Abstract
Ageing changes occur in all the structures of the eye causing varied effects. This article attempts to review the parameters of what is considered within the “normal limits” of ageing so as to be able to distinguish those conditions from true disease processes. Improving understanding of the ageing changes will help understand some of the problems that the ageing population faces.
Keywords: ageing, age related macular degeneration, cataract, arcus senilis
The 2001 census showed that for the first time, there are more people over 60 than there are children in UK.1 Twenty one per cent of the population is 60 years or older. Of these, 1.1 million (1.9%) people are 85 years or older. As time passes, the functional abilities of the eye wane, as do the receptive, storage, and the analytical capacities of the central visual system. Our hope here is to review the parameters of what is considered within the “normal limits” of ageing so as to be able to distinguish those conditions from true disease processes. Both the biological clock theory and wear and tear theory can be used to explain the ageing changes in the body including the eye.
Biological clock theory (Programmed theory)
This planned obsolescence theory focuses on the genetic programming encoded within our DNA. We are born with a unique genetic code, a predetermined tendency to certain types of physical and mental functioning, and that genetic inheritance has a great deal to say about how quickly we age and how long we live. It is as though each of us comes into the world as a machine that is pre‐programmed to self destruct. Each of us has a biological clock ticking away set to go off at a particular time, give or take a few years. When that clock goes off it signals our bodies first to age and then to die.
However, as with all aspects of our genetic inheritance the timing on this genetic clock is subject to enormous variation, depending on what happens to us as we grow up and on how we actually live (the old “nature versus nurture” debate).
Wear and tear theory (Error theory)
Dr August Weismann, a German biologist, first introduced the wear and tear theory in 1882. He believed that the body and its cells were damaged by overuse and abuse. The organs, including the eye are worn down by toxins in our diet and in the environment; by the excessive consumption of fat, sugar, caffeine, alcohol, and nicotine; by the ultraviolet rays of the sun, and by the many other physical and emotional stresses to which we subject our bodies. Wear and tear is not confined to our organs, however; it also takes place on the cellular level. Of course even if one has never touched a cigarette or had a glass of wine, stayed out of the sun, and eaten only natural foods, simply using the organs that nature endowed us is going to wear them out. Abuse will only wear them out more quickly. Likewise as the body ages our very cells feel the effect, no matter how healthy our life style. When we are young the body's own maintenance and repair systems keep compensating for the effects of both normal and excessive wear and tear. With age the body loses its ability to repair damage caused by diet, environmental toxins, bacteria, or a virus. Thus many elderly people die of diseases that they could have resisted when they were younger.
Free radicals are thought to be produced in the body because of wear and tear. “Free radical” is a term used to describe any molecule that differs from conventional molecules in that it possesses a free electron, a property that makes it react with other molecules in highly volatile and destructive ways. Free radicals attack the structure of our cell membranes, creating metabolic waste products, including substances known as lipofuscins. An excess of lipofuscins in the body is shown as a darkening of the skin in certain areas, so called “ageing spots.” Lipofuscins in turn interfere with the cells ability to repair and reproduce themselves. They disturb DNA and RNA synthesis, interfere with synthesis of protein, lower our energy levels, prevent the body from building muscle mass, and destroy cellular enzymes, which are needed for vital chemical processes. This type of free radical damage begins at birth and continues until we die. In our youth its effects are comparatively minor as the body has extensive repair and replacement mechanisms that in healthy young people function to keep cells and organs in working order. With age however the accumulated effects of free radical damage begin to take their toll. Free radical disruption of cell metabolism is part of what ages our cells; it may also create mutant cells leading ultimately to cancer and death. Free radicals attack collagen and elastin, the substances that keep our skin moist, smooth, flexible, and elastic. These vital tissues fray and break under the assaults of free radicals, a process particularly noticeable in the face, where folds of skin and deep cut wrinkles are testaments to the long term effect of free radical damage.
Substances that prevent the harmful effects of oxidation are known as antioxidants. Natural antioxidants include vitamin C, vitamin E, and β carotene, the substance that our body uses to produce vitamin A. These antioxidants have been shown to be of some benefit in prevention of age related macular degeneration in certain group of patients.
We will now discuss the structural and functional ageing changes occurring in various parts of the eye.
Ageing changes of the eyelids and lacrimal system
The involutional changes here mirror similar changes occurring throughout the face and extremities. Gradual tissue atrophy as the mesodermal content of the body begins to shrink; the envelope of the ectoderm becomes too large, and redundant skin folds and wrinkles appear. Loss of adnexal structural support of tarsus, canthal tendons, and orbicularis muscle with thinned skin leads to orbital fat prolapse, eyelid malposition, blepharoptosis, and tearing.
In the lower eyelid, horizontal lid laxity is common. It can be determined by the pinch test, which is the amount the eyelids can be pinched away from the globe and the relative delay and the absence of snap in the lids ability to regain its normal anatomical position. Reduction in the orbital fat with ageing causes the eyes to “sink in” accentuating the lid laxity. Progressive laxity can result in punctual eversion and later eversion of the eyelid margin from the globe (ectropion) and subsequent symptoms of a watery eye. Generalised descent of all of the mid‐face because of ageing further contributes to ectropion formation. If the pretarsal orbicularis muscle is comparatively strong, the eyelid may undergo inversion (entropion) instead causing eyelashes to rub against the cornea and subsequent discomfort. Oculoplastic surgeons operate symptomatic ectropion or entropion. These conditions may also be operated upon before a cataract operation to avoid infection. In entropion, temporary relief may be achieved by simply taping the lid to pull it outwards.
In the upper lids, the disinsertion or attenuation of the levator muscle may cause involutional ptosis.2 Age related descent of the brow (brow ptosis) also contributes to the ptosis formation. Excess upper eyelid skin along with anterior migration of the preaponeurotic fat pads results in dermatochalasis or pseudoptosis. These are operated upon if they are interfering visually.
Deepening of the lines of expression especially at the lateral lid margins cause “crow's feet”, which are sometimes relieved cosmetically by botulinum toxin injections, although not on the NHS in UK.
Watery eye in the elderly, although mainly caused by eyelid malposition, can sometimes result from true lacrimal obstruction. If the nasolacrimal duct obstruction3 causes distressful watering or recurrent infections it can be treated by dacryocystorhinostomy. The other end of the spectrum is reduction in the amount of tears produced by the lacrimal gland causing dry eyes. This is treated with artificial tears or punctual plugs to retain tears in the conjunctival sac.
Corneal changes
Changes in corneal toricity (curvature) cause alteration in refraction in elderly, usually a change from the “with the rule” astigmatism to “against the rule” astigmatism. In with the rule astigmatism, the vertical meridian of the cornea is steeper than the horizontal meridian and the eye has more refractive power (plus cylinder) along the vertical axis, hence the patient has difficulty resolving targets with horizontal lines, for example, letters like E or F. In against the rule astigmatism, the horizontal meridian is steeper than the vertical and the eye has more refractive power (plus cylinder) along the horizontal axis, hence the patient has difficulty focusing vertically oriented targets. Hence, because of presbyopic changes and astigmatism changes regular refraction check ups are advised for the elderly to help them see optimally.
Other corneal changes include decrease in corneal luster and corneal sensitivity and increase in corneal fragility.
Age related dystrophic changes occur in corneal epithelium, stroma, and endothelium (table 1). Hudson‐Stahli line is a pigmented line of iron deposition commonly seen at the junction between middle and lower third of the cornea and is thought to be depostion from the tear film over the opposing lower lid margin. Arcus senilis is the most prominent and frequent ageing change seen in the cornea. These asymptomatic bilateral yellow‐white deposits usually begin inferiorly and then superiorly to form an annular opacity on the peripheral corneal stroma separated from the limbus by a narrow band of clear cornea.4 The deposits are composed of cholesterol esters, cholesterol, and neutral glycerides. Hassall‐Henle bodies are localised thickenings in the periphery of the endothelium of the ageing cornea seen on specular reflection on slit lamp examination. If these descemet membrane excrescences occur axially in the corneal endothelium they are called cornea guttata. Kruckenberg spindle is the deposition of uveal pigment on the corneal endothelium with ageing. All the above mentioned changes by themselves do not interfere in vision.
Table 1 Changes seen in the ageing cornea4.
| Characteristic | Result of change |
|---|---|
| (1) Changes in shape and optical properties | (a) Steepening of keratometry and a shift from with the rule to against the rule astigmatism |
| (b) Transparency is unaffected in central cornea in absence of scar or degeneration | |
| (c) Collagen intramolecular and interfibrillar spacing increases—possibly via increased protein glycation | |
| (d) Increased thickness of Descemet's membrane | |
| (2) Corneal degenerations (influenced by environmental and genetic factors) | (a) Cornea farinata |
| (b) White limbus girdle | |
| (c) Mosaic degeneration | |
| (d) Deep crocodile shagreen | |
| (e) Hassall‐Henle bodies | |
| (f) Arcus senilis | |
| (3) Physical properties | (a) Resistance to infection reduced |
| (b) Failure to upregulate ICAM‐1 and reduced inflammatory cell infiltration | |
| (c) Reduced phagocytically active cells after infection | |
| (d) Decline in high energy metabolism | |
| (e) Increased tear contact time | |
| (f) Increased epithelial permeability to fluorescein |
The corneal endothelium cannot regenerate and hence the endothelial cell density reduces with increasing age. The remaining endothelial cells enlarge to cover the loss of some of the endothelial cells with ageing resulting in pleomorphism. As the endothelial cells maintain the corneal deturgecence, a further decrease in endothelial cell count beyond a certain amount either because of surgical damage or progressive guttata in Fuchs endothelial degeneration, can result in corneal thickening and subsequent opacity and decrease in quality of vision. If visual acuity is reduced penetrating keratoplasty is considered.
Table 1 gives further details of changes in the ageing cornea.
Trabecular meshwork and uveal changes
Gonioscopy shows increased pigmentation of the trabecular meshwork. There is also increase in the resistance to the outflow of aqueous humor. Significant resistance beyond caused by ageing may precipitate glaucoma.
Pupil tends to become smaller and the iris less reactive with age and more difficult to dilate pharmacologically. Pigment loss in the iris may cause iris transillumination on slit lamp examination especially at the pupillary margin.
The shape and tone of ciliary body changes with age and this along with changes in the decrease elasticity of the lens capsule and compactness of the lens fibres causes a decrease in the amplitude of accommodation resulting in presbyopia. Reduction in near vision caused by presbyopia can usually be overcome by holding the objects further away or by near correction glasses (bifocals, reading glasses).
Crystalline lens changes
As we age, the lens selectively absorbs more blue light (410 nm) because of accumulation of yellow pigments in the lens. This decrease in transmission of blue light is a part of cataractogenic process and causes a relative “blue blindness” that is exemplified by the increase in blue colour used in paintings by renowned painters, as they get old.
To the ophthalmic surgeon, one of the most familiar signs of ageing is the hardening (nuclear sclerosis) of the lens caused by various biochemical and photochemical changes. Patients experience symptoms of hardening (presbyopia) many years before frank cataract formation. Presbyopia, as discussed earlier is attributable to change in capsular elasticity and lens hardening because of modifications in cortical fibre cell cytoplasm and nuclear protein solubility.5 The distinguishing line between age related nuclear sclerosis and cataract is blurred. Phacoemulsification and intraocular lens implantation is done in patients whose standard of living and performance of daily routine activities is affected by cataract.
Vitreous ageing
Like any other tissue in the body, the vitreous undergoes the irreversible process of ageing characterised by changes in the collagen fibrils and hyaluronic acid components causing harmless floaters noticed by the elderly. Clinically with ageing there is condensation of the vitreous gel, enhancement of fibrillary structure of vitreous, increased mobility of fibrillary structures, and formation of optically empty spaces called lacunae. As the vitreous liquefaction increases, the resultant lacunae coalesce forming larger cavities, eventually followed by shrinkage of vitreous body from the retina once around 50% liquefaction is reached causing posterior vitreous detachment (PVD). PVD by itself is innocuous and patients note a spider‐like floater in front of the eye, which moves in the direction of gaze. Occasionally if there is strong vitreoretinal adhesion in the peripheral retina, there may occur a retinal tear during acute PVD, which needs to be lasered to prevent retinal detachment. Hence patients with sudden onset flashes (caused by traction on retina during acute PVD) and floaters (caused either by the vitreous debris or pigment dispersion from the torn retina) need to be referred to the ophthalmologist to rule out retinal tears associated with PVD. Onset of a curtain‐like shadow in the field of vision may actually point to a retinal detachment that will need surgical intervention.
Ageing in the retina
Our vision deteriorates with age and virtually every measure of visual function shows declining performance with increasing age including decrease visual acuity, decline in sensitivity of visual field, decreased contrast sensitivity, and increased dark adaptation threshold. Decreased visual function is a combination of mainly ageing changes in neuronal elements of visual system, changes in ocular media, and pupillary miosis. The box lists the neurosensory retinal changes. Retinal pigment epithelium (RPE), which is vital for integrity of the rods and cones, shows with age increased pleomorphism, decrease in number of cells in the posterior pole, decreased melanin content, increased lipofuscin content, and decreased volume of cytoplasm.
Signs of ageing in the retina
Decline in visual function
Decreased visual acuity
Declining sensitivity of visual field
Decreased contrast sensitivity
Increased dark adaptation threshold
Neuronal cell loss and degeneration
Decreased ganglion cells and optic nerve axons
Decreased photoreceptors
Other neurosensory retinal changes
Thickening of basement membrane
Corpora amylacea bodies increase
Lipofuscin increase
Photoreceptors show displaced nuclei and convoluted rod outer segments.
Changes at the macula
Grunwald et al6 have shown that in normal subjects, retinal macular microcirculation reduces with age. The 20% decrease in average velocity with age is similar to the age related decrease in number of cells seen in the human foveal ganglion cell layer. Grunwald et al7 also then showed a systematic decrease in choroidal circulatory parameters with an increase in the severity of AMD (age related macular degeneration) features associated with risk for the development of SRNVM (subretinal neovascular membrane), suggesting a role for ischaemia in its development.
Age related macular degeneration
Bruch's membrane shows some of the most prominent changes in ageing as debris from the overlying retina begins to accumulate. It is hypothesised that apoptosis or bulk shedding of cytoplasmic material by the RPE (retinal pigment epithelium) into the Bruch's membrane may afford a way of disposal of old and damaged membranes or organelles. Subsequent removal of this debris occurs, but accumulation occurs when production overwhelms removal with ageing. Basal laminar deposits accumulate mainly in the macular areas, which eventually manifest as drusen. Drusen looks like specks of yellowish white material under the retina. Drusen can be either hard drusen, which are small solid round deposits with distinct margins or soft drusen, which are larger, pale yellow, and have indistinct borders. Drusen by themselves do not usually cause visual disturbances and cannot be treated, but can progressively worsen and is linked to AMD in some elderly patients. Hard drusen can progress to dry AMD (atrophic) causing gradual distortion of vision and progressive central visual deterioration. There is no treatment, laser or other that can halt or reverse the progression of dry AMD related visual loss. Dry AMD accounts for nearly 90% of all AMD. They are associated with pigmentary maculopathy and in severe cases geographical atrophy. Hard drusen can also sometimes combine together to transform into soft drusen.
Soft drusen are more susceptible to undergo wet AMD (neovascular/exudative) leading to formation of SRNVM. This SRNVM can leak causing central distortion and bleed causing a sudden drop in central visual acuity and exudative maculopathy. As soft drusen build up between the RPE and Bruch's membrane, they lift up the RPE and force the two layers apart. Some evidence suggests soft drusen are instrumental in the spread of abnormal vessels, but whether they stimulate vessel growth or simply provide space for them by lifting up the RPE remains unclear. Risk factors of soft drusen for SRNVM development are:
presence of five or more drusen deposits
drusen size greater than 63 μ
clumping of drusen deposits.
The Beaver Dam study8 in 2002 has shown that in a population more than 75 years of age, about 50% have drusen, 20% have pigmentary changes, 4% have exudative maculopathy, and 3% have geographical atrophy. AMD is the largest cause of blind registration in UK and with the demographic right shift of the population is gaining more importance.
Strong risk factors of AMD are ageing, smoking, family history, and genetic factors. Possible risk factors include exposure to sunlight especially blue light, hypertension, cardiovascular risk factors, female sex, non‐Hispanic white people, and hyperopia (farsightedness).
Advice on diet especially green leafy vegetables and fruits, giving up smoking, certain multivitamins (AREDS study9), and self monitoring with amsler chart has been recommended in patients with high risk of progression to AMD. AREDS researchers found that people at high risk of developing advanced stages of AMD lowered their risk by about 25% when treated with a high dose combination of vitamin C, vitamin E, βcarotene, and zinc. In the same high risk group—which includes people with intermediate AMD, or advanced AMD in one eye but not the other eye—the nutrients reduced the risk of vision loss caused by advanced AMD by about 19%. For those study participants who had either no AMD or early AMD, the supplements did not provide an apparent benefit.
Recent advances in treatment include photodynamic therapy (PDT), macugen therapy, and intravitreal triamcinolone injections, some of which are still in trial stages, to treat wet kind of AMD that fit certain treatment criteria.
Other changes
In the optic nerve, swollen axons at the level of lamina cribrosa have been noted. There is an age related decrease in the number of optic nerve axons and increase in thickness of the connective tissue of the optic nerve and increased elastic fibres.10
Clinically fundal changes visualised with ageing include loss of fundus reflexes, gradual fading of fundus colour, greater visibility of larger choroidal vessels (senile tigroid fundus), peripapillary atrophy, and peripheral retinal degenerations.
Functional changes
Vision is affected in various ways with ageing. Presbyopia is a striking feature. A person with early nuclear sclerosis may complain of glare, especially at night time driving, because of light scattering. At the same time they also need more lighting in the surrounding than a younger person. Reduction in contrast sensitivity is gradual because of progressive media opacities. Decreased contrast sensitivity also diminishes an elderly person's ability to perceive depth. Reduced depth perception makes steps or street curves difficult to manage. Reduction in visual field is normal with ageing and is important to remember while testing visual fields in glaucoma suspects.
Reduction in number of cones at the fovea causes generalised reduction in colour vision. Fovea is the most important part of the macula responsible for our central sharpest vision. For people of all ages, it is harder to tell blues and greens from each other than it is to differentiate reds and yellows. This becomes even more pronounced with ageing. As your age increases, using lots of warm contrasting colours (yellow, orange, and red) in your home can improve your ability to tell where things are and makes it easier to perform daily activities. Many older people find that keeping a red light on in darkened rooms (such as the hallway or bathroom) makes it easier to see than keeping a “regular night light” on.
Furthermore, blue light is attenuated by the ageing lens resulting in shift in appearance of objects towards yellow.
Elderly people find it difficult to adapt in bright light and darkness and also are unable to tolerate glare. Hence elderly patients though are comfortable driving during the day find it difficult to drive at night.
Conclusion
The impact of ageing changes in the eye has a significant effect on the quality of life in the elderly. In considering the effects of ageing on vision, it is important to differentiate between reduced visual function in the normal healthy elderly person and the elderly patient with specific age related disease.11 In the future, interventions may become possible to forestall or prevent many age related visual disabilities.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Photo quiz
Identify the ageing changes in the photographs. Answers after the references.
Acknowledgements
Dr Anne Freeman, whom we thank for giving us an opportunity to be involved in this series.
Answers to the photo quiz:
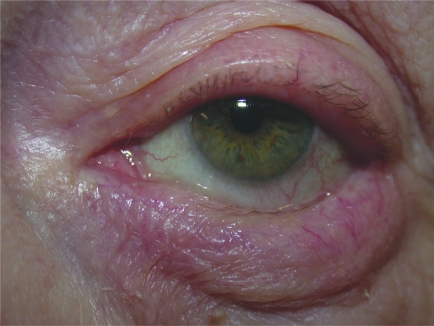
Lower lid entropion
Bilateral cataracts. Also note orbital fat prolapse causing baggy eyelids due to lack of support and thin skin.
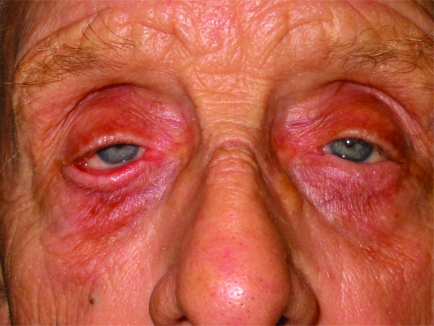
Severe skin laxity and redundant skin, which may cause dermatochalasis.
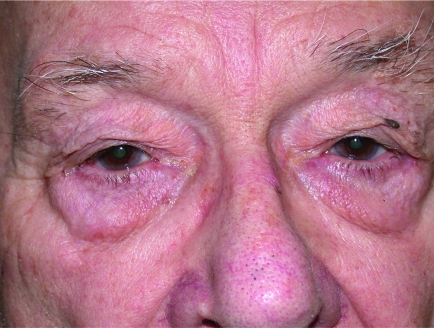
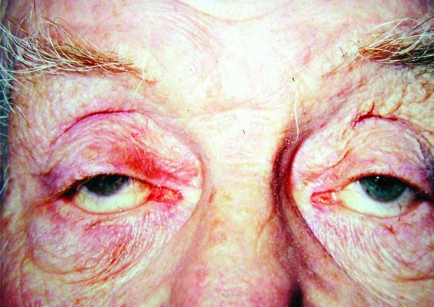
Bilateral ptosis. This degree of ptosis, caused by complete levator muscle disinsertion, is covering the pupillary area and may cause visual obstruction.
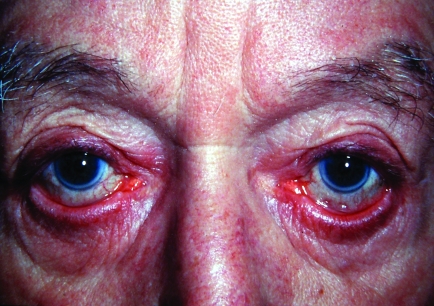
Bilateral ectropion. Note the secondary conjunctival congestion. Also note the midface descent contributing to the ectropion.
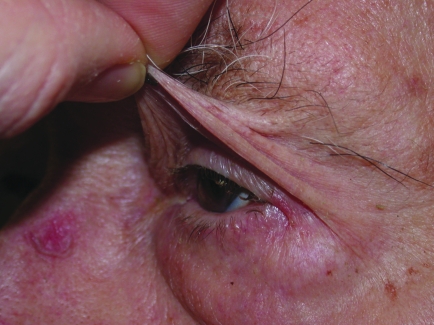
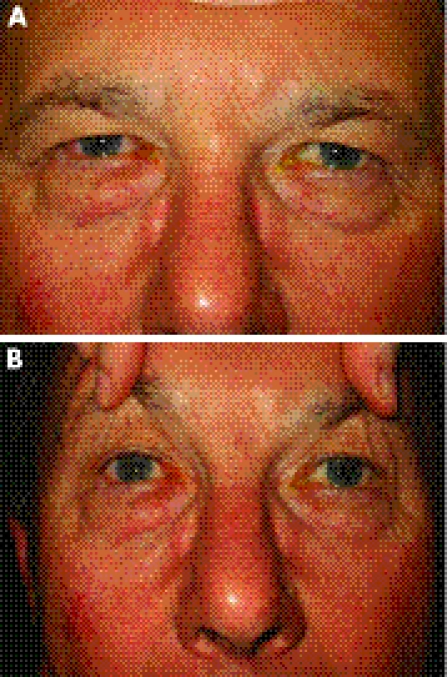
(A) Brow ptosis. This is caused by generalised descent of the brow. (B). Diagnostic test for brow ptosis. The ptosis is relieved by manually lifting up the brow.
Bilateral ptosis; right more than left eyelid. Horizontal lower lid laxity causing lateral canthal droop. Right lower lid ectropion.
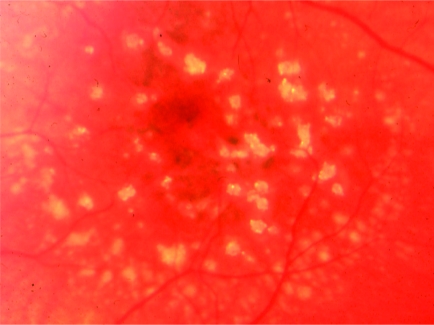
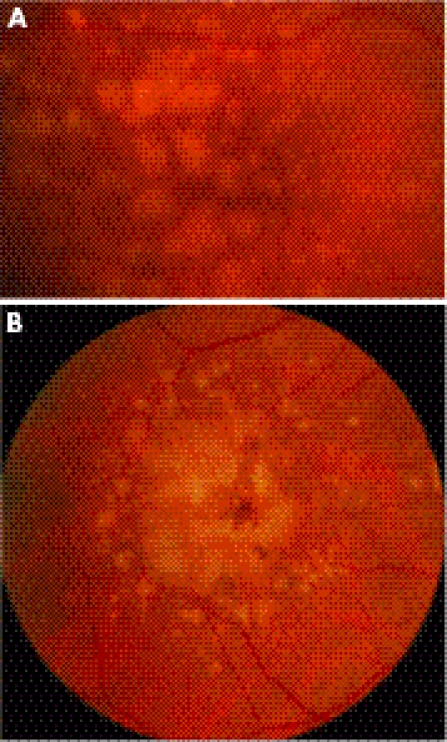
Hard drusen seen on the macula.
(A) Soft drusen on macula. (B) Confluent soft drusen. These are at a high risk of having subsequent subretinal neovascular membrane formation leading to wet age related macular degeneration.
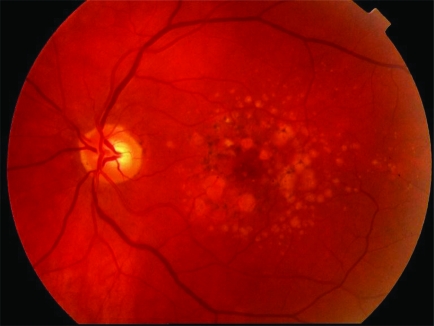
Dry age related macular degeneration.
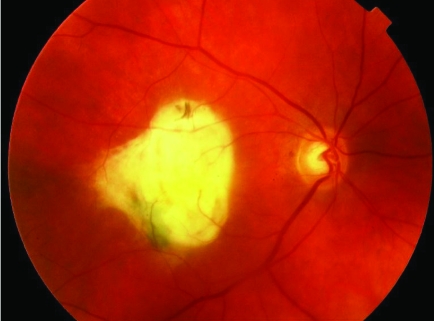
Geographical type of advanced dry age related macular degeneration. These changes occur gradually and cause a gradual loss of central vision. Magnifiers may be of some benefit in reading large print.
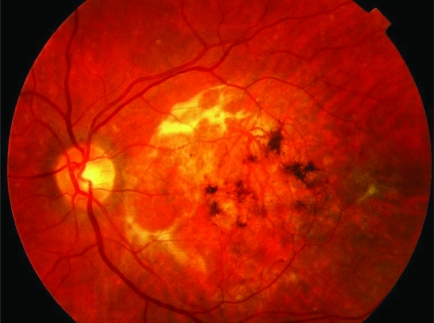
Wet kind of age related macular degeneration showing a fresh subretinal bleed associated with a subretinal neovascular membrane. This causes a sudden drop in central vision following early warning signs of distortion of vision.
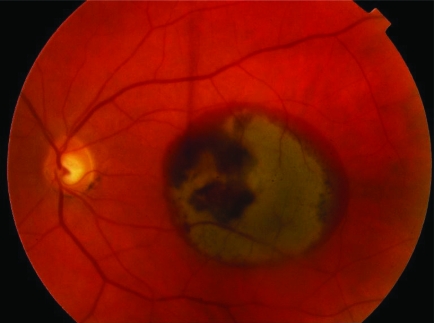
End stage disciform macular scar. Once the subretinal neovascular membrane has fibrosed there is no further bleeding and a large subretinal scar tissue is formed. As the peripheral retina is unaffected the patient retains peripheral navigational vision, but loses central vision.
Footnotes
This article is part of a series on ageing edited by Professor Chris Bulpitt.
Funding: none.
Competing interests: none.
The American Medical Association had nominated aging as the global theme issue for 1997. Accordingly, the October 1997 issue of the British Journal of Ophthalmology had been assigned to papers related to ageing issues hence most references to ageing in the eye can be found in that issue.
References
- 1.National Statistics Census 2001. http://www.statistics.gov.uk
- 2.Van den Bosch W A, Leenders I, Mulder P. Topographic anatomy of the eyelids, and the effects of sex and age. Br J Ophthalmol 199983347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Haeringen N J. Aging and the lacrimal system. Br J Ophthalmol 199781824–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faragher R G, Mulholland B, Tuft S J.et al Aging and the cornea. Br J Ophthalmol 199781814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan G, Wormstone I M, Davies P D. The aging human lens: structure, growth, and physiological behaviour. Br J Ophthalmol 199781818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunwald J E, Piltz J, Patel N.et al Effect of aging on retinal macular microcirculation: a blue field simulation study. Invest Ophthalmol Vis Sci 1993343609–3613. [PubMed] [Google Scholar]
- 7.Grunwald J E, Metelitsina T I, Dupont J C.et al Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci 2005461033–1038. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein B E K, Linton K L P. Prevalence of age related maculopathy. The Beaver Dam study. Ophthalmology 199299933–943. [DOI] [PubMed] [Google Scholar]
- 9.The Age‐Related Eye Disease Research Group A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss. AREDS report no 8. Arch Ophthalmol 20011191417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moya F J, Brigatti L, Caprioli J. Effect of aging on optic nerve appearance: a longitudinal study. Br J Ophthalmol 199983567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester J V. Aging and vision. Br J Ophthalmol 199781809–810. [DOI] [PMC free article] [PubMed] [Google Scholar]