Abstract
Micronutrients play a central part in metabolism and in the maintenance of tissue function. An adequate intake therefore is necessary, but provision of excess supplements to people who do not need them may be harmful. Single micronutrient deficiency states are comparatively easily recognised and treated. Subclinical deficiency, often of multiple micronutrients, is more difficult to recognise, and laboratory assessment is often complicated by the acute phase response. Clinical benefit is most likely in those people who are severely depleted and at risk of complications, and is unlikely if this is not the case. There is little evidence for supplements leading to a reduction in the incidence of infections in the elderly population, in coronary artery disease, or in malignant disease. The best evidence for benefit is in critical illness, and in children in developing countries consuming a deficient diet. More clinical trials are required with good clinical outcomes to optimise intake in prevention and treatment of disease.
Keywords: micronutrients
There is growing interest in the role of the micronutrients (essential trace elements and vitamins) in optimising health, and in prevention or treatment of disease. This stems partly from the increase in knowledge and understanding of the biochemical functions of these nutrients, but also from the extensive but less well founded commercial claims for such substances. It is important that doctors and other health professionals are aware of the evidence for the nutritional essentiality of these substances, and for the situations where an increased intake may lead to clinical benefit.
This review will therefore consider current knowledge of the requirements in health, those people at risk of an inadequate intake, and the conditions where supplements may be clinically required. The review will focus only on the generally accepted essential inorganic micronutrients (trace elements) and organic micronutrients (fat soluble and water soluble vitamins) for which deficiency states, with biochemical, physiological, or structural changes, have been clearly reported—such states occur after prolonged consumption of a diet lacking the single nutrient under consideration, and are uniquely corrected by addition of the nutrient back into the diet. Other nutrients for which there are no confirmed deficiency states, but where supplements are often taken, such as ω‐3 fatty acids, glucosamine, coenzyme Q, will not be considered.
Biochemical functions
Cofactors in metabolism—trace elements are frequently involved in modulating enzyme activity or are an integral part of enzyme prosthetic groups—for example, zinc is a cofactor for over 100 enzymes, whereas selenium is required in the form of selenocysteine within the enzyme glutathione peroxidase.
Coenzymes in metabolism—many vitamins or metabolites of vitamins are required to play an active part within complex biochemical reactions, for example, riboflavin and niacin in the electron transport chain, or folic acid as part of methyl group transfer. These reactions are critical to intermediary metabolism and ensure utilisation of the major nutrients to provide energy, proteins and nucleic acids.
Genetic control—zinc “fingers” are transcription control factors that bind to DNA and regulate transcription of receptors for steroid hormones and other factors.
Antioxidants—much of the popular interest in the micronutrients stems from the recognition that many of the micronutrients have antioxidant properties. Oxidative metabolism inevitably leads to generation of reactive oxygen species (ROS) or “free radicals”, which have the potential to cause further oxidative reactions, especially to those parts of the cell in a relatively reduced state, such as cell membranes or nucleic acids.1 The potential to cause damage is limited by mechanisms that include direct quenching of oxidant activity by tocopherols (vitamin E) or carotenoids (vitamin A), or enzyme systems to dispose of the products of oxidation—superoxide dismutase (either zinc/copper or manganese dependent) and glutathione peroxidase (selenium dependent).
Reference nutrient intakes of micronutrients
Many countries have developed recommendations for intake of micronutrients in the normal diet. These have been based on the observed intakes in the healthy population, coupled with small numbers of detailed nutrient balance studies, and laboratory estimates of blood and tissue status associated with particular levels of intake. Values have been set for the intake of each micronutrient below which a clinical deficiency state is increasingly likely, or above which a toxicity state is likely to develop. Although these are relevant for populations, the difficulty is to determine how adequate is the intake for a particular person. This is especially the case for certain micronutrients where it is recognised that there may be additional benefits in terms of tissue function if the intake is somewhat greater than that needed only to prevent a deficiency state from developing. An excellent discussion of this is provided in the most recent editions of the Dietary Reference Intakes published by the Institute of Medicine in the USA.2,3
For practical purposes, the Reference Nutrient Intakes (RNI) in the UK,4 and Dietary Reference Intakes (DRI) in the USA2,3 are defined as the intakes of each micronutrient that meet the requirements of almost all (97%–98%) of persons in the group.
People at risk of an inadequate intake
As the RNIs were largely established from the nutritional intake of the healthy population, it follows that the typical diet of the healthy population provides the necessary range and amount of these nutrients. This is the basis of the widely accepted dietary advice that five portions of fruit and vegetables a day, as part of a mixed diet providing otherwise adequate energy and protein will, over a period of time, provide sufficient amounts of all trace elements and vitamins.5 There are however a number of situations where the intake may be less than adequate even in the healthy population.
Are there sufficient amounts of the nutrient in the typical healthy diet?
The national diet and nutrition survey has confirmed that adequate intakes of most micronutrients can be obtained from a typical diet in the UK, both in adults aged 19–646 and in people aged 65 years and older7 although in young people aged 4–18 there may be significant numbers with intakes lower than the lower RNI for certain minerals.8 Of interest in the main study in the 19–64 year group was the finding that only 13% of men and 15% of women met the five a day recommendation for fruit and vegetables, the mean intakes being 2.7 portions and 2.9 portions respectively, but biochemical evidence of poor status of vitamins was rare.6 However, laboratory tests of micronutrient status are relatively insensitive, and absence of biochemical evidence of deficiency does not imply optimal function.
In the case of selenium, it is now apparent that the content of this element in the typical UK diet has been falling progressively over the past 15–20 years.9 This has resulted from the switch from use of wheat imported from North America, where the soil has a high content of selenium, to wheat grown in Europe and UK, where the soil content of selenium is comparatively low. It is therefore now estimated that the typical selenium intake in the UK is of the order of 35 μg/day, which is roughly 50% of the RNI for adults in the UK (70 μg/day for men, 55 μg/day for women). This has led to concentrations of plasma selenium that are not high enough to provide optimum activity of the antioxidant enzyme glutathione peroxidase.9 It can be concluded therefore that the dietary intake in the UK for selenium is probably inadequate and this may well have harmful consequences.
There have however been few good clinical trials of the benefits of additional selenium intake in the general population, but a trial of selenium supplements (200 μg/day in patients with a past history of skin cancer), showed that this intake over a 4.5 year period led to a significant reduction in the incidence of a number of types of cancer.10 This study took place in America where selenium status is much higher than in the UK, and therefore it is essential it should be repeated in other parts of the world.
Socioeconomic effects
Many groups in the population have a poor intake of nutrients as a result of a complex interaction between social and economic circumstances. Thus people from a poorer background may well take less fresh fruit and vegetables.6,7 Such groups may benefit from some form of micronutrient supplement, although direct comparisons are lacking of provision of supplements rather than improving dietary intake.
Population groups with known poor intake or increased requirements
Certain groups within the population are known to have a poor or inadequate intake. For example, adolescents and teenagers may have an inadequate intake of milk and other sources of calcium,11 and elderly people in nursing homes and residential care have an inadequate vitamin D intake.7 Furthermore, despite there being an adequate supply of most micronutrients in the food available in nursing homes, there is a high incidence of biochemical deficiency for most micronutrients, including zinc, iron, vitamin C, and riboflavin.7 There are many possible causes of insufficient intake or absorption, including anorexia, inability to cut up food, or poor food presentation.
Moreover, certain groups have increased requirements—expectant mothers require increased folate both before conception and in the first trimester,2 smokers require additional vitamin C,3 and people who are recovering from an acute illness or after surgery probably have multiple requirements.12
It would be entirely appropriate for groups of this sort to take supplements to ensure their total intake, including their diet, was adequate.
Changes in micronutrient status as a result of disease
Nutritional status is profoundly affected by most disease states, usually by a combination of increasing demand at a time when there is reduced intake. This can particularly affect micronutrient status:
Reduced intake
Anorexia is common as a result of disease, for example, because of chronic inflammation, acute infection, or neoplastic disease. This is especially likely in the institutionalised elderly population. As mentioned above, the lower the protein energy intake, the lower the micronutrient intake.
Chronic alcohol misuse leads to severe malnutrition with multiple micronutrient depletion from inadequate intake. Patients are at risk of acute vitamin deficiency as soon as carbohydrates are provided—acute thiamine deficiency leading to Wernicke's encephalopathy is well reported. This can be part of the more generalised refeeding syndrome, with hypophosphataemia, hypokalaemia, and fluid overload.13
Inadequate supplements during parenteral nutrition (PN). When patients are dependent on an intravenous supply, adequate amounts of all vitamins and trace elements must be provided. Many cases have been reported when this has not been done, sometimes with very serious clinical consequences.12
Increased requirements for metabolism
The catabolism associated with acute infection, surgery, or trauma leads to increased energy expenditure and net protein breakdown. Requirements for water soluble vitamins, as coenzymes for these metabolic pathways, will be increased, as will the requirements for various trace elements.4 Some intracellular elements, such as zinc will be released during cell breakdown,14 so that increased amounts may not be required at this time.
Anabolism increases the requirement for all nutrients; hence increased micronutrients should be supplied when patients are gaining weight. Trace element deficiencies are more likely when patients become anabolic, after a prolonged period of catabolism.15
Increased losses
Any loss of body fluid will lead to loss of some micronutrients. The commonest deficiency is, of course, iron deficiency as a result of menstrual or other blood loss. Diarrhoea can be a significant cause of zinc deficiency,16 setting up a vicious circle of worsening diarrhoea as a result of zinc deficiency. Losses from fistulas, burn exudates, or dialysis, all lead to depletion of water soluble vitamins or trace elements.
It is therefore important to recognise the various situations where micronutrient status may be impaired as result of disease, and ensure that patients receive an adequate intake, either from their food, or from separate supplements.
Consequences of a micronutrient intake insufficient to meet requirements
Classic nutritional deficiency usually results in a complex syndrome of typical signs and symptoms, and these have now been fully characterised for each of the vitamins and trace elements.17 It is however now clear that the full blown clinical deficiency syndrome is the endpoint of a prolonged pathway. With the exception of certain single nutrient deficiencies, most clinical deficiency states are comparatively uncommon in clinical practice in Western Europe. The most common deficiencies are for iron, vitamin D, folate, and vitamin B12. Such single nutrient deficiency states are comparatively easy to recognise, diagnose, confirm with suitable laboratory tests, and treat with the appropriate supplement.
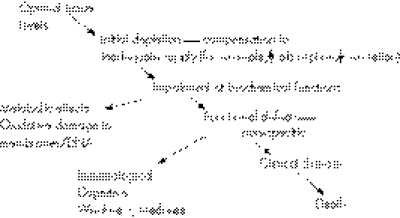
However, milder forms of deficiency, often of multiple micronutrients, are much more common and more difficult to recognise. As a person develops progressively more severe depletion of one or more micronutrients, they will pass through a series of stages with biochemical or physiological consequences. The metabolic or physiological penalty of such a suboptimal status is usually not clear but the assumption remains that this impaired metabolism is likely to have detrimental effects. Similarly, specific and localised tissue deficiencies can occur and lead to pathological changes. Such situations can be defined as “subclinical deficiency”. The time course for development of a subclinical deficiency varies for each micronutrient, and depends upon the nature and amount of body stores. Figure 1 shows more clearly the consequences of an inadequate intake.
Figure 1 Development of micronutrient deficiency.
The consequences of a subclinical deficiency
Metabolic effects with clinical consequences
As the intracellular concentration of micronutrients falls, metabolic pathways in particular tissues will begin to be affected. Three examples of this are as follows:
Folate and homocysteine
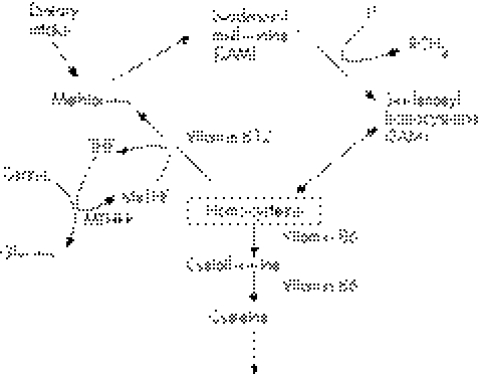
The essential requirement for folate, in the form of methyltetrahydrofolate, in ensuring homocysteine is converted to methionine has been recognised for some time (fig 2). Homocysteine metabolism also requires vitamin B12 and vitamin B6. Homocysteine is an independent risk factor for coronary artery disease, and a number of trials are currently underway to investigate clinical benefit of reducing homocysteine plasma concentration. In one study of 205 patients who had had successful coronary angioplasty,18 six months' treatment with folic acid (1 mg/day) B12 (400 μg/day), B6 (10 mg/day) in comparison with placebo, led to a reduction in mean plasma homocysteine from 11.1 to 7.2 μmol/l, and a significant reduction in restenosis rate and the need for revascularisation. Moreover a large meta‐ analysis reviewed 72 studies (involving 16 849 cases and controls), investigating the prevalence of mutation in the methyl tetrahydrofolate reductase (MTHFR) gene, which increases homocysteine, and 20 prospective studies (3820 participants) of serum homocysteine and disease risk.19 There was a high degree of association between homocysteine and cardiovascular disease. It was estimated that lowering homocysteine concentration by 3 μmol/l from current levels, which could be achieved by increasing folic acid intake by about 0.8 mg/day, would reduce the risk of ischaemic heart disease by 16%, deep vein thrombosis by 25%, and stroke by 24%.
Figure 2 Homocysteine‐metabolic pathways.
However, an almost identical study using similar supplements was subsequently published but this time in high risk patients after coronary stenting.20 The group receiving folate, B6, and B12 supplements had an increase in the need for another operation over one year. These conflicting results probably reflect heterogeneity within the study populations.
Recently, two further trials involving large numbers of patients have been published. The Norwegian vitamin trial looked at various combinations of folate and B vitamins, or placebo in 3749 patients who had had an acute myocardial infarction.21 After a median of 40 months, there was a 27% reduction in homocysteine in those treated with folate and B12, but no effect on myocardial events was seen. In the HOPE‐2 study, 5522 patients with vascular disease or diabetes were studied over five years.22 Despite a reduction in homocysteine, again vitamin treatment had no effect on myocardial events, but had a marginally significant effect on stroke. It seems clear therefore that folate does not reduce the risk of atherosclerosis by reducing homocysteine. The relation between intermediates in the homocysteine pathway and mediators of atherosclerosis is therefore complex and requires much further study.23
Chromium and glucose tolerance
An interesting interaction between a trace element and substrate utilisation is the role of chromium in improving insulin action. A low molecular weight intracellular octapeptide, known as chromomodulin, binds trivalent chromium and increases the response of the insulin receptors.24 This has been seen especially in NIDDM patients,25 but also in non‐diabetic obese subjects with a family history of type II diabetes mellitus,26 as well as during long term total parenteral nutrition (TPN).27 There is growing evidence for the value of added chromium in maintaining glucose tolerance, reducing body fat, and increasing lean tissue mass,28 although some of the evidence is conflicting.29 This may be partly because of the dose used, a study with 100 μg chromium/day as chromium picolinate being ineffective,29 whereas other studies have found the effective dose to vary between 200 μg/day and 1000 μg/ day.30 It is clear that further studies are required to clarify the optimal dose and type of chromium supplement, and the patient population or individual patients most likely to benefit.
Zinc and protein synthesis
In an elegant study on 24 stable patients requiring TPN, Wolman et al31 determined the effects of different amounts of intravenous zinc provision. The key finding was that most patients lost substantial amounts of zinc from the gastrointestinal tract, but that zinc balance could be achieved by supplying adequate intravenous zinc. A positive zinc balance was associated with improved nitrogen retention, and a better nitrogen balance. Interestingly, plasma insulin was also found to increase when a negative zinc balance was converted to a positive one. Adequate zinc provision is therefore necessary not only to stimulate protein synthesis, probably as a result of increasing the activity of the many zinc dependent enzymes in the protein synthetic pathway, but also to stimulate an adequate insulin response and utilisation of glucose as well as amino acids.
Other biochemical effects—uncertain clinical consequences
As so many of the micronutrients are involved in parts of the antioxidant defence mechanisms, it has been assumed that suboptimal antioxidant activity will lead to oxidative damage to tissues or to parts of the cell, with potentially serious results.
Oxidative damage and coronary artery disease.
Because of the strong link between oxidised low density lipoprotein and development of coronary artery disease, together with the lower incidence of coronary artery disease in countries where there is a high intake of antioxidants, it has been expected that an increased intake of antioxidants would reduce the incidence and complications of heart disease. Some small studies did initially suggest beneficial effects from taking vitamin E supplements.32,33
However, more recently a number of large scale trials have failed to show such a benefit, for example, the HOPE study34 studied 9541 patients with high risk of coronary heart disease, who received 400 IU vitamin E plus ACE inhibitors and showed no benefit from vitamin E supplements. The GISSI study35 in Italy failed to show any benefit from the use of vitamin E in 11 324 patients after myocardial infarction, although patients did benefit from an increased intake of polyunsaturated fatty acids.
Moreover, the Medical Research Council/British Heart Foundation study36 of 20 333 high risk UK adults showed that an intake of vitamin E (600 mg), vitamin C (250 mg), and β carotene (20 mg), over a five year period had no significant benefit on incidence or progression of coronary heart disease.
A comprehensive review of studies by the American Heart Association concluded that cardiovascular disease reduction can be achieved by long term consumption of a well balanced diet together with regular physical activity, and that there was no additional benefit from consumption of micronutrients at levels exceeding these obtained from such a diet.37
Furthermore, Miller and coworkers38 have published a meta‐analysis of 19 clinical trials examining the role of vitamin E, either alone (nine studies) or together with other antioxidants (10 studies) in a total of 135 000 participants. They concluded that high intake of vitamin E at doses of >400 IU/day or more carried no benefit, and indeed may increase all cause mortality. They estimated that the increased risk might start at doses as low as 150 IU/day. Supplements of even lower doses than this may however carry a marginal benefit. A particularly important feature of this meta analysis is the dose effect of vitamin E. It has long been believed that the higher the dose of vitamin E the better would be any potential benefit, and that there were no harmful effects associated with a high dose. This meta analysis makes it clear that high doses of any nutrient are likely to be associated with harmful side effects. If beneficial effects are obtained by correction of a sub‐clinical deficiency, then the dose needed to do so must be carefully established.
Oxidative damage and neoplastic disease
It has also been assumed that an increased intake of antioxidants would be beneficial in reducing the incidence of various forms of cancer, by reducing oxidation induced mutations in DNA. Indeed this is the most common reason given in the USA for consumption of such supplements.39 Apart from the studies of selenium, including that mentioned above, and a study on liver cancer in China40 other studies have been disappointing. In particular, a study over a five to eight year period of 29 133 Finnish male smokers who received β carotene (20 mg), α tocopherol (50 mg), or both, showed an 18% higher incidence of lung cancer in the β carotene group, whereas α tocopherol had no effect.41 A study from the USA of 18 314 smokers, former smokers, and asbestos workers who received 30 mg β carotene and 25 000 IU vitamin A42 over a four year period, also showed that the supplemented group had a higher relative risk of lung cancer of 1.28.
Other studies have provided conflicting evidence. The nurse health study in USA of 88 758 women showed that folate intake or supplements for less than 15 years did not significantly reduce the risk of colon cancer, but greater than 15 years' supplementation did reduce risk.43 The benefit of folate seems to be greatest in those taking more than two alcoholic drinks per day.44 However, in the same group of women there was a higher incidence of non‐Hodgkin's lymphoma in those supplemented.45 Other studies have shown that long term vitamin E (greater than 10 years) may reduce bladder cancer mortality.46
The concern about supplements and cancer was compounded in a further meta‐analysis that showed that with the possible exception of selenium, antioxidant supplements did not reduce the risk of gastric or intestinal cancers, but rather, if anything, somewhat increased that risk by about 6%.47 This led to the Lancet highlighting in an editorial—“The prospect that vitamin pills may not only do no good but also kill their customers is a scary speculation given the vast quantities that are used in certain communities”.48
However, interestingly, and on a more positive note, a recent study of the diet of those who took part in the lung cancer study performed in Finland41 showed that when an antioxidant index was used that combined all antioxidants in foods eaten by the participants, the subjects in the highest quintiles of antioxidant intakes had a lower incidence of lung cancer.49 So it would seem that when taken in foods antioxidants may have a beneficial effect, whereas high dose purified supplements may be harmful.
There have also been some interesting findings from the SU.VI.MAX study on 13 000 adults, aged more than 60 years, from the general population in France.50 A supplement of comparatively low doses of individual antioxidants (vitamin C 120 mg, vitamin E 30 mg, β carotene 6 mg, selenium 100 µg, zinc 20 mg) was studied over a median follow up time of 7.5 years. Although no important differences were seen in total cancer incidence, ischaemic heart disease, or all cause mortality, a significant difference was seen between male and female responses. The sex stratified analysis showed lower cancer incidence and all cause mortality in men who took the supplement but not in women. A further analysis of the data suggested that this might be a result of the poorer baseline status of β carotene in men, which was progressively corrected during the period of supplementation. A further interesting result from this study was that the men receiving supplements had a lower incidence of prostate cancer if PSA at the beginning of the study was normal, but an increased incidence if PSA at baseline was raised.51 The significance of these results in relation to development of prostate cancer requires further investigation.
In summary, the situation regarding antioxidant supplements and cancer is currently confused. There would seem little doubt that a diet rich in antioxidants is likely to minimise the risk of certain types of cancer, but the situations where specific supplements may be beneficial requires much further work to target the populations and the intakes of micronutrients that may be beneficial, and to limit the possibility that these may be harmful.
Oxidative damage and eye disease.
The Age Related Eye Disease Study (AREDS) Group52,53 reported the results from a large prospective double blind placebo controlled study across 11 centres in the USA. The supplements were of vitamin C (500 mg) plus vitamin E (400 IU) plus β carotene (15 mg), or zinc (80 mg) plus copper (2 mg), or both of these. 4629 patients were followed up for a mean period of 6.3 years. The important conclusions from this study were firstly that there was no significant difference in development or progression of age related cataract. However, there was a significant reduction in the progression of age related macular degeneration, the combination of antioxidants together with zinc being most effective and leading to a reduction of about 25%.
Non‐specific functional effects
Immune function
There are many lines of in vitro evidence that have shown the essential effect of trace elements and vitamins on all aspects of immune function. It has therefore been assumed that patients who have subclinical deficiencies of trace elements and vitamins may be at risk of impaired immune function and hence an increased risk of infection. This may well be the case in specific at risk populations such as intensive care or in some developing countries with chronic poor status of micronutrients.
Outcome in critically ill
Of much interest currently in clinical nutrition is the situation in critically ill patients, especially those with burns or in intensive care where the need for antioxidants is likely to be greatest as a result of the hypermetabolism. This is compounded by the likelihood of increased losses from aspirates, fistulas, dialysis, or through damaged skin. A recent meta‐analysis has been published on studies on antioxidants in the critically ill.54 Most of the studies had small numbers of subjects, and used quite different supplements, but the meta‐analysis did suggest that overall there may be a reduction in mortality from the use of antioxidants. In an analysis of single supplements, either selenium or zinc, there was still a significant effect on mortality.
In a small study on patients in intensive care with severe infection, large doses of selenium were given for a nine day period, and were found to lead to a reduced requirement for renal replacement therapy.55 Our group has also recently completed a study following the same protocol but we failed to show any benefit on renal replacement therapy.56
In a trial of high dose vitamin C and E given as supplements to enteral nutrition, Crimi and coworkers57 found a reduction in mortality from 67.5% in the control group to 45.7% in the antioxidant supplemented group. This study is limited by the surprisingly high mortality in the placebo group. In the same study however, it is interesting to note the potential value of markers of oxidative damage. Although both groups started the study with high levels of plasma isoprostanes and thiobarbituric acid reacting substances (TBARS), the group receiving antioxidants showed a significant reduction in both these oxidative damage markers by the end of the study in contrast with the group receiving placebo.
Some of the best evidence of benefit in the critically ill has come from Mette Berger and her colleagues in Lausanne. In a landmark prospective randomised placebo controlled trial, high dose zinc, copper, and selenium were supplemented intravenously over an eight day period after severe burn injury.58 There was a significant reduction in the number of episodes of infection per patient, and also reduced length of stay in the intensive care unit, when this was standardised for the extent of body surface area burned. Recently, they have repeated this study with a larger dose of supplements. Combining the two studies shows a dramatic reduction in the incidence of hospital acquired pneumonia.59 This study clearly needs to be confirmed in a multi‐centre trial, but has great potential to reduce nosocomial pneumonia, especially in burns patients.
Infections in children
Zinc deficiency is prevalent in children in developing countries where diarrhoea is also an important problem. In six of nine trials, zinc supplementation significantly reduced the incidence of diarrhoea, and in five of these there was a lower incidence of pneumonia.60 Moreover, in acute diarrhoea trials, zinc supplemented children had a 15% lower probability of continuing diarrhoea on a given day, and in persistent diarrhoea trials, there was a 24% lower probability of continuing diarrhoea.
Vitamin A supplements in populations with poor vitamin A status, have been shown to reduce mortality from diarrhoea in community studies, and deaths from pneumonia in measles studies.61
A possible interaction between zinc and vitamin A status has been explored by Rahman and colleagues.62 In a study on 800 children (aged 12–35 months) in Bangladesh, a two week supplement of zinc, vitamin A, both, or placebo was given to children who were then followed up for six months. Combined zinc and vitamin A synergistically reduced the prevalence of persistent diarrhoea and dysentery. Interestingly, zinc alone was associated with a significant increase in acute lower respiratory infection, but this adverse effect was reduced by interaction between zinc and vitamin A. In a study of tuberculosis in Indonesia, supplementation with zinc and vitamin A led to much earlier resolution of radiological changes and time to sputum negativity.63
In a large recent study in India on residential schoolchildren with biochemical evidence of poor status for several micronutrients, a multi‐micronutrient supplement did not reduce the incidence of common childhood infections, but did reduce the duration of such illnesses.64
Key points
Micronutrients play a central part in metabolism and in the maintenance of tissue function.
An adequate intake therefore is necessary to sustain metabolism and tissue function
Provision of excess supplements to people who do not need them may be harmful.
Clinical benefit of micronutrient supplements is most probable in those people who are severely depleted and at risk of complications, and is unlikely if this is not the case.
Most clinical trials have failed to show a benefit from supplements in reducing the incidence of infections in the elderly population, coronary artery disease, or malignant disease.
Supplements of zinc and vitamin A in children in developing countries have led to reduced diarrhoea and pneumonia.
The evidence for benefit from supplements in HIV is weak.
There is some evidence for benefit of supplements on cognitive function in marginally malnourished children, but no evidence of such benefit in the elderly population.
There is good evidence of benefit in critical illness. There is special interest in selenium supplements.
Most benefit from micronutrients seems to come from a well balanced diet.
The situations where supplements are beneficial in prevention or treatment of disease requires clarification by clinical trials.
Accurate assessment of micronutrient status is difficult, especially in disease.
HIV infection and AIDS
Weight loss is a common problem in HIV, and patients are frequently found to have abnormalities of plasma mineral and trace element concentrations, especially of zinc, selenium, and magnesium.65 There are a number of interacting factors, including loss of appetite, decreased absorption, diarrhoeal and urinary losses, and the effects of redistribution from plasma to tissues as a result of the response to infection.66
Of special significance is the role of trace elements and other micronutrients in antioxidant defence. Zinc and copper are essential for cytoplasmic superoxide dismutase, manganese for the mitochondrial enzyme, and selenium is part of the prosthetic group of glutathione peroxidase. Loss of antioxidant activity will lead to increased activation of nuclear factor‐κB (NF‐κB), which is a key regulator of HIV replication.67 There is evidence that decline in plasma selenium parallels the loss of CD4+ cells, and that low levels of selenium in children is related to faster disease progression and to mortality.9 Although supplementation may lead to biochemical improvement, showing that this leads to improved clinical outcome remains controversial.68 However, one study does suggest that supplementation will delay progression of the severity of HIV.69
Selenium deficiency and virulence of infection
Although not directly relating to immune function, another line of investigation has potential consequences on the incidence of infection. This is the finding that when a normally benign strain of Coxsackie B3 virus is injected into selenium deficient mice, the virus mutates to a more virulent form that may cause severe cardiomyopathy.70 The viral genome was found to have mutated in six regions when the virus was cultured at the end of the study. The same group has also found that influenza virus causes more severe lung disease in selenium deficient mice, probably also as a result of genome mutation—they identified 29 nucleotide changes in the M1 matrix protein, an internal viral protein.71 The relevance of these findings to human infectious disease remains to be established, but if there are comparable effects in humans, there are implications for optimisation of selenium status.
Key references
Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. Br J Nutr 2001;85(suppl 2):S67–74.
Kris‐Etherton PM, Lichtenstein AH, Howard BV, et al. Antioxidant vitamin supplements and cardiovascular disease. Circulation 2004;110:637–641.
Bjelakovic G, Nikolova D, Simonetti RG, et al. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta‐analysis. Lancet 2004;364:1219–28.
Heyland DK, Dhaliwal R, Suchner U, et al. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med 2005;31:327–37.
Woodside JV, McCall D, McGartland C, et al. Micronutrients: dietary intake v. supplement use. Proc Nutr Soc 2005;64:543–53.
Infections in the elderly population
There has also been a belief that elderly people in general, who often have poor micronutrient status and deteriorating immune function, would particularly benefit from supplements. This field of work has suffered badly from the recent revelations regarding falsification of work by Dr Ranjit Chandra—his publication in the Lancet was one of the few studies to give credence to the hypothesis that supplements would reduce infectious disease in an elderly population.72 This study has now been discredited.73
One important study involved institutionalised elderly patients in France, where 725 patients over 65 years old took part in a two year double blind placebo controlled trial, where they received either trace elements (20 mg zinc/100 μg selenium), vitamins (vitamin C 120 mg, β carotene 6 mg, α tocopherol 15 mg) or both of these.74 The results were suggestive, but did not reach statistical significance, of a reduction in respiratory infections over the two year period in the group receiving trace elements, with intermediate results for the vitamin groups. This result was however particularly striking in one individual nursing home with regards to combined respiratory and urogenital infections, where in the 80 patients studied, the group receiving trace elements alone had a significant reduction in infections, whereas the groups receiving vitamins, either alone or together with trace elements did not.75 Of particular interest in the main study was the finding that sero‐conversion to influenza vaccine was significantly better in the trace element group alone, and significantly worse in the group receiving vitamins. This finding urgently requires to be investigated in future studies, as it would have implications on whether the elderly population would benefit more from targeted specific supplements rather than a general multivitamin and trace element supplements. We have however been unable to detect any improvement in sero‐conversion by one month's supplementation with a balanced trace element and vitamin preparation.76
Taken overall, a meta‐analysis showed that there is little if any benefit from a multivitamin and mineral supplement, in terms of incidence of infections in an elderly population,77 and this has recently been confirmed in a large free living population in Scotland.78
Cognitive function
It has long been thought that supplements of vitamins and trace elements may improve aspects of cognitive function, and studies of well school children have suggested that concentration ability may be improved as a result of such supplements.79 A large recent study involving residential schoolchildren in India has shown that 14 months' supplementation with a well balanced vitamin and trace element supplement, led to significant improvement in attention‐concentration, but not in IQ, memory, or school achievement scores.80 A low selenium diet for a 14 week period has been shown to have a significant negative effect on profile of mood score.81 A further striking study on prison inmates has shown that three weeks' supplementation with vitamins and trace elements led to a reduction in the number of disciplinary offences by about 30%.82
An analysis of cognitive function in elderly people taking part in the AREDS trial, did not show any benefit on cognitive function of a vitamin C, vitamin E, β carotene, and zinc supplement.83 On the other hand, in a small study of a supplement providing energy and micronutrients, some significant improvements were seen after six months.84 Studies that have used folic acid, vitamin B12, and vitamin B6, three of the vitamins most likely to improve cognitive function, found no evidence for any improvement of cognition or dementia.85 Moreover, a recent study by Rayman and coworkers86 strongly suggests that in contrast with previous reports, selenium supplementation does not improve mood or quality of life in healthy volunteers.
Taken together, these results are inconclusive as to whether there may be certain sub‐groups of the population with borderline low intakes for which cognitive function may be beneficially affected by supplements.
Bone function
Postmenopausal women in particular are at risk of osteoporosis, and although the disease is not caused by lack of calcium and vitamin D, adequate provision of these micronutrients is beneficial in maintaining and indeed increasing bone mass.87 There is a positive correlation between zinc intake and bone mineral density in middle aged pre‐menopausal women.88 A controlled trial of copper supplementation in middle aged women showed no loss in bone mineral density (BMD) in the copper supplemented group compared with a significant decrease in BMD in the control group.89 A small trial of calcium, zinc, manganese, and copper supplements showed a positive effect on spinal BMD in post‐menopausal women.90 In addition, there is growing evidence of the importance of optimal vitamin K intake in carboxylation of bone proteins and complexation of calcium, to increase bone mass.91 Specific evidence to optimise dietary intake is still lacking, and pending the results of future trials, the current best advice is to ensure a diet high in fruit and vegetables to ensure adequate intakes of all vitamins and trace elements.92
How to optimise provision of trace elements and vitamins
In reaching a decision regarding the amount of provision for a particular patient, a number of factors should be taken into account:
For people who are taking oral diets, whether in the general population or in hospital, ideally an adequate intake of micronutrients should be obtained from a well balanced diet. If such a well balanced diet cannot be consumed and there is evidence of inadequate micronutrient intake, a well balanced supplement of all trace elements and vitamins designed to deliver at most the RNI should be given. Indeed, some authorities have recommended provision of a supplement containing roughly half the RNI for most vitamins and trace elements,93 to allow for some intake from the oral diet. Whether there are particular benefits or advantages from use of diets rich in micronutrients, from a fortified diet, or from artificial supplements remains to be proved.94
If a clinical deficiency state is present, the single nutrient concerned clearly needs to be provided in adequate doses to correct this. If there is uncertainty whether a deficiency state is present, then a trial of supplements can reasonably be given for a two week period.
In disease, sufficient amounts of all micronutrients must be provided to prevent clinical deficiency states from occurring. This entails appropriate use of knowledge of requirements in health, modified by metabolic state and increased losses to assess the approximate ongoing daily requirement. This can then be adjusted for the history of the patient in terms of the probable recent intake and estimated losses, to permit an estimate of the amount required for repletion and maintenance.
Subclinical deficiency states should be avoided by using intakes that have been proved to have beneficial clinical effects in controlled clinical studies.
Plasma concentrations of trace elements and vitamins should be interpreted together with an assessment of the acute phase response, to determine if the trend of results is likely to be beneficial.66 During an acute phase response to trauma, infection, or inflammation, there is a fall in serum zinc, iron, and selenium, and a rise in serum copper. Red blood cell glutathione peroxidase may be helpful in evaluating whole body selenium status.
Biochemical indicators of the efficacy of antioxidant systems should be considered—although not yet in widespread use, there is substantial evidence of increased production of markers of oxidative damage such as malondialdehyde in catabolic illness, and that these can be reduced by providing extra selenium and zinc, and other antioxidants.95
As all micronutrients are potentially harmful if given in excessive amounts, special care must be used when providing them intravenously. In any case, high doses should only be used when there is definite evidence of such a requirement, for example, in severe burns.
Conclusion
Micronutrients play a central part in metabolism and in the maintenance of tissue function. An adequate intake therefore is necessary but provision of excess supplements to people who do not need them may be harmful. Clinical benefit is most probable in those people who are severely depleted and at risk of complications, and is unlikely if this is not the case. Much more research is needed to characterise better markers of micronutrient status both in terms of metabolic effects and antioxidant effects, so that at risk patients can be identified and supplementation modified accordingly. Large scale trials of different doses of micronutrients are required with precise outcome markers to optimise intakes in different groups of patients as well as in the general population.
Self assessment questions (true (T)/false (F); answers after the references)
-
Which of the following are regarded as important dietary antioxidants?
thiamine
ascorbic acid
selenium
zinc
α tocopherol (vitamin E)
-
Which of the following are likely to be inadequate in the typical UK diet?
thiamine
ascorbic acid
selenium
zinc
α tocopherol (vitamin E)
-
In which of the following is there good evidence of benefit from micronutrient supplements
vitamin E and coronary artery disease
vitamin C and gastric neoplasm
Multivitamin supplements and community acquired infections in the elderly population
Mulivitamin supplements and progression of HIV/AIDS
Pneumonia in children in areas of zinc deficiency
-
Which of the following changes are associated with inflammation
a fall in serum iron
a fall in serum zinc
a rise in serum ferritin
a fall in serum α tocopherol
a fall in serum copper
-
Excess provision of which of the following vitamins has been associated with increased mortality?
α tocopherol
ascorbic acid
thiamine
riboflavine
pyridoxine
Answers
1. (A) F, (B) T, (C) T, (D) T, (E) T; 2. (A) F, (B) F, (C) T, (D) F, (E) F; 3. (A) F, (B) F, (C) F, (D) T, (E) T; 4. (A) T, (B) T, (C) T, (D) T, (E) F; 5. (A) T, (B) F, (C) F, (D) F, (E) F.
Footnotes
Funding: none.
Conflicts of interest: none.
References
- 1.Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. Br J Nutr 200185(suppl 2)S67–S74. [PubMed] [Google Scholar]
- 2.Food and Nutrition Board IoM Dietary reference intakes for thiamin, roboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed]
- 3.Food and Nutrition Board IoM Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press, 2000 [PubMed]
- 4.Panel of Dietary Reference Values, Department of Health Dietary reference values for food energy and nutrients for the United Kingdom. London: HMSO, 1991 [PubMed]
- 5.Food Standards Agency Eat well, be well. London: FSA, 2006
- 6.Hoare J, Henderson L, Bates C J.et al The national diet and nutrition survey: adults aged 19–64. London: The Stationery Office, 2004
- 7.Finch S, Doyle W, Lowe C.et alNational diet and nutrition survey: people aged 65 and over. London: The Stationery Office, 1998
- 8.Food Standards Agency National diet and nutrition survey: young people aged 4 to 18 years, 1997. London: The Stationery Office, 2000
- 9.Rayman M P. The argument for increasing selenium intake. Proc Nutr Soc 200261203–215. [DOI] [PubMed] [Google Scholar]
- 10.Clark L C, Combs G F, Jr, Turnbull B W.et al Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 19962761957–1963. [PubMed] [Google Scholar]
- 11.Weaver C. Adolescence:the period of dramatic bone growth. Endocrine 20021743–48. [DOI] [PubMed] [Google Scholar]
- 12.Shenkin A. Adult micronutrient requirements. In: Payne‐James J, Grimble G, Silk D, eds. Artificial nutrition support in clinical practice. London: GMM, 2001193–212.
- 13.Solomon S M, Kirby D F. The refeeding syndrome: a review. JPEN J Parenter.Enteral Nutr 19901490–97. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbertson D P, Fell G S, Smith C.et al Metabolism after injury. 1. Effects of severity,nutrition,and environmental temperature on protein, potassium, zinc and creatine. Br J Surg 197259926–931. [DOI] [PubMed] [Google Scholar]
- 15.Kay R G, Tasman‐Jones C, Pybus J.et al A syndrome of acute zinc deficiency during total parenteral alimentation in man. Ann Surg 1976183331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo‐Duran C, Vial P, Uauy R. Trace mineral balance during acute diarrhea in infants. J Pediatr 1988113452–457. [DOI] [PubMed] [Google Scholar]
- 17.Geissler C, Powers H.Human nutrition. Edinburgh: Churchill Livingstone, 2005
- 18.Schnyder G, Rouvinez G. Total plasma homocysteine and restenosis after percutaneous coronary angioplasty: current evidence. Ann Med 200335156–163. [DOI] [PubMed] [Google Scholar]
- 19.Wald D S, Law M, Morris J K. Homocysteine and cardiovascular disease: evidence on causality from a meta‐analysis. BMJ 20023251202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange H, Suryapranata H, De Luca G.et al Folate therapy and in‐stent restenosis after coronary stenting. N Engl J Med 20043502673–2681. [DOI] [PubMed] [Google Scholar]
- 21.Bonaa K, Njolstad I, Ueland P.et al Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 20063541578–1588. [DOI] [PubMed] [Google Scholar]
- 22. The Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 20063541567–1577. [DOI] [PubMed] [Google Scholar]
- 23.Loscalzo J. Homocysteine trials‐clear outcomes for complex reasons. N Engl J Med 20063541629–1632. [DOI] [PubMed] [Google Scholar]
- 24.Vincent J B. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc 20046341–47. [DOI] [PubMed] [Google Scholar]
- 25.Anderson R A, Cheng N, Bryden N A.et al Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 1997461786–1791. [DOI] [PubMed] [Google Scholar]
- 26.Cefalu W T, Bell‐Farrow A D, Stigner‐Jet al Effect of chromium picolinate on insulin sensitivity in vivo. J Trace Elem Exp Biol 19991271–84. [Google Scholar]
- 27.Jeejeebhoy K N, Chu R C, Marliss E B.et al Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long‐term total parenteral nutrition. Am J Clin Nutr 197730531–538. [DOI] [PubMed] [Google Scholar]
- 28.Anderson R A. Chromium in the prevention and control of diabetes. Diabetes Metab 20002622–27. [PubMed] [Google Scholar]
- 29.Trow L G, Lewis J, Greenwood R H.et al Lack of effect of dietary chromium supplementation on glucose tolerance, plasma insulin and lipoprotein levels in patients with type 2 diabetes. Int J Vitam Nutr Res 20007014–18. [DOI] [PubMed] [Google Scholar]
- 30.Kalman D S. Chromium picolinate and type 2 diabetes. Am J Clin Nutr 200378192–193. [DOI] [PubMed] [Google Scholar]
- 31.Wolman S L, Anderson G H, Marliss E B.et al Zinc in total parenteral nutrition: requirements and metabolic effects. Gastroenterology 197976458–467. [PubMed] [Google Scholar]
- 32.Stephens N G, Parsons A, Schofield P M.et al Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge heart antioxidant study (CHAOS). Lancet 1996347781–786. [DOI] [PubMed] [Google Scholar]
- 33.Fang J C, Kinlay S, Beltrame J.et al Effect of vitamins C and E on progression of transplant‐associated arteriosclerosis: a randomised trial. Lancet 20023591108–1113. [DOI] [PubMed] [Google Scholar]
- 34.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet 2000355253–259. [PubMed] [Google Scholar]
- 35.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Lancet 1999354447–455. [PubMed] [Google Scholar]
- 36.Heart Protection Study Collaborative Group MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 200236023–33.12114037 [Google Scholar]
- 37.Kris‐Etherton P M, Lichtenstein A H, Howard B V.et al Antioxidant vitamin supplements and cardiovascular disease. Circulation 2004110637–641. [DOI] [PubMed] [Google Scholar]
- 38.Miller ER I I I, Pastor‐Barriuso R, Dalal D.et al Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Ann Intern Med 200514237–46. [DOI] [PubMed] [Google Scholar]
- 39.American Institute for Cancer Research Vitamin supplements don't help prevent cancer. Washington, DC: AICR 2003
- 40.Li W, Zhu Y, Yan X.et al [The prevention of primary liver cancer by selenium in high risk populations]. Zhonghua Yu Fang Yi Xue Za Zhi 200034336–338. [PubMed] [Google Scholar]
- 41.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers The Alpha‐Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 19943301029–1035. [DOI] [PubMed] [Google Scholar]
- 42.Omenn G S, Goodman G E, Thornquist M D.et al Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 19963341150–1155. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E, Stampfer M J, Colditz G A.et al Multivitamin use, folate, and colon cancer in women in the nurses' health study. Ann Intern Med 1998129517–524. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs E J, Connell C J, Patel A V.et al Multivitamin use and colon cancer mortality in the cancer prevention study II cohort (United States). Cancer Causes Control 200112927–934. [DOI] [PubMed] [Google Scholar]
- 45.Zhang S M, Giovannucci E L, Hunter D J.et al Vitamin supplement use and the risk of non‐Hodgkin's lymphoma among women and men. Am J Epidemiol 20011531056–1063. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs E J, Henion A K, Briggs P J.et al Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am J Epidemiol 20021561002–1010. [DOI] [PubMed] [Google Scholar]
- 47.Bjelakovic G, Nikolova D, Simonetti R G.et al Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta‐analysis. Lancet 20043641219–1228. [DOI] [PubMed] [Google Scholar]
- 48.Forman D, Altman D. Vitamins to prevent cancer: supplementary problems. Lancet 20043641193–1194. [DOI] [PubMed] [Google Scholar]
- 49.Wright M E, Mayne S T, Stolzenberg‐Solomon R Z.et al Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol 200416068–76. [DOI] [PubMed] [Google Scholar]
- 50.Hercberg S, Galan P, Preziosi P.et al The SU.VI. MAX study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals, Arch Intern Med 20041642335–2342. [DOI] [PubMed] [Google Scholar]
- 51.Meyer F, Galan P, Douville P.et al Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer 2005116182–186. [DOI] [PubMed] [Google Scholar]
- 52.Age‐Related Eye Disease Study Research Group A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E and beta carotene for age‐related cataract and vision loss: AREDS report no 9. Arch Ophthalmol 20011191439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Age‐Related Eye Disease Study Research Group A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no 8. Arch Ophthalmol 20011191417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heyland D K, Dhaliwal R, Suchner U.et al Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med 200531327–337. [DOI] [PubMed] [Google Scholar]
- 55.Angstwurm M W, Schottdorf J, Schopohl J.et al Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 1999271807–1813. [DOI] [PubMed] [Google Scholar]
- 56.Mishra V, Baines M, Perry S.et al Selenium supplementation and outcome in septic ICU patients. Clin Chim Acta 2005355S45–S46. [Google Scholar]
- 57.Crimi E, Liguori A, Condorelli M.et al The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double‐blind, placebo‐controlled trial. Anesth Analg 200499857–863. [DOI] [PubMed] [Google Scholar]
- 58.Berger M M, Spertini F, Shenkin A.et al Trace element supplementation modulates pulmonary infection rates after major burns: a double‐blind, placebo‐controlled trial. Am J Clin Nutr 199868365–371. [DOI] [PubMed] [Google Scholar]
- 59.Berger M M, Eggimann P, Revelly J P.et al Selenium supplements reduce the incidence of nosocomial pneumonia after major burns. Clin Nutr 200524614 [Google Scholar]
- 60.Black R E. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 20031331485–9S. [DOI] [PubMed] [Google Scholar]
- 61.Glasziou P P, Mackerras D E. Vitamin A supplementation in infectious diseases: a meta‐analysis. BMJ 1993306366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman M M, Wahed M A, Fuchs G J.et al Synergistic effect of zinc and vitamin A on the biochemical indexes of vitamin A nutrition in children. Am J Clin Nutr 20027592–98. [DOI] [PubMed] [Google Scholar]
- 63.Karyadi E, West C E, Schultink W.et al A double‐blind, placebo‐controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr 200275720–727. [DOI] [PubMed] [Google Scholar]
- 64.Sarma K V, Udaykumar P, Balakrishna N.et al Effect of micronutrient supplementation on health and nutritional status of schoolchildren: growth and morbidity. Nutrition 200622S8–14. [DOI] [PubMed] [Google Scholar]
- 65.Cunningham‐Randles S. Trace element and mineral nutrition in HIV infection and AIDS. In: Bogden JD, Kleavey LM, eds. Clinical nutrition of the essential trace elements and minerals. Clifton: Humana Press, 2000333–352.
- 66.Shenkin A. Trace elements and inflammatory response: implications for nutritional support. Nutrition 199511100–105. [PubMed] [Google Scholar]
- 67.Conner E M, Grisham M B. Inflammation, free radicals, and antioxidants. Nutrition 199612274–277. [DOI] [PubMed] [Google Scholar]
- 68.Irlam J H, Visser M E, Rollins N.et al Micronutrient supplementation in children and adults with HIV infection. Cochrane Library. Chichester: Wiley, 2005 [DOI] [PubMed]
- 69.Fawzi W W, Msamanga G I, Spiegelman D.et al A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med 200435123–32. [DOI] [PubMed] [Google Scholar]
- 70.Beck M A, Shi Q, Morris V C.et al Rapid genomic evolution of a non‐virulent coxsackievirus B3 in selenium‐deficient mice results in selection of identical virulent isolates. Nat Med 19951433–436. [DOI] [PubMed] [Google Scholar]
- 71.Beck M A, Levander O A, Handy J. Selenium deficiency and viral infection. J Nutr 20031331463–7S. [DOI] [PubMed] [Google Scholar]
- 72.Chandra R K. Effect of vitamin and trace‐element supplementation on immune responses and infection in elderly subjects. Lancet 19923401124–1127. [DOI] [PubMed] [Google Scholar]
- 73.Carpenter K J, Roberts S, Sternberg S. Nutrition and immune function: a 1992 report. Lancet 20033612247–2248. [DOI] [PubMed] [Google Scholar]
- 74.Girodon F, Galan P, Monget A L.et al Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX geriatric network. Arch Intern Med 1999159748–754. [DOI] [PubMed] [Google Scholar]
- 75.Girodon F, Blache D, Monget A L.et al Effect of a two‐year supplementation with low doses of antioxidant vitamins and/or minerals in elderly subjects on levels of nutrients and antioxidant defense parameters. J Am Coll Nutr 199716357–365. [DOI] [PubMed] [Google Scholar]
- 76.Allsup S J, Shenkin A, Gosney M A.et al Can a short period of micronutrient supplementation in older institutionalized people improve response to influenza vaccine? A randomized, controlled trial. J Am Geriatr Soc 20045220–24. [DOI] [PubMed] [Google Scholar]
- 77.El Kadiki A, Sutton A J. Role of multivitamins and mineral supplements in preventing infections in elderly people: systematic review and meta‐analysis of randomised controlled trials. BMJ 2005330 pp 871-4 correction BMJ 2005331142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avenell A, Campbell M K, Cook J A.et al Effect of multivitamin and multimineral supplements on morbidity from infections in older people (MAVIS trial): pragmatic, randomised, double blind, placebo controlled trial. BMJ 2005331324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benton D. Micro‐nutrient supplementation and the intelligence of children. Neurosci Biobehav Rev 200125297–309. [DOI] [PubMed] [Google Scholar]
- 80.Vazir S, Nagalla B, Thangiah V.et al Effect of micronutrient supplement on health and nutritional status of schoolchildren: mental function. Nutrition 200622S26–S32. [DOI] [PubMed] [Google Scholar]
- 81.Finley J W, Penland J G. Adequacy or deprivation of dietary selenium in healthy men: clinical and psychological findings. J Trace Elem Exp Biol 19981111–27. [Google Scholar]
- 82.Gesch C B, Hammond S M, Hampson S E.et al Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners. Randomised, placebo‐controlled trial. Br J Psychiatry 200218122–28. [DOI] [PubMed] [Google Scholar]
- 83.Yaffe K, Clemons T E, McBee W L.et al Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology 2004631705–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wouters‐Wesseling W, Wagenaar L W, Rozendaal M.et al Effect of an enriched drink on cognitive function in frail elderly persons. J Gerontol A Biol Sci Med Sci 200560265–270. [DOI] [PubMed] [Google Scholar]
- 85.Ellinson M, Thomas J, Patterson A. A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J Hum Nutr Diet 200417371–383. [DOI] [PubMed] [Google Scholar]
- 86.Rayman M, Thompson A, Warren‐Perry M.et al Impact of selenium on mood and quality of life: a randomized, controlled trial. Biol Psychiatry 200659147–154. [DOI] [PubMed] [Google Scholar]
- 87.Sub Group on Bone health CoMAoFP Nutrition and bone health: with particular reference to calcium and vitamin D. Report on health and social subjects no 49. London: The Stationery Office, 1998 [PubMed]
- 88.Reid D M, New S A. Nutritional influences on bone mass. Proc Nutr Soc 199756977–987. [DOI] [PubMed] [Google Scholar]
- 89.Eaton‐Evans J, McIlrath E M, Jackson E V.et al Copper supplementation and the maintenance of bone mineral density in middle‐aged women. J Trace Elem Exp Biol 1996987–94. [Google Scholar]
- 90.Strause L, Saltman P, Smith K T.et al Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J Nutr 19941241060–1064. [DOI] [PubMed] [Google Scholar]
- 91.Vermeer C, Shearer M, Zitterman A.et al Beyond deficiency:potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr 200643325–335. [DOI] [PubMed] [Google Scholar]
- 92.Nieves J. Osteoporosis:the role of micronutrients. Am J Clin Nutr 2005811232–9S. [DOI] [PubMed] [Google Scholar]
- 93.Dror Y, Stern F, Berner Y N.et al Recommended micronutrient supplementation for institutionalized elderly. J Nutr Health Aging 20026295–300. [PubMed] [Google Scholar]
- 94.Woodside J V, McCall D, McGartland C.et al Micronutrients: dietary intake v. supplement use. Proc Nutr Soc 200564543–553. [DOI] [PubMed] [Google Scholar]
- 95.Berger M M. Chiolero R. Relations between copper, zinc and selenium intakes and malondialdehyde excretion after major burns. Burns 199521507–512. [DOI] [PubMed] [Google Scholar]