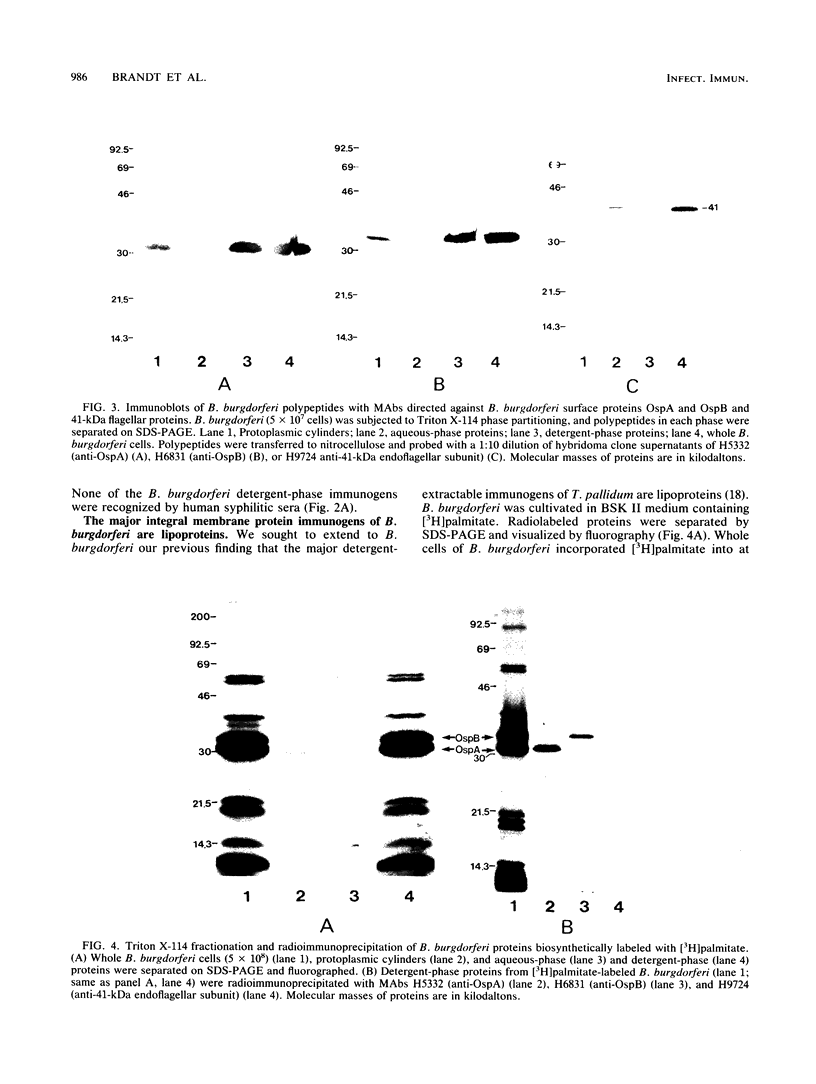
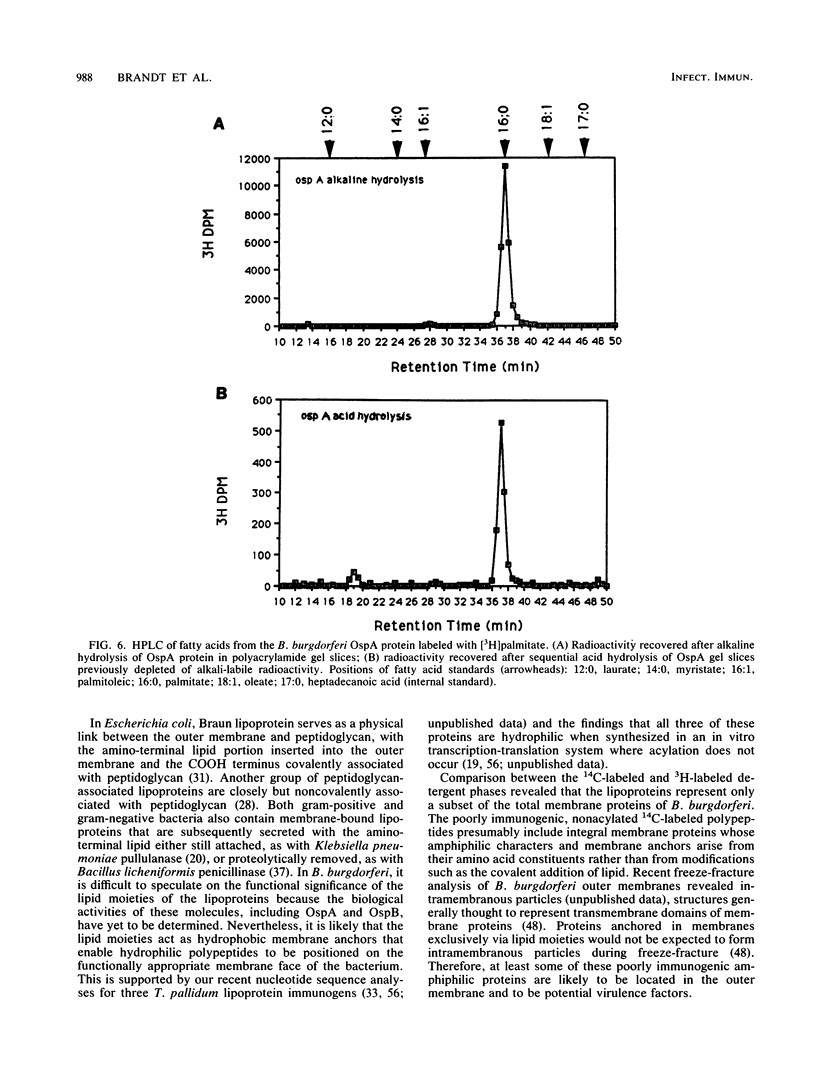
The pathogenic spirochete Borrelia burgdorferi contains a set of integral membrane proteins which were selectively extracted into the detergent phase upon solubilization of intact B. burgdorferi with the nonionic detergent Triton X-114. Virtually all of these hydrophobic proteins were recognized by antibodies in pooled sera from patients with chronic Lyme arthritis, demonstrating that proteins partitioning into the detergent phase of Triton X-114 encompass the major B. burgdorferi immunogens. Furthermore, most of these immunogenic proteins, including the previously characterized OspA and OspB membrane antigens, could be biosynthetically labeled when B. burgdorferi was incubated in vitro with [3H]palmitate. The OspA and OspB antigens were radioimmunoprecipitated from [3H]palmitate-labeled detergent-phase proteins with monoclonal antibodies, and [3H]palmitate was recovered unaltered from these proteins after sequential alkaline and acid hydrolyses. The combined results provide formal confirmation that the major B. burgdorferi immunogens extracted by Triton X-114 are lipoproteins. The demonstration that B. burgdorferi integral membrane antigens are lipoproteins may explain the basis of their immunogenicity and may help to improve our understanding of the surface topology of B. burgdorferi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Immunochemical analysis of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):581–586. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev. 1988 Oct;1(4):399–414. doi: 10.1128/cmr.1.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Golightly M. G. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J Immunol. 1988 Jan 1;140(1):265–272. [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bessler W. G., Suhr B., Bühring H. J., Muller C. P., Wiesmüller K. H., Becker G., Jung G. Specific antibodies elicited by antigen covalently linked to a synthetic adjuvant. Immunobiology. 1985 Sep;170(3):239–244. doi: 10.1016/S0171-2985(85)80095-4. [DOI] [PubMed] [Google Scholar]
- Biesert L., Scheuer W., Bessler W. G. Interaction of mitogenic bacterial lipoprotein and a synthetic analogue with mouse lymphocytes. Isolation and characterization of binding proteins. Eur J Biochem. 1987 Feb 2;162(3):651–657. doi: 10.1111/j.1432-1033.1987.tb10687.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., DeOgny L., Slaughter C., Radolf J. D., Norgard M. V. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989 Sep;57(9):2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C., Raibaud O. Structure of two divergent promoters located in front of the gene encoding pullulanase in Klebsiella pneumoniae and positively regulated by the malT product. J Bacteriol. 1985 Nov;164(2):639–645. doi: 10.1128/jb.164.2.639-645.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L., Beck G., Habicht G. S. Isolation of the outer envelope from Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):123–126. doi: 10.1016/s0176-6724(86)80112-2. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Grodzicki R. L., Shrestha M., Fischer D. K., García-Blanco M., Steere A. C. The antibody response in Lyme disease. Yale J Biol Med. 1984 Jul-Aug;57(4):561–565. [PMC free article] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Proteolipid formation in Mycoplasma capricolum. Influence of cholesterol on unsaturated fatty acid acylation of membrane proteins. J Biol Chem. 1983 Oct 10;258(19):11814–11818. [PubMed] [Google Scholar]
- Dattwyler R. J., Halperin J. J. Failure of tetracycline therapy in early Lyme disease. Arthritis Rheum. 1987 Apr;30(4):448–450. doi: 10.1002/art.1780300414. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Halperin J. J., Luft B. J., Thomas J., Golightly M. G. Specific immune responses in Lyme borreliosis. Characterization of T cell and B cell responses to Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:93–102. doi: 10.1111/j.1749-6632.1988.tb31842.x. [DOI] [PubMed] [Google Scholar]
- Deich R. A., Metcalf B. J., Finn C. W., Farley J. E., Green B. A. Cloning of genes encoding a 15,000-dalton peptidoglycan-associated outer membrane lipoprotein and an antigenically related 15,000-dalton protein from Haemophilus influenzae. J Bacteriol. 1988 Feb;170(2):489–498. doi: 10.1128/jb.170.2.489-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer M. N., Halperin J. J., Dattwyler R. J. Lyme meningoencephalitis: report of a severe, penicillin-resistant case. Arthritis Rheum. 1987 Jun;30(6):705–708. [PubMed] [Google Scholar]
- Golightly M., Thomas J., Volkman D., Dattwyler R. Modulation of natural killer cell activity by Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:103–111. doi: 10.1111/j.1749-6632.1988.tb31843.x. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Howe T. R., Mayer L. W., Barbour A. G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985 Feb 8;227(4687):645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- Hunter E. F., Russell H., Farshy C. E., Sampson J. S., Larsen S. A. Evaluation of sera from patients with Lyme disease in the fluorescent treponemal antibody-absorption test for syphilis. Sex Transm Dis. 1986 Oct-Dec;13(4):232–236. doi: 10.1097/00007435-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Sarvas M., Brammar W. J., Neugebauer K., Wu H. C. Bacillus licheniformis penicillinase synthesized in Escherichia coli contains covalently linked fatty acid and glyceride. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3506–3510. doi: 10.1073/pnas.78.6.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastavica C. C., Wilson M. L., Berardi V. P., Spielman A., Deblinger R. D. Rapid emergence of a focal epidemic of Lyme disease in coastal Massachusetts. N Engl J Med. 1989 Jan 19;320(3):133–137. doi: 10.1056/NEJM198901193200301. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz L. E., Steere A. C., Benach J. L., Slade J. D., Broome C. V. Lyme disease during pregnancy. JAMA. 1986 Jun 27;255(24):3394–3396. [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Chamberlain N. R., Swancutt M. A., Goldberg M. S. Cloning and expression of the major 47-kilodalton surface immunogen of Treponema pallidum in Escherichia coli. Infect Immun. 1986 Nov;54(2):500–506. doi: 10.1128/iai.54.2.500-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect Immun. 1988 Jul;56(7):1825–1828. doi: 10.1128/iai.56.7.1825-1828.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Cox C. D. Catabolism of glucose and fatty acids by virulent Treponema pallidum. Infect Immun. 1977 Apr;16(1):60–68. doi: 10.1128/iai.16.1.60-68.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger P. A., Duray P. H., Burke B. A., Steere A. C., Stillman M. T. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann Intern Med. 1985 Jul;103(1):67–68. doi: 10.7326/0003-4819-103-1-67. [DOI] [PubMed] [Google Scholar]
- Schmid G. P. The global distribution of Lyme disease. Yale J Biol Med. 1984 Jul-Aug;57(4):617–618. [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Bartenhagen N. H., Craft J. E., Hutchinson G. J., Newman J. H., Rahn D. W., Sigal L. H., Spieler P. N., Stenn K. S., Malawista S. E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983 Jul;99(1):76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Bartenhagen N. H., Spieler P. N., Newman J. H., Rahn D. W., Hutchinson G. J., Green J., Snydman D. R., Taylor E. The clinical spectrum and treatment of Lyme disease. Yale J Biol Med. 1984 Jul-Aug;57(4):453–461. [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Schoen R. T., Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987 Nov;107(5):725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- Swancutt M. A., Riley B. S., Radolf J. D., Norgard M. V. Molecular characterization of the pathogen-specific, 34-kilodalton membrane immunogen of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3314–3323. doi: 10.1128/iai.57.11.3314-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Twehous D. A., Norgard M. V. Monoclonal antibody selection and analysis of a recombinant DNA-derived surface immunogen of Treponema pallidum expressed in Escherichia coli. Infect Immun. 1986 Apr;52(1):110–119. doi: 10.1128/iai.52.1.110-119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]