Abstract
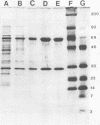
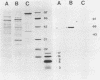
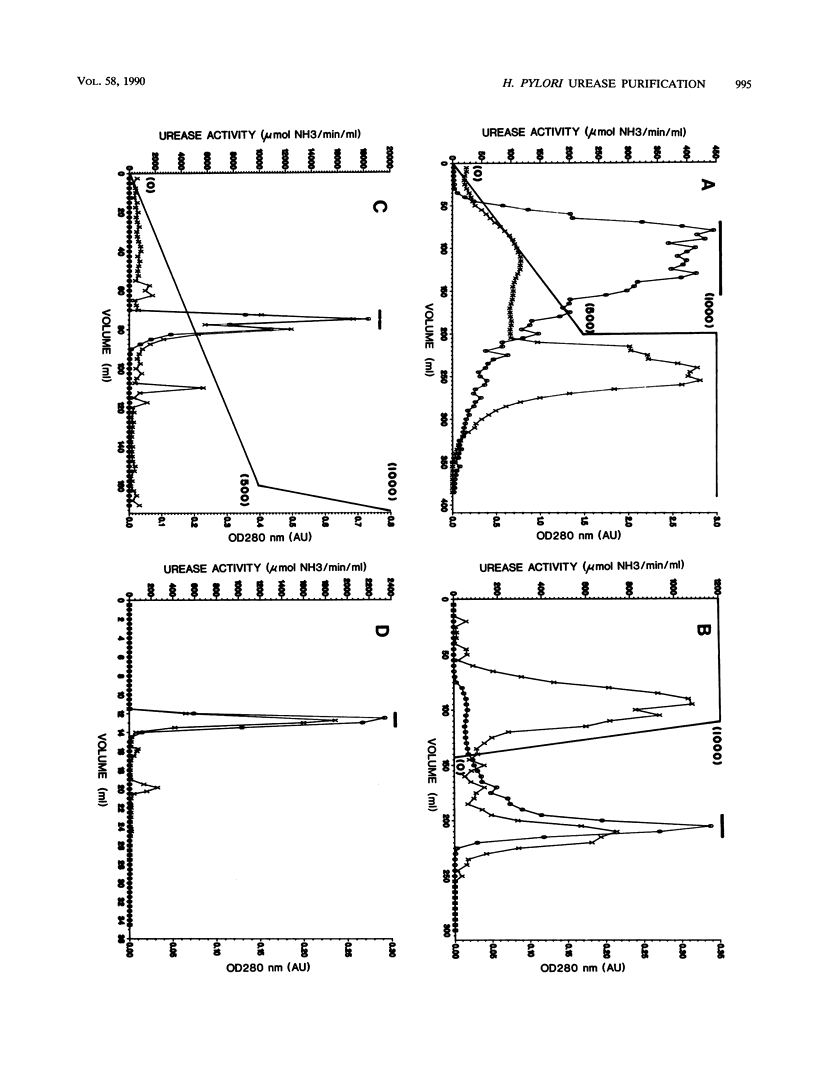
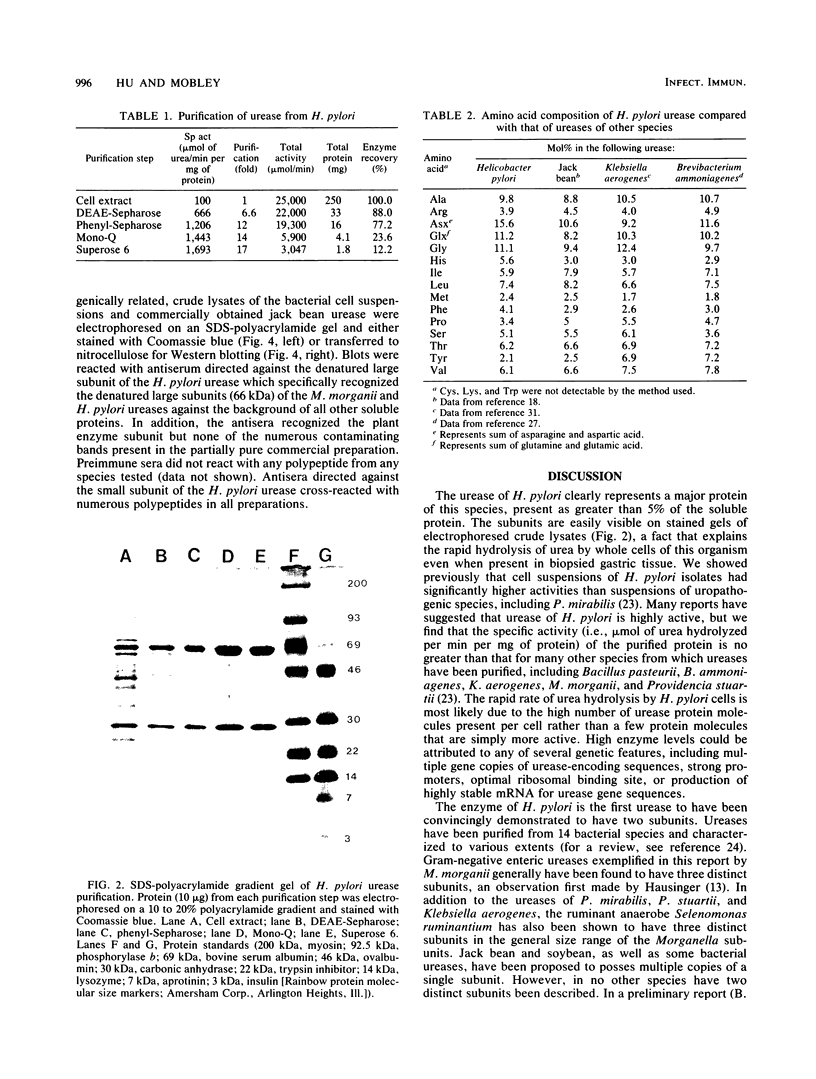
Urease of Helicobacter pylori (formerly Campylobacter pylori) is believed to represent a critical virulence determinant for this species. Ammonia generated by hydrolysis of urea may protect the acid-sensitive bacterium as it colonizes human gastric mucosa. An H. pylori strain, cultured from a gastric biopsy of a patient with complaints of abdominal pain and a history of peptic ulcer disease, was isolated on selective medium and cultured in Mueller-Hinton broth supplemented with 4% fetal calf serum. Whole cells were ruptured by French pressure cell lysis, and soluble protein was chromatographed on DEAE-Sepharose, phenyl-Sepharose, Mono-Q, and Superose 6 resins. Purified urease represented 6% of the soluble protein of crude extract, was estimated to have a native molecular size of 550 kilodaltons (kDa), and was composed of two distinct subunits of apparent molecular sizes of 66 and 29.5 kDa. On the basis of subunit size, a 1:1 subunit ratio as measured by scanning densitometry of Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels, and estimated native molecular size, the data are consistent with a stoichiometry of (29.5 kDa-66 kDa)6 for the structure of the native enzyme. Km for urea was estimated at 0.2 mM. By N-terminal analysis, the 29.5-kDa subunit of H. pylori urease was found to share significant amino acid sequence similarity with the smallest of three subunits of the Proteus mirabilis and Morganella morganii ureases, as well as to the amino terminus of the unique jack bean subunit. The 66-kDa subunit also shared up to 80% similarity with the largest of three subunits of P. mirabilis, M. morganii, and Klebsiella aerogenes ureases and to internal sequences (amino acids 271 to 285) of the jack bean urease subunit. Thus, the amino acid sequence is conserved among ureases with one, two, and three distinct subunits, suggesting a common ancestral urease gene. Also, urease subunits of M. morganii and jack bean were specifically recognized by antisera raised against the 66-kDa subunit of H. pylori urease, demonstrating that at least some antigenic determinants were conserved among ureases from different species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. K., Blakeley R. L., Zerner B. Urea and urease. Adv Inorg Biochem. 1984;6:245–283. [PubMed] [Google Scholar]
- Barer M. R., Elliott T. S., Berkeley D., Thomas J. E., Eastham E. J. Cytopathic effects of Campylobacter pylori urease. J Clin Pathol. 1988 May;41(5):597–597. doi: 10.1136/jcp.41.5.597-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. G., Correa P., Offerhaus J., Rodriguez E., Janney F., Hoffmann E., Fox J., Hunter F., Diavolitsis S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am J Clin Pathol. 1986 Nov;86(5):575–582. doi: 10.1093/ajcp/86.5.575. [DOI] [PubMed] [Google Scholar]
- Clayton C. L., Wren B. W., Mullany P., Topping A., Tabaqchali S. Molecular cloning and expression of Campylobacter pylori species-specific antigens in Escherichia coli K-12. Infect Immun. 1989 Feb;57(2):623–629. doi: 10.1128/iai.57.2.623-629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Cox J. A., Theibert J. L. Amino acid sequence of a sarcoplasmic calcium-binding protein from the sandworm Nereis diversicolor. J Biol Chem. 1988 Oct 25;263(30):15378–15385. [PubMed] [Google Scholar]
- Czinn S., Carr H., Sheffler L., Aronoff S. Serum IgG antibody to the outer membrane proteins of Campylobacter pylori in children with gastroduodenal disease. J Infect Dis. 1989 Mar;159(3):586–589. doi: 10.1093/infdis/159.3.586. [DOI] [PubMed] [Google Scholar]
- Dent J. C., McNulty C. A., Uff J. S., Gear M. W., Wilkinson S. P. Campylobacter pylori urease: a new serological test. Lancet. 1988 Apr 30;1(8592):1002–1002. doi: 10.1016/s0140-6736(88)91827-2. [DOI] [PubMed] [Google Scholar]
- Ferrero R. L., Hazell S. L., Lee A. The urease enzymes of Campylobacter pylori and a related bacterium. J Med Microbiol. 1988 Sep;27(1):33–40. doi: 10.1099/00222615-27-1-33. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Armstrong J. A. Will antibacterial chemotherapy be efficacious for gastritis and peptic ulcer? J Antimicrob Chemother. 1986 Jan;17(1):1–3. doi: 10.1093/jac/17.1.1. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Purification of a nickel-containing urease from the rumen anaerobe Selenomonas ruminantium. J Biol Chem. 1986 Jun 15;261(17):7866–7870. [PubMed] [Google Scholar]
- Hazell S. L., Lee A. Campylobacter pyloridis, urease, hydrogen ion back diffusion, and gastric ulcers. Lancet. 1986 Jul 5;2(8497):15–17. doi: 10.1016/s0140-6736(86)92561-4. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989 Dec;171(12):6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata J. H., Elashoff J. D., Haile B. M., Honda G. D. A reappraisal of time trends in ulcer disease: factors related to changes in ulcer hospitalization and mortality rates. Am J Public Health. 1983 Sep;73(9):1066–1072. doi: 10.2105/ajph.73.9.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall B. J., McGechie D. B., Rogers P. A., Glancy R. J. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985 Apr 15;142(8):439–444. doi: 10.5694/j.1326-5377.1985.tb113444.x. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Morgan D. R., Freedman R., Depew C. E., Kraft W. G. Growth of Campylobacter pylori in liquid media. J Clin Microbiol. 1987 Nov;25(11):2123–2125. doi: 10.1128/jcm.25.11.2123-2125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G. Identification of the outer membrane proteins of Campylobacter pyloridis and antigenic cross-reactivity between C. pyloridis and C. jejuni. J Gen Microbiol. 1987 Jan;133(1):163–170. doi: 10.1099/00221287-133-1-163. [DOI] [PubMed] [Google Scholar]
- Newell D. G., Stacey A. Antigens for the serodiagnosis of Campylobacter pylori infections. Gastroenterol Clin Biol. 1989;13(1 Pt 1):37B–41B. [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Purification and characterization of the nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem. 1987 May 5;262(13):5963–5967. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]