Abstract
Inflammation induced by inhalation of air pollutant particles has been implicated as a mechanism for the adverse health effects associated with exposure to air pollution. The inflammatory response is associated with upregulation of various pro-inflammatory cytokines and chemokines. We have previously shown that diesel exhaust particles (DEP), a significant constituent of air pollution particulate matter in many urban areas, bind and concentrate IL-8, an important human neutrophil-attracting chemokine, and that the chemokine remains biologically active. In this report, we examine possible mechanisms of this association and the effects on clearance of the chemokine. The binding appears to be the result of ionic interactions between negatively charged particles and positively charged chemokine molecules, possibly combined with intercalation into small pores in the particles. The association is not limited to diesel exhaust particles and IL-8: several other particle types also adsorb the chemokine and several other cytokines are adsorbed onto the diesel particles. However, there are wide ranges in the effectiveness of various particle types and various cytokines. Finally, male Fisher 344 rats were intratracheally instilled with chemokine alone or combined with diesel exhaust or silica particles under isofluorane anesthesia. In contrast to silica particles, which do not bind the chemokine, the presence of diesel exhaust particles, which bind the chemokine, prolonged the retention of the chemokine.
Keywords: Diesel, particle, inflammation, cytokines, adsorption, zeta potential
Introduction
Epidemiological studies have demonstrated that increased levels of air pollution are associated with a wide range of health effects. Many of these effects involve altered inflammatory responses in the respiratory system, the initial target of inhaled pollutants. Specifically, exacerbation of asthma, increased pathology in response to respiratory infections, reduced lung function, and increased respiratory mortality have been observed (Brunekreef et al., 1997;van Vliet et al., 1997;Samet et al., 2000;Pope, III et al., 2002;Hoek et al., 2001). Several studies have specifically implicated fossil fuel combustion products in general, and mobile source emissions from heavy-duty vehicles (primarily diesel engines) in particular, as strong contributors to the health burden (Janssen et al., 2002;Laden et al., 2000;Mar et al., 2000). However, many questions remain regarding the specific cellular and molecular mechanisms by which these emission products could produce health effects.
Pulmonary inflammation is one potentially important type of response to inhalation of air pollution constituents. Experimental results have confirmed that inflammation does occur in response to exposure to inhaled diesel exhaust or intratracheally instilled diesel exhaust particles (DEP). Inhalation of diesel exhaust (Mauderly et al., 1994;Hashimoto et al., 2001) or instillation of DEP (Ichinose et al., 1997;Ito et al., 2000;Miyabara et al., 1998;Murphy et al., 1998;Sadakane et al., 2002;Seagrave et al., 2002) causes lung inflammation in animals. Animal models also provide evidence for adjuvant effects of DEP (recently reviewed in (Boland et al., 2001). DEP are believed to cause inflammation at least partially by inducing pro-inflammatory cytokine expression and secretion by the lung cells (Ohtoshi et al., 1998;Steerenberg et al., 1998;Juvin et al., 2002;Boland et al., 1999;Boland et al., 2000;Kawasaki et al., 2001;Abe et al., 2000;Stringer et al., 1998;Quay et al., 1998).
We have previously shown that DEP selectively adsorb a key pro-inflammatory chemokine (IL-8) involved in recruitment and activation of lung neutrophils and that this binding is modestly sensitive to ionic strength, but does not require the organic fraction of the DEP. Furthermore, the DEP-associated chemokine is biologically active (Seagrave et al., 2004). A substantial fraction of inhaled or instilled DEP persists in the lungs long after the cessation of exposure (Wolff et al., 1986), with considerable retention at low exposure concentrations (Strom et al., 1990). These observations suggest the hypotheses that DEP exacerbate inflammatory responses (a) by concentrating pro-inflammatory mediators, thus providing increased localized activation of the target cells, or (b) by extending the pro-inflammatory chemokine lifetime in the lung. Thus, damage may be related to strong activation of cells at the sites of high particle deposition, prolongation of the inflammatory response, or both. Particle properties resulting in binding of a “corona” of associated biomolecules (Cedervall et al., 2007) may be a common phenomenon, which may mediate at least some of the biological effects of nanoscale particles (Kendall et al., 2004a;Kendall, 2007;Dutta et al., 2007). Given the considerable existing literature regarding the pro-inflammatory and adjuvant-like effects of the particulate fraction of this environmentally ubiquitous pollutant, it is important to understand the potential mechanisms for these effects. The current study was designed to investigate the mechanisms of the DEP/chemokine interactions and the biological implications of the binding.
Materials and Methods
Suspension assays for binding
Particles were suspended in PBS containing 0.02% Tween-80, a non-toxic, non-ionic surfactant, and sonicated for 1 min at setting 4 on a Branson Sonifier equipped with a “cup-”style horn, as previously described (Seagrave et al., 2004). Particle suspensions, diluted as required in PBS with 0.02% Tween-80 were combined with equal volumes of a 2× concentrated solution of the chemokines in PBS without Tween-80 (final Tween-80 concentration 0.01%), incubated for the required time and temperature, and centrifuged for 5 min at 14,000 × g in an Eppendorf microfuge. Chemokine remaining in the supernatant was assayed by ELISA or, for radiolabeled IL-8, by gamma counting. Data were fit to a competitive binding model: y = 100/1+ 10(x-logk) where x is the concentration of the particles, y is the percent remaining in the supernatant, and k is the EC50. To assess maximal binding and affinity, tracer amounts of radiolabeled IL-8 were combined with increasing concentrations of unlabeled IL-8. The counts in the supernatant and the pellet were analyzed in a Packard Cobra II Auto-gamma Model 5002 Single Detector gamma counter. Bound IL-8 was calculated from the total and the tracer and plotted against the total concentration. Data were fit to a hyperbolic function, after correction for non-specific binding obtained in the presence of a 10,000-fold excess of unlabeled IL-8.
ELISA assays
These analyses were performed as recommended by the supplier.
Luminex Immunomultiplex assays
A Biosource Luminex kit for analysis of 30 human cytokines, chemokines, and growth factors was used as recommended by the supplier, and analyzed on a BioPlex 100 instrument. The standards for the kit were used as the source of cytokines for the DS binding.
Zeta potential and size
The hydrodynamic diameter and zeta potential of the various particles were measured under experimental conditions (i.e., vehicle, time points (2h), effective concentration, temperatures (25° C) that paralleled the biological response. The apparent zeta potential of each material was measured with a Zeta Sizer Nano ZS (Malvern, Inc., Southborough, MA) using the Smoluchowski equation to correlate particle mobility to zeta potential. Particle size distributions (PSD) were determined by dynamic light scattering as a function of time and reported as volume %. Both the zeta potential and the PSD were determined using 25 μg/ml dispersions of each sample). For calculating particle size (volume average) the refractive index of magnetite (the oxidation product of iron) was used (2.42). At least 10 replicates were collected for each material to determine the size distribution and zeta potential. Reported values are the mean and standard deviation of these measurements.
Rat studies
The protocol for all animal studies was approved by Lovelace Respiratory Research Institute’s Institutional Animal Care and Use Committee. Male Fisher 344 rats, approximately 8 wk old at shipping, were purchased from Charles River, and quarantined in a barrier facility for 2 wk prior to use in experiments. They were housed 2 rats/cage under controlled temperature and humidity with a 12:12 light cycle, and provided with tap water and standard rodent diet (Lab Bloks from Harlan Teklad, Madison, WI) ad libitum. Intratracheal instillations of the 125I-IL-8/particle mixtures suspended in 0.9% NaCl with 0.01% Tween-80 were performed under isoflurane anesthesia as previously described (Seagrave et al., 2002). At the indicated times following instillation, the rats were killed with an overdose of a pentobarbital-based euthanasia solution (Euthasol, Virbac Labs, Ft. Worth, TX). Lungs and other organs were removed. Lungs were lavaged with PBS, and the lavage fluid was separated into supernatant and cell pellet by centrifugation. 125I-IL-8 in the various organs was measured using a Packard Cobra II Auto-gamma Model 5002 Single Detector gamma counter. To determine whether the radiolabel remained associated with the chemokine, a portion of the lung from selected animals was thoroughly homogenized in 1% Triton X-100 in PBS. The homogenate was centrifuged and the supernatant was placed in MicroCentricon ultrafiltration units with a 5kDa cut-off membrane. The fraction of the label passing through the membrane was assumed to be lost from the chemokine.
Materials
MIP-2, IL-8, and ELISA kits for IL-8 were purchased from R and D Systems (Minneapolis, MN). All other ELISA kits and multiplex (Luminex) kits were purchased from Biosource International (Carlsbad, CA). 125I-labeled IL-8 was purchased from Amersham (Piscataway, NJ). DEP (Standard Reference Materials [SRM] 2975 and 1650) and ambient particulate matter from Washington, DC (SRM 1649a) and St. Louis, MO (SRM 1648) were obtained from the National Institute of Standards and Technology (NIST: Gaithersburg, MD). Ambient particulate matter from Ottawa, CA (EHC-93) was a gift from R. Vincent (Health Canada, Ottawa). Silica particles (Min-U-Sil 5) were obtained from U.S. Silica (Berkeley Springs, WV). Carbon black (Elftex-12, Monarch 900, and Monarch 1000) were provided by Cabot Corporation (Boston MA). Additional samples of DEP were collected during ongoing studies from the exhaust of a Cummins 5.9-L Turbo engine, run on a repeating heavy duty cycle, and from the exhaust of a Yanmar 5500-W generator run under either full load or 75% load conditions. Particulate matter collected from a set of normal emitter gasoline vehicles and a black smoker gasoline vehicle were collected and extracted as previously described (Seagrave et al., 2002). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Statistical analyses and model fitting: Statistical analyses (ANOVA with Tukey Kramer post-test) and curve fitting (models defined in the text) were performed using Graph Pad Prism software, version 4.
RESULTS
Comparison of Particle Characteristics
Table 1 presents a summary of the characteristics of the particles as received from the supplier or as determined in the laboratory.
Table 1.
Particle Characteristics
| Particle | Zeta potential |
Native size (nm) provided by supplier |
Suspension Size (nm) |
Organic content |
Surface area |
|---|---|---|---|---|---|
| NIST 2975 | −6.47 ± 0.54 | 1620 | 402 ± 15.7 | 2.7% | 91 m2/g |
| NIST 1650 | −7.99 ± 0.52 | 1550 | 1346 ±145 | 20.2% | 108 m2/g |
| Truck | −6.27 ± 1.76 | 140 | 525 ± 8.9 | 30% | NA |
| Generator lowa | −2.38 ± 0. 65 | NA | 1229 ± 270 | 86% | NA |
| Generator higha | −7.04 ± 1.14 | NA | 347 ± 19 | 26% | NA |
| Ottawa | −18.3 ± 1.20 | 500 | 870 ±144 | NA | NA |
| St. Louis | −15.07 ± 1.64 | 5900 | 2432 ± 318 | 2.3% | NA |
| Wash. DC | −10.00 ± 1.98 | 12900 | 1745 ± 423 | 4.5% | NA |
| Black Smoker | −12.16 ± 1.98 | NA | 80 ± 38 | 68% | NA |
| Avg Gasoline | −7.14 ± 1.40 | NA | 860 ± 268 | 52% | NA |
| Elftex 12 | −4.89 ± 1.05 | 12 | 336 ± 10 | Lowb | 443 m2/g |
| Monarch 900 | −4.42 ± 0.29 | 15-16 | 178 ± 4.7 | Lowb | 320 m2/g |
| Monarch 1000 | −4.47 ± 0.57 | 15-16 | 705 ± 156 | Lowb | 340 m2/g |
| Silica | −12.36 ± 0.36 | 1700 | 856 ± 91.7 | < 0.5% | NA |
Low and high refer to the load imposed on the generator.
Organic content was not measured, but is assumed to be low.
NA: not available.
DEP Removes Immunodetectable IL-8 and MIP-2 from Solution
We previously showed that IL-8 was lost from suspensions of DEP following centrifugation, suggesting adsorption to the particles (Seagrave et al., 2004). It was therefore of interest whether a similar phenomenon was observed with MIP-2, a functionally homologous rodent chemokine. Figure 1 shows that incubation of a 500 pg/ml solution of IL-8 or MIP-2 (physiologically relevant concentrations) with increasing concentrations of DEP SRM2975 resulted in similar dose-dependent decreases in the chemokine available for assay in the supernatant of the reaction mixture. The effective concentrations of DEP SRM 2975 for removal of half of the chemokine (EC50) were 22.5 μg/ml for IL-8 and 12.1 μg/ml for MIP-2.
Figure 1.
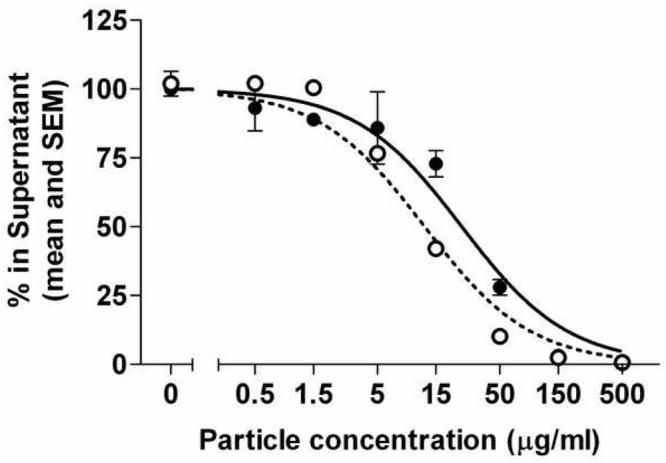
Depletion of soluble IL-8 and MIP-2 by diesel soot particles. SRM2975 was suspended by sonication in PBS with 0.01% Tween-80. Serial dilutions were made in the same buffer. IL-8 (solid circles) or MIP-2 (open circles) were then added to the particles and incubated for 2 h. The suspensions were centrifuged and the chemokine remaining in the supernatant was measured by ELISA. Curves show fits to a competitive binding model.
Loss of IL-8 Is Due to Adsorption, Not Loss of Epitopes
One possible mechanism for the loss of chemokines detectable by ELISA from suspensions of DEP is that chemical modification of the chemokines occurred that destroyed epitopes required for recognition by the capture and/or detection antibodies. To confirm that IL-8 was directly bound to the DEP SRM 2975, we performed a similar experiment using tracer levels of radiolabeled 125I-IL-8 with unlabeled IL-8 at 500 pg/ml. Results are shown in Figure 2. The EC50 for depletion of the radiolabel from the supernatant, 22.9 μg/ml, was essentially identical to that obtained using ELISA to detect the remaining chemokine, thus confirming that the chemokine was adsorbed to the particles.
Figure 2.
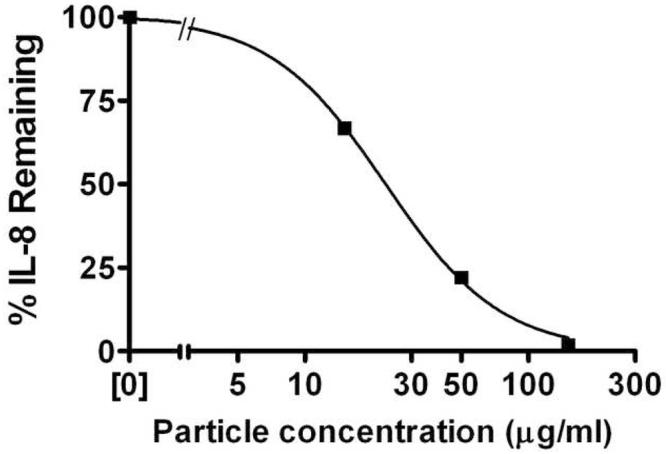
Association of radiolabeled IL-8 with diesel soot particles. SRM2975 was suspended as described in the legend to Figure 1 and incubated with 500 pg/ml IL-8 containing tracer amounts of 125I-IL-8 for 2 h. The suspension was centrifuged and the radiolabel in the supernatant was determined by gamma counting. The remaining label was detected in the pellet (data not shown). Standard errors for triplicate measurements were smaller than the size of the symbols. Curves were fit to a competitive binding model.
Some Other Forms of Particulate Matter Adsorb IL-8
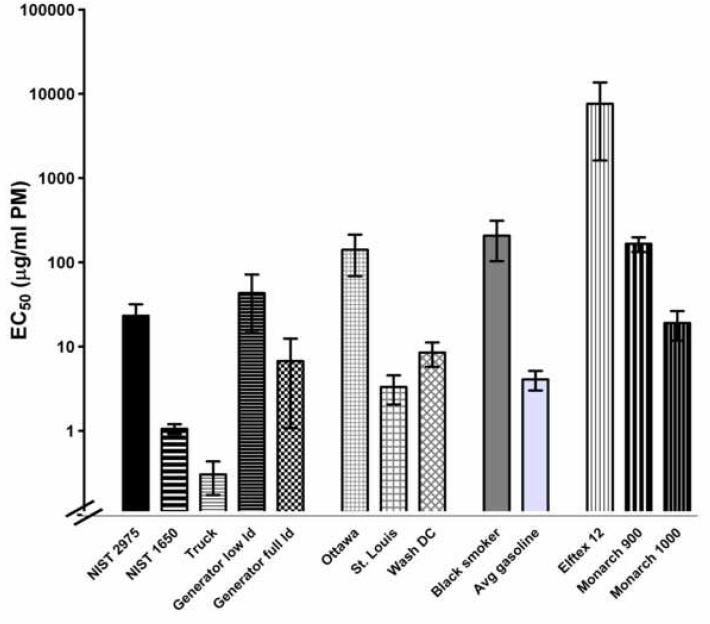
We repeated the experiment using a variety of other types of particulate matter, including three forms of carbon black (Elftex-12, Monarch 900, and Monarch 1000), three forms of ambient particulate matter (from Washington DC [SRM 1649a], St. Louis, MO [SRM 1648], and Ottawa Canada [EHC93]), silica particles (Min-U-Sil 5), DEP from four additional sources: SRM 1650, and particles collected on teflon-coated glass fiber filters from a dilution tunnel associated with a Cummins 2.9-L Turbo Diesel engine on a dynamometer run on a cycle, burning certification fuel (Reed et al., 2003), or from a Yanmar 5500 W generator run on the same fuel under either full load [5500 W] or low load [4000 W] conditions, or particles from normal emitting gasoline engines or a high emitter “black smoker” gasoline engine. Each type of particle was tested using the ELISA detection system, in at least three independent experiments. Results are shown in Figure 3. These results clearly show a wide range in the effectiveness, assessed as the EC50, of clearing IL-8 from the solutions, from < 1 μg/ml for the DEP from the Cummins (truck) engine to > 10,000 μg/ml for the Elftex carbon black. Even among the five types of DEP there was greater than a 100-fold difference in the EC50’s. In four of six experiments with silica no significant depletion from the supernatant was observed even at 500 μg/ml, so an EC50 could not be calculated.
Figure 3.
Binding of IL-8 by other forms of particulate matter. Various forms of particulate matter were suspended in PBS at a wide range of concentrations as described in the legend to Figure 1 and incubated with 500 pg/ml of IL-8 for 2 h. The particles were removed by centrifugation and the IL-8 in the supernatant was determined by ELISA. The EC50s for the various particles were determined as described in the legend to Figure 1. At least three independent experiments were performed for each type of particle, and the results shown are the average and SEM of the resulting EC50 determinations.
IL-8 Association with DEP Is Saturable
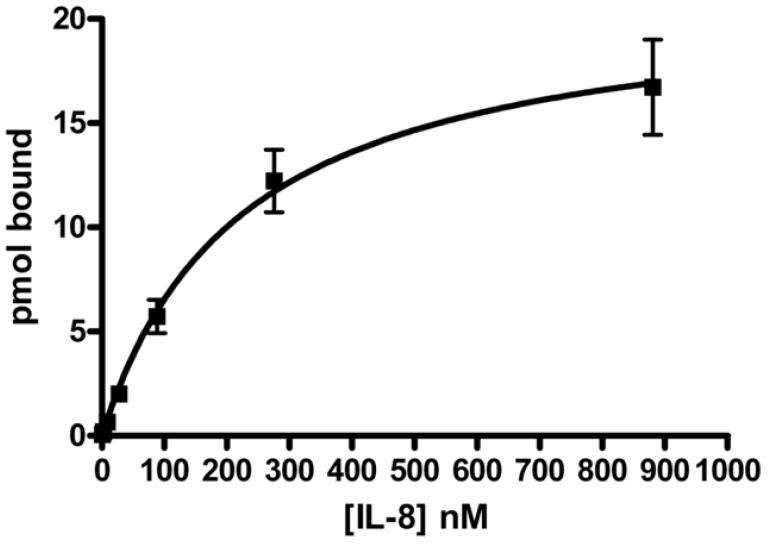
Tracer levels of 125I-IL-8 were combined with unlabeled IL-8 at a range of concentrations and allowed to adsorb to 2.5 μg DEP SRM 2975 in 1 ml of PBS with 0.01% Tween-80 for 2 h. The radiolabel remaining in the supernatant and that bound to the DEP pellet were determined, and the concentrations of the bound and free IL-8 were calculated. The results were analyzed by fitting a nonlinear regression, assuming a single site binding model, corrected for non-specific binding in the presence of a 10,000-fold molar excess of unlabeled IL-8. The results indicated that the association was saturable, with a maximum binding of 11.7 ± 1.6 μmoles of IL-8 per gram of DEP and an association constant of 240 ± 60 nM. Results of these experiments are shown in Figure 4.
Figure 4.
IL-8 association with diesel soot is saturable. Radiolabeled IL-8 at tracer levels was combined with a range of concentrations of unlabeled IL-8. The solutions were incubated with SRM2975 for 2 h and then centrifuged. The fraction associated with the particles and that in the supernatant were determined and the data were plotted as pmol bound as a function of the free concentration. Data were analyzed using a single-site binding model. Data shown are the mean and standard error of four independent experiments, each conducted in duplicate, for BSA and three independent experiments for cytochrome C.
Mechanisms of Adsorption
Competition by proteins
Given that the binding appeared to be saturable, we next determined whether other proteins would compete for the binding sites. Inclusion of bovine serum albumin (BSA) at concentrations between 3 and 300 μg/ml actually appeared to enhance the binding of 500 pg/ml 125I-IL-8 to DEP SRM2975. Competition was observed at higher concentrations, with an apparent EC50 of > 3 mg/ml, or approximately 50 μM. Cytochrome C, however, competed with an EC50 of 77 μg/ml, or approximately 6 μM (Figure 5). These correspond to molar excesses of approximately 5 (cyto C) or 6 (BSA) orders of magnitude greater than the IL-8.
Figure 5.
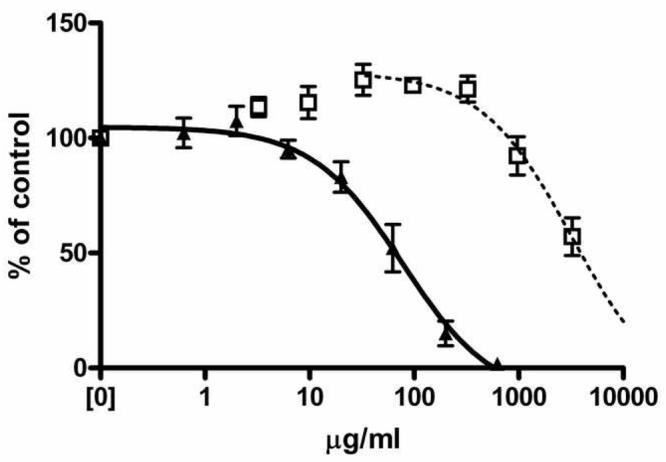
Competition for binding of IL-8 by other proteins. SRM2975 (25 μg/ml) was incubated with radiolabeled IL-8 at 500 pg/ml in the presence of a range of concentrations of either BSA (open squares) or cytochrome C (closed triangles). Following centrifugation, the amount of labeled IL-8 associated with the particles was determined. Curves indicate the fit to a one-site competition model, using the maximum binding as the baseline. Data shown are from one of three similar experiments. Error bars represent the SEM of duplicate measurements.
Competition by peptides
We then tested the effects of inclusion of poly-tyrosine (uncharged at neutral pH, and hydrophobic), poly-glutamic acid (negatively charged at neutral pH) or poly-lysine (positively charged at neutral pH) of several sizes on the association of IL-8 with DEP SRM2975. Neither poly-glutamic acid of either 1.5-5 kDa or 3 - 15 kDa, nor poly-tyrosine (10-40 kDa) at concentrations up to 100 μg/ml had any effect on the association. Poly-lysine was tested in four size fractions. All sizes of the polymer competed with the labeled IL-8, as shown in Figure 6. Interestingly, the size dependence of the competition showed a biphasic effect, with the most effective size being the 4-15 kDa size, that is, a size range similar to the chemokines. The smallest polymer was the least effective competitor, while larger polymers became less effective with increasing size.
Figure 6.
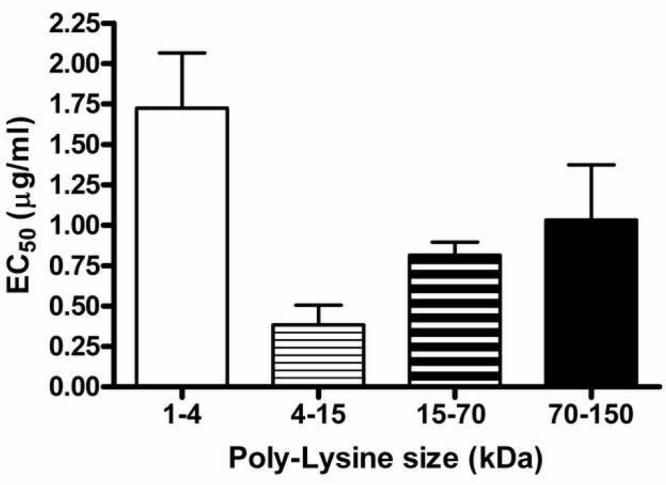
Poly-lysine competes for association of IL-8 with diesel soot particles. Radiolabeled IL-8 was incubated with SRM2975 in the presence of a range of concentrations of poly-L-lysine of several size classifications. The competition curves were fit with a one-site model and the resulting EC50 values and SEM for at least three experiments for each size class are shown.
Lack of effects of organic or sulfhydryl reagents
Neither inclusion of DMSO at concentrations up to 50% nor pre-treatment of the DEP SRM2975 with 100 mM dithiothreitol for 1 h had significant effects on the adsorption of IL-8 (data not shown).
Release of 125I-IL-8 from DEP
We next evaluated the desorption of 125I-IL-8 from DEP SRM2975. Aliquots of DEP (25 μg/ml) were incubated with the labeled IL-8 for 2 h. The particles were then washed twice by centrifugation and resuspended either in PBS, PBS with 1 or 10 mg/ml BSA, or 50 μg/ml cytochrome C. At intervals thereafter, the tubes were centrifuged, the supernatant was removed for gamma-counting, and fresh aliquots of the appropriate solution (PBS, with or without BSA or cytochrome C) were added back to the pellets. The cumulative release of the radiolabel was plotted and the curves were fit as a monotonic exponential decline. The data in Figure 7 clearly show very little release of the label into buffer over a 96-h period in the absence of competing proteins: the curve fit to the data indicated a plateau of 83% of the initial binding, with a half-life of 51 h for the 17% that was released. In the presence of competing proteins to minimize rebinding, the plateaus were between 59 and 65% of the label remaining bound, with half lives of 11-16 h for the fraction released. The R2 (coefficient of determination) for these curves were 0.94-0.96. Attempts to fit to an equation constrained to 100% release were far worse, with R2 values of < 0.25.
Figure 7.
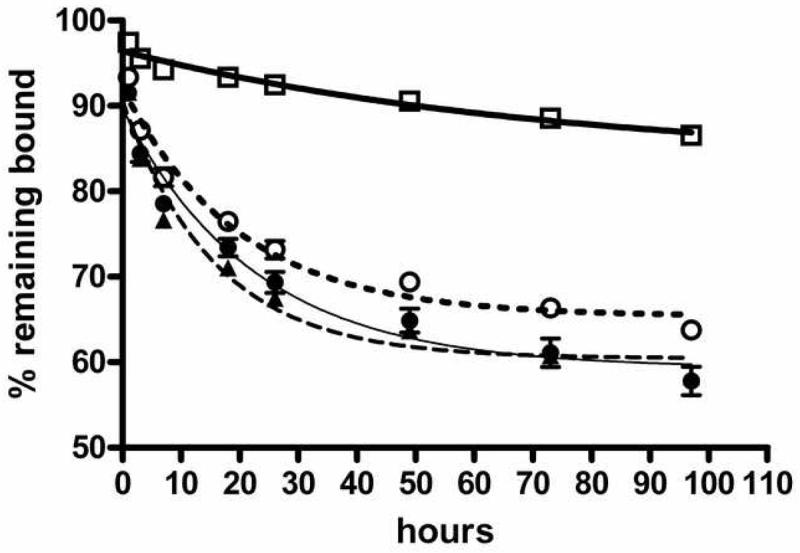
Assocation of IL-8 with diesel soot is reversible. SRM2975/radiolabeled IL-8 mixtures were incubated for 2 h. The particles were removed by centrifugation, washed twice in PBS with 0.01% Tween-80, and resuspended in the same buffer, alone (open squares, heavy line) or in the presence of BSA at 1 mg/ml (open circles, dotted line) or 10 mg/ml (solid circles, thin line), or 50 μg/ml cytochrome C (solid triangles, dashed line). At intervals thereafter, suspensions were centrifuged and the label released to the supernatant was determined. The fraction remaining bound was plotted as a function of time. Curves are the best fit to a single exponential decay model. Data shown are from one of three similar experiments. Error bars represent the SEM of duplicate measurements.
DEP Adsorbs Some, But Not All, Other Cytokines
To determine whether the adsorption of IL-8/MIP-2 is a unique function of these cytokines, we used the standards provided with the human 30-plex Luminex at a 1:4 dilution. This has the advantage of testing all of the cytokines under identical conditions, although the precise composition of the stabilizing buffer included with the standard mixtures is proprietary. The cytokine mixture was combined with a range of concentrations of DEP SRM2975 as described above. The cytokines fell into three classes: those for which complete loss of the cytokine at the highest concentration of the DEP was observed (including IL-8); those for which the maximal depletion was between 40 and 60%; and those for which no depletion was observed (Figure 8). Importantly, when plotted as a function of the isoelectric point and size, the three classes segregated such that those with alkaline isoelectric points and smaller sizes were generally highly depleted, and those with acidic isoelectric points were not adsorbed to a significant degree. There appeared to be a trend for those with alkaline isoelectric points but larger sizes to be in the class of partially depleted cytokines. Unfortunately, the lack of any large cytokines with strongly alkaline isoelectric points makes this less clear.
Figure 8.
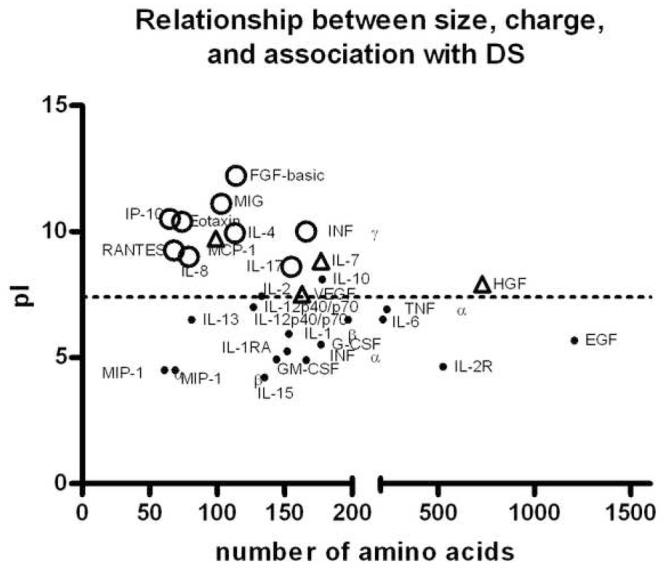
Association of other cytokines with diesel soot particles. SRM2975 was suspended in PBS at a wide range of concentrations as described in the legend to Figure 1 and combined with a cocktail of 30 human cytokines and growth factors. Recovery in the supernatant as determined by Luminex multiplex analysis was determined for each cytokine. Depletion was determined as a function of the DEP concentration, using one-site model as described for IL-8 in Figure 1. Cytokines are plotted as functions of the number of amino acids in the mature form (sizes and amino acid sequences from the Entrez protein database (National Center for Biotechnology Information) and isoelectric points (pI’s) calculated from published sequences using software from the Sequence Manipulation Site: http://www.bioinformatics.vg/sms/protein_iep.html. Cytokines (including IL-8) for which < 10% of the cytokine remained in the supernatant at the highest concentration of DEP are shown as open circles, those for which between 40 and 60% remained in the supernatant at the highest concentration are shown as shaded triangles, and those for which no significant depletion was observed are shown as dots.
Effects in Rats
Pilot study for inflammatory responses
Given the apparently very high EC50 for binding of IL-8 to silica, this particle was selected as a low affinity particle for comparison with DEP. However, silica is a potent pro-inflammatory particle, and inflammatory cells may alter the kinetics of chemokine clearance (Frevert et al., 2002). We therefore performed a pilot study to confirm the appropriate levels of silica and DEP to produce similar inflammatory responses in the lungs of the rats. Five rats per group were intratracheally instilled with doses of 300 μg of DEP SRM 2975/rat or 75 μg of silica/rat and then killed either 6 or 24 h later. None of the treatments caused marked effects on lavage fluid LDH, protein, or alkaline phosphatase, with none of these effects reaching the criterion for statistical significance.
DEP and silica caused small increases in the numbers of macrophages recovered in lavage fluid at 6 hr with a resolution to control levels at 24 hr, but the effects did not reach statistical significance. In contrast, significant increases in neutrophils were observed at 6 hr, rising from levels not significantly different from zero for naïve animals to 0.38 ± 0.16 × 106 cells/ml for vehicle instillations, and to 0.88 ± 0.20 × 106 cells/ml for DEP and 0.85 ± 0.28 × 106 cells/ml for silica. The effect of the two particle suspensions was significantly different from the vehicle, but the two particles were not significantly different from each other. By 24 hr, all neutrophil counts had returned to the baseline levels, not significantly different from each other or from uninstilled animals. These results confirm the similar inflammatory responses of these doses of DEP and silica.
125I-IL-8 instillations with DEP or Silica
Retention of IL-8 in the lungs was followed using intratracheal instillation of 125I-IL-8, alone or in combination with DEP SRM 2975 or silica. Clearance of IL-8 administered alone from the lungs was rapid, with an estimated time to clear 50% of the label (t1/2) of 1.4 h (Figure 9). Silica did not substantially alter this clearance rate: the t1/2 for removal of the label from the lungs for this condition was 1.3 h. In striking contrast, when the 125I-IL-8 was administered associated with the DEP, approximately 40% of the label remained in the lungs at 18 h, and this level was maintained to the 54 h time point.
Figure 9.
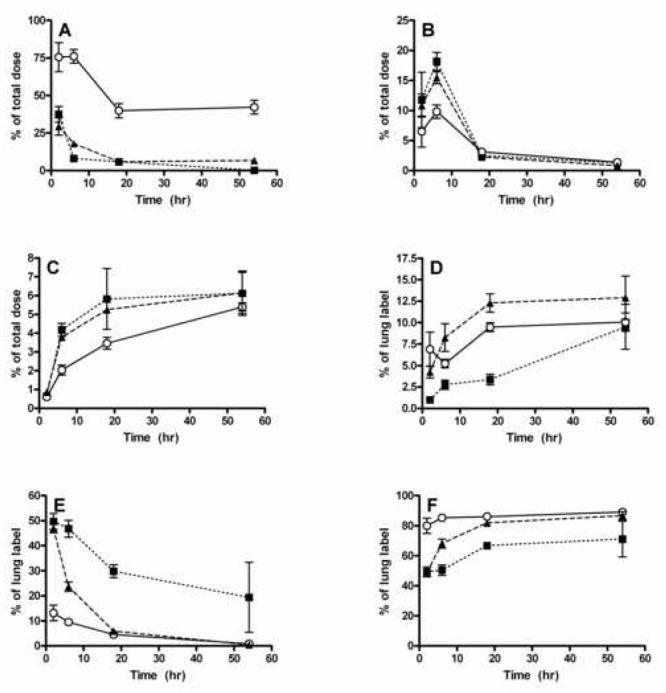
Distribution of 125I-IL-8 following intratracheal instillation alone or in the presence of DEP or silica particles. Rats were instilled with the suspensions and killed at intervals thereafter. The various organs were isolated, lungs were lavaged, and the fraction of the initial level of the label present in each fraction is plotted as a function of time. A: percent of administered label in the lung. B: percent of administered label in the GI tract. C: percent of administered label in the thyroid. D: percent of the lung label in BAL pellet. E: percent of the lung label in BAL fluid supernatant. F: percent of lung label in lung tissue. IL-8 alone: solid squares, dotted lines. IL-8 + DEP: open circles, solid line. IL-8 + silica: solid triangles, dashed line.
At the earliest time (3 h), approximately 50% of the free IL-8 and that instilled with silica in the lung was in the supernatant of the lavage fluid, with most of the remaining label associated with the lung tissue. The amount associated with the tissue increased with time, and this increase was slightly faster when the chemokine was administered with silica. The fraction of the lung-associated label associated with the lavage cells increased slowly over time, with a slightly greater rate of uptake into the cells when silica particles were present. However, when the chemokine was administered associated with DEP, almost 80% was associated with the lung tissue at the earliest time, and this fraction of the lung-associated label remained constant for the entire period. To determine whether the label was still associated with the chemokine, a portion of the lungs from animals killed 54 h after instillation was homogenized with detergent, and the fraction of the label that passed through a 5kDa cut-off ultrafiltration membrane was determined. The very low levels of the label remaining in the lungs of rats instilled with the free chemokine precluded accurate analysis of the form of the label in these animals, but in the case of the DEP + IL-8 instilled lungs, only 1.2 ± 0.3% and for Si + IL-8, 4.6 ± 0.8%, passed through the filter. These results suggested that the 125I remained associated with the chemokine, although the possibility that the label was hydrolyzed from the chemokine and adsorbed to other materials, possibly including the DEP, cannot be excluded.
In all cases, a small amount of the label was found in the gastrointestinal tract, as expected from mucociliary clearance. This fraction peaked at 6 h, followed by a decrease at 18 h, with little remaining in the GI tract at 54 h. Finally, a gradual increase to a maximum of approximately 6% of the label was observed in the thyroid, most likely as the result of hydrolysis of the 125I from the IL-8. The rate of increase of the label in the thyroid was much slower in the case where the chemokine was administered with DEP. Results of these experiments are shown in Figure 9.
Discussion
Human IL-8 and rodent MIP-2 are major pro-inflammatory chemokines responsible for neutrophil recruitment. This recruitment is essential for protection against pathogens, but potentially damaging when extremely high or excessively prolonged. Alterations in neutrophil recruitment and activation can have profound health effects. The experiments described here provide important new information on the mechanisms by which exposure to air pollutant particles, as modeled by DEP, causes adverse health effects.
The results demonstrate that the previously observed (Seagrave et al., 2004) loss of IL-8 from the supernatant of suspensions of DEP and the chemokine were due to direct binding of the chemokine, not loss of epitopes; that the binding was saturable; and that the effect was not unique to DEP. For the particle type most extensively studied, SRM2975 DEP, the estimated Bmax (maximum density of binding sites) was 11.7 μmol/g of particles. These particles have a reported surface area of 91 m2/g, resulting in a surface density of approximately 1 molecule of IL-8 for each 13 nm2 of surface area. Assuming a crosssectional area of 2.25 nm2 for this 8.7kDa protein, 17% of the particle surface would be covered with IL-8 molecules at saturation.
The three types of carbon black formed an especially interesting group of particles. The primary particle diameters and Stokes radii (measure of aggregate size) of the three types of particles are 37 nm/198.2 nm for Elftex-12, and 15-16 nm/30-40 nm for the two Monarch samples (information provided by the suppliers). However, the surface areas, as determined by nitrogen adsorption, are similar: 443, 320 and 340 m2/g, for Elftex-12, Monarch 900, and Monarch 1000 respectively. Monarch 1000 is derived from Monarch 900 by a proprietary oxidative process that produces a variety of carbonyl, aldehyde, ketone, and lactone surface groups. The much higher affinity of this sample compared with the “graphite-like” surface chemistry of Elftex-12 and Monarch 900 could be related to these increased polar groups. Among the five types of DEP, the average chemical composition of the Cummins emissions and the Yanmar generator emissions run at high load are considered to be similar (McDonald et al., 2004). However, because the Cummins engine was run on a cycle, its emissions are probably composed of a wider range of individual particle types. Emissions from the generator under low-load conditions are enriched in organic compounds compared with high load-conditions. These two samples showed fairly similar binding characteristics with a trend to slightly less effective binding (higher EC50) to DEP generated under the low load (higher organic content) conditions. The two NIST DEP samples also differ in the extractable mass, primarily as organic compounds (2.7% for SRM2975, vs. 20.2% for SRM1650). However in this case, the sample with the higher organic content had higher binding. The two NIST DEP samples have similar surface areas (91 vs. 108 m2/g respectively): both have substantially lower surface area and greater organic content than the carbon black samples, but the binding to the NIST 2975 was intermediate between the two Monarch samples. Thus, neither organic content nor surface area directly correlates with the ability to adsorb IL-8.
We next investigated the possible mechanisms for the chemokine:particle association. The data shown here, in combination with previously reported observations that the interaction is suppressed in the presence of increasing salt concentrations (Seagrave et al., 2004), suggests that the interaction is ionic. Specifically, it results from a net negative charge on the particles and a net positive charge of the chemokine. IL-8 has an isoelectric point (pI) of +8.7, and a net charge of +5 at pH 7. All of the particles tested had a negative zeta potential, supporting the possibility of electrostatic association with a positively charged protein. However, there was no correlation between the zeta potential and the EC50 among the different particle types. For example, the two forms of Monarch carbon black had essentially identical zeta potentials, but Monarch 1000 was substantially more effective in adsorbing IL-8. As mentioned above, Monarch 1000 has more polar groups due to the processing, and it is possible that these polar groups, or the specific arrangements of charges or polar groups, are more important than actual net charge on the particle. Alternatively, it is important to recognize that, except possibly for the silica and carbon black particles, these are fairly heterogeneous types of particles, and it is possible that there are populations of particles within the suspensions that have very high or very low binding. In this case, further characterization of the properties of the particles that define the binding potential would require novel methods to characterize single particles and their association with the chemokines.
Competition with other proteins suggested relatively high selectivity for IL-8. BSA has an acidic isoelectric point, and is thus negatively charged at neutral pH, while cytochrome C’s isoelectric point is approximately 10, with a net charge of +9 at neutral pH. Given these parameters, however, it would be expected that if charge were the only factor mediating the association of IL-8 with DEP, cytochrome C would be a very effective competitor. In contrast, the results indicate that at steady state, it requires approximately 5 orders of magnitude excess of cytochrome C to reduce the binding of IL-8 by 50%. Competition with the synthetic peptides provides additional support for the concept that the binding is primarily ionic, in that neither poly-tyrosine (pI ∼ 6.05, phenolic hydroxyl essentially uncharged at neutral pH) nor poly-glutamic acid (pI 3.29, γ-carboxyl groups negatively charged at neutral pH) competed with the IL-8 binding, but poly-lysine (pI ∼ 11, ε-amino positively charged at neutral pH) did. The effect of the size of the poly-lysine peptides suggests that positively charged molecules below a certain size are less effective, perhaps due to an inability to span the negatively charged sites on the DEP resulting in less effective electrostatic interactions. Peptides in the range of the size of IL-8 are quite effective with EC50 in the ng/ml (nM) range, and larger peptides compete less effectively. These results suggest that at least a portion of the adsorption sites are located in pores within the DEP, analogous to gel filtration beads, into which only smaller peptides can permeate.
Second, these studies indicate that association with DEP prolongs the half-life of IL-8 in the rat lung. One limitation of this experiment is that IL-8 is not a natural chemokine for rats. Unfortunately, radiolabeled MIP-2 is not commercially available and the rat chemokine lacks any tyrosines (Murakami et al., 1997), thus precluding radio-iodination by convenient methods. However, IL-8 has been shown to attract and activate rat neutrophils in vivo and in vitro (Rot, 1991;Henke et al., 2001), albeit less efficiently than human neutrophils, and has previously been used as a surrogate for studying clearance mechanisms in rat lungs (Frevert et al., 2002). The latter study implicated neutrophils in the processes resulting in clearance. We therefore included a group of rats treated with silica particles, which did not significantly bind to the chemokine, at a dose to induce a similar low level of neutrophilic inflammation as the selected dose of DEP. Co-instillation with diesel particles dramatically slowed clearance of the 125I label compared with free 125I-IL-8 or 125I-IL-8 in the presence of silica particles. Our previously published evidence indicates that the DEP-associated chemokine is biologically active (Seagrave et al., 2004). The present results indicate that there is a slow dissociation of bound IL-8, with rapid re-binding in the absence of competing DEP-binding proteins. This suggests that there could be a “corona” of active cytokine in the immediate vicinity of the particles, as previously suggested by others (Cedervall et al., 2007).
Finally, these studies suggest possible mechanisms for exacerbation of inflammatory responses by exposure to diesel exhaust. It is important to consider that these mechanisms could exacerbate the effects of chemokines (or potentially, other cytokines) present as a result of pre-existing conditions (infection, irritation, or asthma). Although epithelial secretion of chemokines is predominantly to the basolateral compartment, macrophages activated by pathogens or pollutants would be expected to secrete their chemokines into the epithelial lining fluid, where they could interact with inhaled particulate matter. Specifically, adsorption of chemokines on DEP could cause the local concentration to exceed a threshold for activation of neutrophils. This concept is consistent with the principle of a “kinetic proofreading mechanism” (McKeithan, 1995), wherein interactions of multiple cell surface receptors with multiple particulate matter-associated chemokine molecules results in a higher effective affinity. Furthermore, simultaneous activation of multiple receptors would presumably result in increased peak levels of the secondary messengers (e.g., intracellular free Ca2+), with a greater potential for full activation of the cells. Activation of the neutrophils by the concentrated chemokine could cause localized release of additional damaging or pro-inflammatory mediators by the target cells. Alternatively, the association of the chemokine with the DEP could extend the biological half-life of the chemokine in the lung, resulting in sustained inflammation. Clearly these two possible effects are not mutually exclusive, and both may function to amplify inflammatory responses resulting from DEP-induced or pre-existing chemokines. An alternative hypothesis is that sequestration of the chemokines results in a lower overall concentration of the free active chemokine, and thus suppresses the inflammatory responses. Further studies will be required to distinguish between these two possible outcomes.
Although there is a substantial literature in the biomaterials and drug delivery fields documenting association of particles with proteins (Cai et al., 2008;Luck et al., 1998;Roach et al., 2006;Mejias et al., 2008;Gaumet et al., 2008;Kingsley et al., 2006), only recently has this phenomenon been appreciated as a possible factor in the biological effects of inhaled particles (Dutta et al., 2007;Kendall et al., 2004b;Kendall et al., 2004a). The current study advances this field by showing differences in the adsorption of a number of biologically significant proteins to DS, and of the key neutrophil chemoattractant, IL-8 to several different forms of particles.
ACKNOWLEDGEMENTS
The author gratefully acknowledges the technical contributions of Brenda Pacheco, Sandy Dunaway, and Guy Herbert; the analysis of the zeta potential and suspension sizes of the suspensions of the various particles by Dr. Bellina Veronesi, United States Environmental Protection Agency; gifts of carbon black by Cabot Corporation and EHC93 by Dr. Renaud Vincent, Health Canada (Ottawa Canada); collection of gasoline engine emission particles by Kevin Whitney, Southwest Research Institute; characterization of gasoline vehicle exhaust particles and collection and characterization of truck and generator diesel exhaust particles by Dr. Jacob D. McDonald, LRRI; and useful discussions with John Foster of Cabot Corp. regarding the properties of the carbon black samples.
FUNDING Health Effects Institute and National Institutes of Health (AI058109)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT The author has no conflicts of interest to disclose.
Reference List
- 1.Abe S, Takizawa H, Sugawara I, Kudoh S. Diesel exhaust (DE)-induced cytokine expression in human bronchial epithelial cells: a study with a new cell exposure system to freshly generated DE in vitro. Am. J. Respir. Cell Mol. Biol. 2000;22:296–303. doi: 10.1165/ajrcmb.22.3.3711. [DOI] [PubMed] [Google Scholar]
- 2.Boland S, Baeza-Squiban A, Fournier T, Houcine O, Gendron MC, Chevrier M, Jouvenot G, Coste A, Aubier M, Marano F. Diesel exhaust particles are taken up by human airway epithelial cells in vitro and alter cytokine production. Am. J. Physiol. Lung Cell Mol. Physiol. 1999;276:L604–L613. doi: 10.1152/ajplung.1999.276.4.L604. [DOI] [PubMed] [Google Scholar]
- 3.Boland S, Baeza-Squiban A, Marano F. Respiratory toxicity of Diesel exhaust particles: cellular and molecular mechanisms. M S-Medecine Sciences. 2001;17:596–603. [Google Scholar]
- 4.Boland S, Bonvallot V, Fournier T, Baeza-Squiban A, Aubier M, Marano F. Mechanisms of GM-CSF increase by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L25–L32. doi: 10.1152/ajplung.2000.278.1.L25. [DOI] [PubMed] [Google Scholar]
- 5.Brunekreef B, Janssen NA, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997;8:298–303. doi: 10.1097/00001648-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Cai C, Bakowsky U, Rytting E, Schaper AK, Kissel T. Charged nanoparticles as protein delivery systems: a feasibility study using lysozyme as model protein. Eur J Pharm. Biopharm. 2008;69:31–42. doi: 10.1016/j.ejpb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Cedervall T, Lynch I. t., Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. PNAS. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta D, Sundaram SK, Teeguarden JG, Riley BJ, Fifield LS, Jacobs JM, Addleman SR, Kaysen GA, Moudgil BM, Weber TJ. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol Sci. 2007;100:303–315. doi: 10.1093/toxsci/kfm217. [DOI] [PubMed] [Google Scholar]
- 9.Frevert CW, Goodman RB, Kinsella MG, Kajikawa O, Ballman K, Clark-Lewis I, Proudfoot AEI, Wells TNC, Martin TR. Tissue-specific mechanisms control the retention of IL-8 in lungs and skin. J. Immunol. 2002;168:3550–3556. doi: 10.4049/jimmunol.168.7.3550. [DOI] [PubMed] [Google Scholar]
- 10.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm. Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto K, Ishii Y, Uchida Y, Kimura T, Masuyama K, Morishima Y, Hirano K, Nomura A, Sakamoto T, Takano H, Sagai M, Sekizawa K. Exposure to diesel exhaust exacerbates allergen-induced airway responses in guinea pigs. Am. J. Respir. Crit. Care Med. 2001;164:1957–1963. doi: 10.1164/ajrccm.164.10.2011070. [DOI] [PubMed] [Google Scholar]
- 12.Henke PK, Wakefield TW, Kadell AM, Linn MJ, Varma MR, Sarkar M, Hawley A, Fowlkes JB, Strieter RM. Interleukin-8 administration enhances venous thrombosis resolution in a rat model. J Surg. Res. 2001;99:84–91. doi: 10.1006/jsre.2001.6122. [DOI] [PubMed] [Google Scholar]
- 13.Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Ichinose T, Takano H, Miyabara Y, Yanagisawa R, Sagai M. Murine strain differences in allergic airway inflammation and immunoglobulin production by a combination of antigen and diesel exhaust particles. Toxicology %19. 1997;122:183–192. doi: 10.1016/s0300-483x(97)00096-6. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Ikeda M, Yamasaki H, Sagai M, Tomita T. Peroxynitrite formation by diesel exhaust particles in alveolar cells: Links to pulmonary inflammation. Environ. Toxicol. Pharmacol. 2000;9:1–8. doi: 10.1016/s1382-6689(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 16.Janssen NA, Schwartz J, Zanobetti A, Suh HH. Air conditioning and source-specific particles as modifiers of the effect of PM(10) on hospital admissions for heart and lung disease. Environ. Health Perspect. 2002;110:43–49. doi: 10.1289/ehp.0211043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juvin P, Fournier T, Boland S, Soler P, Marano F, Desmonts JM, Aubier M. Diesel particles are taken up by alveolar type II tumor cells and alter cytokines secretion. Arch. Environ. Health. 2002;57:53–60. doi: 10.1080/00039890209602917. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki S, Takizawa H, Takami K, Desaki M, Okazaki H, Kasama T, Kobayashi K, Yamamoto K, Nakahara K, Tanaka M, Sagai M, Ohtoshi T. Benzene-extracted components are important for the major activity of diesel exhaust particles: effect on interleukin-8 gene expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;24:419–426. doi: 10.1165/ajrcmb.24.4.4085. [DOI] [PubMed] [Google Scholar]
- 19.Kendall M. Fine airborne urban particles (PM2.5) sequester lung surfactant and amino acids from human lung lavage. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00131.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kendall M, Brown L, Trought K. Molecular adsorption at particle surfaces: a PM toxicity mediation mechanism. Inhal Toxicol 16. 2004a;1(Suppl):99–105. doi: 10.1080/08958370490443187. [DOI] [PubMed] [Google Scholar]
- 21.Kendall M, Guntern J, Lockyer NP, Jones FH, Hutton BM, Lippmann M, Tetley TD. Urban PM2.5 surface chemistry and interactions with bronchoalveolar lavage fluid. Inhal Toxicol 16. 2004b;1(Suppl):115–129. doi: 10.1080/08958370490443204. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley JD, Dou H, Morehead J, Rabinow B, Gendelman HE, Destache CJ. Nanotechnology: a focus on nanoparticles as a drug delivery system. J Neuroimmune. Pharmacol. 2006;1:340–350. doi: 10.1007/s11481-006-9032-4. [DOI] [PubMed] [Google Scholar]
- 23.Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ. Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luck M, Paulke BR, Schroder W, Blunk T, Muller RH. Analysis of plasma protein adsorption on polymeric nanoparticles with different surface characteristics. J Biomed. Mater. Res. 1998;39:478–485. doi: 10.1002/(sici)1097-4636(19980305)39:3<478::aid-jbm19>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995-1997. Environ. Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauderly JL, Snipes MB, Barr EB, Belinsky SA, Bond JA, Brooks AL, Chang IY, Cheng YS, Gillett NA, Griffith WC, Henderson RF, Mitchell CE, Nikula KJ, Thomassen DG. Pulmonary toxicity of inhaled diesel exhaust and carbon black in chronically exposed rats. Part I: Neoplastic and nonneoplastic lung lesions. Res. Rep. Health Eff. Inst. 1994;68(Pt 1):1–75. [PubMed] [Google Scholar]
- 27.McDonald JD, Barr EB, White RK. Design, characterization and evaluation of a small scale diesel exhaust exposure system. Aerosol Sci. Technol. 2004;38:62–78. [Google Scholar]
- 28.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mejias R, Costo R, Roca AG, Arias CF, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Del Puerto MM, Serna CJ, Manes S, Barber DF. Cytokine adsorption/release on uniform magnetic nanoparticles for localized drug delivery. J Control Release. 2008 doi: 10.1016/j.jconrel.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Miyabara Y, Yanagisawa R, Shimojo N, Takano H, Lim HB, Ichinose T, Sagai M. Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur. Respir. J. 1998;11:291–298. doi: 10.1183/09031936.98.11020291. Ref Type: Abstract
- 31.Murakami K, Shibata F, al Mokdad M, Nakagawa H, Ueno A, Kondo T. Identification and characterization of receptor for cytokine-induced neutrophil chemoattractant-3 on rat neutrophils. Biochem. Biophys. Res. Commun. 1997;232:562–567. doi: 10.1006/bbrc.1997.6136. [DOI] [PubMed] [Google Scholar]
- 32.Murphy SA, BeruBe KA, Pooley FD, Richards RJ. The response of lung epithelium to well characterised fine particles. Life Sci. 1998;62:1789–1799. doi: 10.1016/s0024-3205(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 33.Ohtoshi T, Takizawa H, Okazaki H, Kawasaki S, Takeuchi N, Ohta K, Ito K. Diesel exhaust particles stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitro. J. Allergy Clin. Immunol. 1998;101:778–785. doi: 10.1016/S0091-6749(98)70307-0. [DOI] [PubMed] [Google Scholar]
- 34.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappaB activation. Am. J. Respir. Cell Mol. Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- 36.Reed MD, Gigliotti AP, McDonald JD, Seagrave JC, Seilkop SK, Mauderly JL. Health effects of subchronic exposure to environmental levels of diesel exhaust. Inhalation Toxicology. 2003;16:177–193. doi: 10.1080/08958370490277146. [DOI] [PubMed] [Google Scholar]
- 37.Roach P, Farrar D, Perry CC. Surface tailoring for controlled protein adsorption: effect of topography at the nanometer scale and chemistry. J Am Chem Soc. 2006;128:3939–3945. doi: 10.1021/ja056278e. [DOI] [PubMed] [Google Scholar]
- 38.Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991;3:21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- 39.Sadakane K, Ichinose T, Takano H, Yanagisawa R, Sagai M, Yoshikawa T, Shibamoto T. Murine Strain Differences in Airway Inflammation Induced by Diesel Exhaust Particles and House Dust Mite Allergen. Int. Arch. Allergy Immunol. 2002;128:220–228. doi: 10.1159/000064255. [DOI] [PubMed] [Google Scholar]
- 40.Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, Schwartz J, Zanobetti A. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res. Rep. Health Eff. Inst. 2000;94:5–70. [PubMed] [Google Scholar]
- 41.Seagrave JC, Knall C, McDonald JD, Mauderly JL. Diesel particulate material binds and concentrates a proinflammatory cytokine that causes neutrophil migration. Inhalation Toxicology 16. 2004;1(suppl):93–98. doi: 10.1080/08958370490443178. [DOI] [PubMed] [Google Scholar]
- 42.Seagrave JC, McDonald JD, Gigliotti AP, Nikula KJ, Seilkop SK, Gurevich M, Mauderly JL. Mutagenicity and in vivo toxicity of combined particulate and semivolatile organic fractions of gasoline and diesel engine emissions. Toxicol. Sci. 2002;70:212–226. doi: 10.1093/toxsci/70.2.212. [DOI] [PubMed] [Google Scholar]
- 43.Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, Scheepers PT, van Loveren H. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp. Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- 44.Stringer B, Kobzik L. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of preexisting inflammation and oxidant stress. J. Toxicol. Environ. Health A. 1998;55:31–44. doi: 10.1080/009841098158601. [DOI] [PubMed] [Google Scholar]
- 45.Strom KA, Garg BD, Johnson JT, D'Arcy JB, Smiler KL. Inhaled particle retention in rats receiving low exposures of diesel exhaust. J Toxicol. Environ. Health. 1990;29:377–398. doi: 10.1080/15287399009531399. [DOI] [PubMed] [Google Scholar]
- 46.van Vliet P, Knape M, de Hartog J, Janssen N, Harssema H, Brunekreef B. Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ. Res. 1997;74:122–132. doi: 10.1006/enrs.1997.3757. [DOI] [PubMed] [Google Scholar]
- 47.Wolff RK, Henderson RF, Snipes MB, Sun JD, Bond JA, Mitchell CE, Mauderly JL, McClellan RO. Lung retention of diesel soot and associated organic compounds. Dev. Toxicol. Environ. Sci. 1986;13:199–211. [PubMed] [Google Scholar]