Abstract
One of the major roles of seminal plasma is to provide antimicrobial protection for the spermatozoa in the female reproductive tract. We found that the bactericidal activity of seminal plasma was highest after resolution of the seminal clot and that this antibacterial activity subsequently became greatly diminished. The antibacterial activity was derived from peptides generated by fragmentation of the semenogelins while the semenogelin holoproteins displayed no antibacterial activity. After ejaculation the semenogelin-derived peptides were fragmented to smaller and smaller fragments over time and thereby lost antibacterial activity. This paralleled the loss of antibacterial activity of whole seminal plasma both in vitro and after sexual intercourse. Moreover, the antibacterial activity of the semenogelin-derived peptides generated in seminal plasma was strictly zinc-dependent both at neutral and low pH. These data provide novel roles for the resolution of seminal clots and for the high zinc concentration in human seminal plasma.
Keywords: Bacterial infections, human, Reproductive Immunology, Antigens/Peptides/Epitopes
Introduction
Seminal plasma contains a wide range of inorganic and organic constituents produced by the seminal vesicles, the prostate, the epididymis and the bulbourethral glands (1). The major proteins in seminal plasma are semenogelin I and II (SgI and SgII). They are synthesized in the seminal vesicles and are present in seminal plasma with concentrations of ∼50g/L of SgI and ∼10g/L of SgII. Semenogelins are believed to affect the sperm capacitation and motility (2). They are expressed in the seminal vesicles, and also a small amount of SgII is expressed in the epididymis (3). The sequence similarity between SgI and SgII is 78% and they contain similar repeats of 60 amino acids. SgI (50 kD) contains six such repeats and SgII (63 kD) contains eight repeats (3). After ejaculation SgI, SgII and fibronectin aggregate and form a coagulum that traps and immobilizes the spermatozoa (4,5). The coagulum is then liquefied within 15-20 min as the semenogelins are degraded into smaller fragments mainly by prostate specific antigen (PSA) (6,7), thus freeing the spermatozoa.
One of the major roles of seminal plasma is to provide nutrients and protection for the spermatozoa in the female reproductive tract. The protection of the spermatozoa is of vital importance for reproduction. In order to ensure fertilization, the integrity of the spermatozoa needs to be maintained both while still in the male genital tract and, after ejaculation, in the female genital tract. While the male reproductive tract is sterile, the healthy female vaginal tract is colonized mostly by lactobacilli that produce lactic acid and thereby cause an acidic environment in the vagina. The lactic acid produced by the lactobacilli is the major antibacterial component of vaginal fluid (8) and the low pH constitutes a major barrier to colonization with pathogenic bacteria. However, since the vagina is not sterile, it reasons that seminal plasma contains antibacterial components to ensure that the spermatozoa ascend into the sterile uterine environment devoid of microbes.
Many antibacterial components have been identified in seminal plasma, such as lysozyme (9), lactoferrin (10), phospholipase A2 (11), secretory leukocyte protease inhibitor (SLPI) (12), and semenogelin I-derived peptides (13). Nevertheless, there is scant information available on the role of these polypeptides in host defense during and after coitus. A recent study from our group demonstrated that, upon interaction of seminal plasma and vaginal fluid, an antimicrobial peptide ALL-38 is generated following coitus (14). In seminal plasma, ALL-38 exists in abundant amounts as the inactive precursor protein hCAP-18 (15). Proteolytic cleavage of hCAP-18 by semen-derived gastricsin occurs when the seminal plasma pH drops in the vagina after coitus, which subsequently liberates the mature and active ALL-38 peptide (14). However, since gastricsin is activated by low pH 2-7 hours after sexual intercourse (16), and the majority of viable spermatozoa enter the uterus approximately 30 min after sexual intercourse (17), these sperm might not benefit from the full antimicrobial potential of coitally activated ALL-38.
Herein, we sought to identify which components of seminal plasma are biologically relevant for its intrinsic bactericidal activity. We found that the majority of the bactericidal activity of seminal plasma was a result of peptides derived proteolytically from semenogelin (SgI and SgII). This bactericidal activity was dependent on zinc ions, which are present in seminal plasma in high concentrations and have previously been reported to bind the histidine-rich semenogelins (18).
Materials and methods
Reagents
Monoclonal mouse anti-semenogelin antibodies were generated as previously described (19). Polyclonal goat anti-SLPI antibodies were from R&D Systems (Minneapolis, USA). Polyclonal rabbit anti-hCAP-18 antibodies were generated as previously described (20). HRP-conjugated antibodies were from DAKO (Glostrup, Denmark) and prostate specific antigen (PSA) was from Sigma (St. Louis, MI).
Bacterial strains and growth conditions
Overnight cultures of the bacterial strains Streptococcus pyogenes M5 Manfredo, Escherichia coli (strain 37.4), Staphylococcus aureus (strain 5120), Streptococcus agalactiae SB1, Pseudomonas aeruginosa (strain 1553), and Enterococcus faecalis (strain 2374) were grown in THY broth (Todd Hewitt with addition of yeast). N. gonorrhoeae (strain 34327) was grown over night on GAB plates.
Seminal plasma
Freshly ejaculated semen was collected from healthy volunteers and patients at Malmö University Hospital Fertility Clinic. The semen was allowed to liquefy for 1h at room temperature followed by centrifugation at 1 000 × g at 4 °C for 10 min. The supernatant was collected, aliquoted and stored at −20 °C. 4 pools of seminal plasma from 4-5 healthy donors were used in the experiments. Furthermore, seminal plasmas from 6 individual donors were used in verification experiments. Before the seminal plasma was used in a CFU assay it was dialyzed (MWCO 3500 Da) against 25 mM ammonium acetate pH 7 at 4°C. Post coital seminal plasma in vaginal fluid was collected from healthy donors 10 hours following sexual intercourse and stored at −20°C as previously described (14).
Extraction of cationic peptides from seminal plasma
Seminal plasma was dialyzed against 25 mM ammonium acetate pH 7, mixed with pre-equilibrated CM-beads (Macro-Prep CM Cation Exchange Support; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and incubated over night at 4°C. The beads were washed with 25 mM ammonium acetate pH 7 and cationic peptides were eluted with 30% acetic acid. The eluate was dried down and dissolved to its starting volume in 10 mM tris 5 mM glucose pH 7.5 and this was repeated until the acetic acid was removed and pH of the solution was 7.5.
Semenogelin purification and cleavage
Semenogelin I and II were purified from human seminal plasma as previously described (18) and the purified SgI was cleaved by incubation with PSA in 50 mM Tris-HCl, 0.5 M urea pH 8.0 as previously described (21).
Depletion of semenogelin from seminal plasma
Divalent ions were depleted from seminal plasma by incubation with 125 mM EDTA. Subsequently the EDTA was removed by dialysis. Ni-NTA Agarose (Qiagen) was equilibrated with coupling buffer (50 mM NaH2PO4, 2 M urea, pH 7.4) and the seminal plasma sample was diluted in coupling buffer and incubated under rotation with the Ni-agarose over night at 4°C. The flow-through was collected and the agarose washed with coupling buffer and the semenogelin was eluted with coupling buffer with addition of 500 mM imidazole. The eluate was dried down to its starting volume and then dialyzed against coupling buffer to remove the imidazole.
Gel overlay assay
Gel overlay assay was performed essentially as described by Lehrer et al. (22). Briefly, duplicate samples were run on non-denaturing Acid Urea (AU-PAGE) gels in 5% acetic acid at 100 V for 1h 15 min. Bacteria were grown over night in THY broth, inoculated and grown for 3 h. The bacteria were washed and resuspended in 10 mM NaPO4 pH 7.4. 4×106 bacteria were added to 12 ml melted underlay agarose (10 mM NaPO4, pH 7.4, 0.03% TH, 1% Agarose type 1 (Sigma)) and poured into a square Petri dish. One AU-gel was stained with Coomassie brilliant blue and one AU gel was washed 3×4min in 10 mM NaPO4 pH 7.4 and then placed on top of the agarose and incubated for 3 h at 37°C. The AU gel was then removed and an overlay agarose (6% THY, 1% agarose type 1 (Sigma)) was poured on top of the underlay and incubated over night at 37°C. To make the clearing zones more visible the agarose was stained with Coomassie brilliant blue and then destained.
Antibacterial activity assay/Colony forming unit (CFU) assay
Except for N. gonorrhoeae bacteria were grown overnight, inoculated and grown for 3 h, washed, and resuspended in 10 mM Tris, 5 mM glucose, pH 7.5. Approximately 8×104 CFU of bacteria were incubated together with different seminal plasma samples in tris glucose buffer for 1 h at 37 °C. After incubation the suspension was diluted and plated. The plates were incubated over night and the colonies counted. N. gonorrhoeae was grown on GAB plates and bacteria were taken directly from the plates and resuspended in 0.7% casamino acids (CA). The antimicrobial assays were performed in the same way as for the other bacteria with the exceptions of using CA buffer instead of tris glucose and incubating for 3 h instead of 1 h. The longer incubation time was chosen to allow proper killing of the slow growing N. gonorrhoeae.
Western blot
Samples were run on an AU-gel and transferred onto a PVDF membrane. The membrane was fixed in 0.05% glutaraldehyde in PBS, washed in PBST (PBS with 0.05% Tween 20), and blocked in 3% skimmed milk in PBST. The membrane was incubated with primary antibodies in 1% skimmed milk for 1h at room temperature, washed in PBST and incubated with secondary HRP-conjugated antibodies in 1% skimmed milk for 1h at room temperature, washed in PBST and developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Peptide identification
Seminal plasma samples were run on two identical AU-gels. One was stained with Coomassie brilliant blue and the other was used in a gel overlay assay. Bands corresponding to the clearing zones in the agarose gel were cut out from the stained gel and sent to Alphalyse (Alphalyse A/S, Odense, Denmark) where the gel band was treated with trypsin that cleaves the protein after Lysine and Arginine residues and the resulting peptides were analysed by MALDI TOF/TOF mass spectrometry. The masses of the peptides were used to query sequence databases for proteins with matching peptide masses.
Synthetic semenogelin peptides
Two peptides from semenogelin II with the sequence: HKQEGRDHDKSKGHFHMIVIHHKGGQAHHG-OH (SgII peptide A) and HQDQEHGRKAHKISYPSSRTEERQLHHGE-OH (SgII peptide B) were purchased from Schafer-N (Copenhagen, Denmark). The peptides were synthesized using the Fmoc(N-(9-fluorenyl)methoxycarbonyl) strategy, purified by reverse phase chromatography, and analyzed by HPLC coupled to a QP800 spectrometer. The purities were greater than 95%.
Transmission electron microscopy
An over night culture of S. pyogenes was inoculated and grown for 3 hours, washed twice in 10 mM Tris, 5 mM glucose, pH 7,5 and resuspended in the same buffer. The bacteria (2×107 CFU) were incubated for one hour at 37°C in buffer only or with addition of cationic peptides extracted from seminal plasma. Samples of S. pyogenes suspensions were adsorbed onto carbon-coated copper grids for 2 min, washed briefly on two drops of water, and negatively stained on two drops of 0.75% uranyl formate. The grids were rendered hydrophilic by glow discharge at low pressure in air. Specimens were observed in a Jeol JEM 1230 electron microscope operated at 60 kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 CCD camera.
2D gel
2-dimensional gel electrophoresis was performed essentially as described (23). The samples were mixed 1:1 with (0.2% CETAB and 0.1% acetic acid) and then 1:1 with 3X AU loading dye (9M urea in 5% acetic acid with methyl green). 7 μl of each sample was electrophoresed on a 1st dimension AU-PAGE gel. 1st dimension strips were stained in 10% amido black, washed in ddH20, and 50 mM Tris pH 8.8 and then stored in equilibrium buffer containing 10 mg/ml DTT at −80C. 1st dimension strips were subsequently electrophoresed on a 2nd dimension Tris-Tricine SDS-PAGE gel, and the gel was stained with SYPRO Ruby stain (Bio-Rad, Hercules, CA) and imaged under UV light.
Results
Antibacterial activity of human seminal plasma
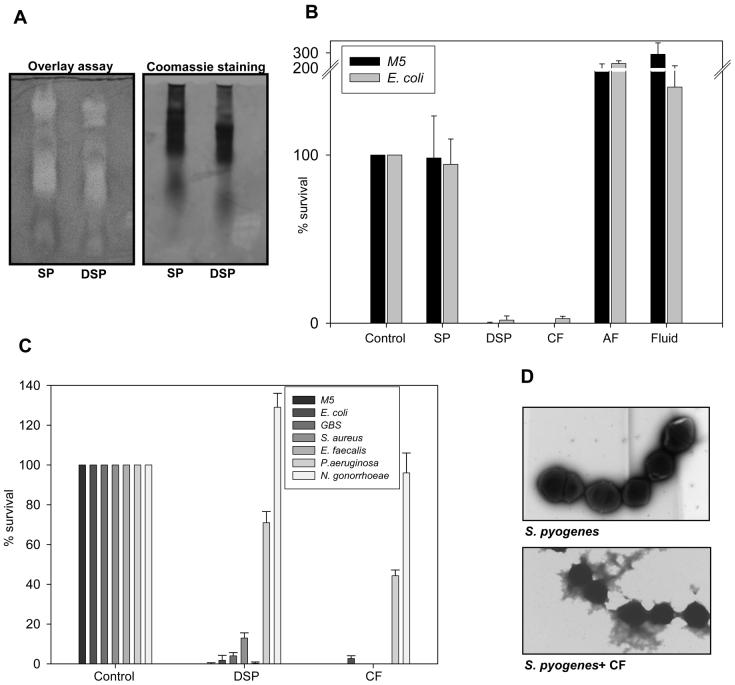
We examined the bactericidal activity of seminal plasma by gel overlay and CFU assays. In the gel overlay assay we found several bands with activity against E.coli (figure 1A) while little or no antibacterial activity was observed in seminal plasma by the CFU assay (figure 1B). The activity of antimicrobial (poly)peptides is often inactivated by physiological salt concentration in vitro. To investigate whether the lack of antibacterial activity in the CFU assay was due to the salt concentration, seminal plasma was dialyzed against ammonium acetate prior to performing the CFU assay. After dialysis we found prominent antibacterial activity of seminal plasma even after diluting the sample to 2% of the original concentration (figure 1B). In the gel overlay assay seminal plasma displayed similar antibacterial activity before and after dialysis (figure 1A). To test whether the antibacterial activity was due to cationic (poly)peptides, these cationic (poly)peptides were extracted from seminal plasma and CFU assays were performed. The cationic fraction was found to be equally active to dialyzed seminal plasma (figure 1BC). No antibacterial activity was found in the seminal plasma after extraction of the cationic fraction or in protein free seminal fluid (figure 1C), demonstrating that the cationic fraction was solely responsible for the antibacterial activity of seminal plasma.
Figure 1.
Seminal plasma has broad bactericidal activity. A. Seminal plasma (SP) and dialyzed seminal plasma (DSP) were run on an AU-PAGE gel and analyzed in an antibacterial gel overlay assay with E. coli. Clearing zones depict antibacterial activity. Salt causes retardation of the samples in AU gels. This can explain the difference between the SP and DSP sample.
B. Seminal plasma (SP), dialyzed seminal plasma (DSP), cationic fraction (CF), anionic fraction from extraction of cationic peptides (AF) and protein free seminal fluid (Fluid) were tested in a CFU-assay in a concentration of 2% of that found in seminal plasma and displayed as bacterial survival compared to buffer control. Results are shown as average from three independent experiments.
C. Dialyzed seminal plasma (DSP) and the cationic fraction (CF) from seminal plasma were tested in a CFU-assay in a concentration corresponding to 2% of the concentration in seminal plasma. Results are shown as average from three independent experiments.
D. S. pyogenes M5 were incubated with or without cationic fraction (CF) from seminal plasma and visualized by transmission electron microscopy.
The antibacterial activity of dialyzed seminal plasma as well as the cationic fraction was tested against a variety of microbes. We found prominent activity against group A and group B streptococci, E.coli, S. aureus and E. faecalis as well as modest activity against P. aeruginosa (figure 1C) by CFU assay. No activity found against N. gonorrhoeae. Increasing the concentration of cationic peptides did not increase the antibacterial activity against P. aeruginosa or N. gonorrhoeae (data not shown).
To examine the bacterial killing mediated by the seminal plasma Group A streptococci S. pyogenes M5 were incubated with cationic fraction from seminal plasma and analyzed by electron microscopy. The antibacterial components of seminal plasma caused lysis of the bacteria (figure 1D).
Identification of antibacterial components in seminal plasma
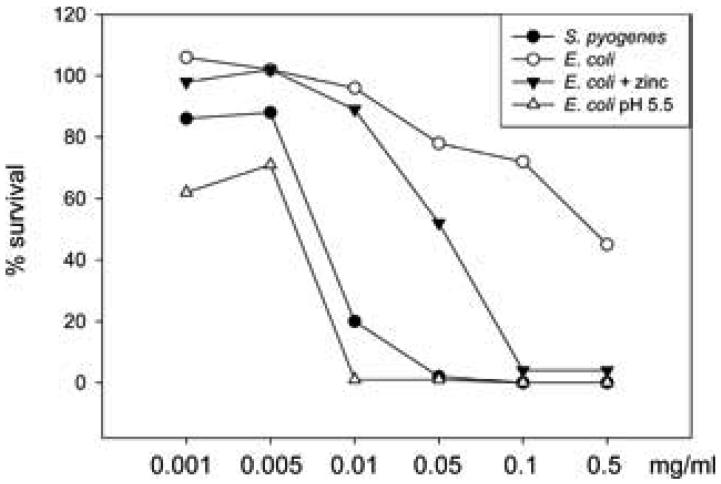
In order to identify the bactericidal (poly)peptides, seminal plasma and extracted cationic peptides were tested in gel overlay assays against E. coli and bands corresponding to the zones of clearance were excised and identified by trypsin digestion followed by mass spectrometry. 20 bands in total were identified. Out of these 2 bands were identified as secretory leukocyte inhibitor (SLPI), 2 bands as lactoferrin and 1 band as phospholipase A2. However, 15 bands were identified as semenogelins. 9 bands were semenogelin I (SgI), and 6 bands were SgII. Except for SgII, all these (poly)peptides have known antibacterial activity (13,12,10,11). To demonstrate that a peptide derived from semenogelin II was functionally antimicrobial we tested the antibacterial activity of a synthetic histidine-rich peptide derived from the sequence of SgII (H-KQEGRDHDKSKGHFHMIVIHHKGGQAHHG-OH) named SgII peptide A. SgII peptide A exhibited antibacterial activity in CFU assay in a concentration of 0.32 μM against S. pyogenes (figure 2). In CFU assays the antibacterial activity of the peptide against E. coli was modest but potentiated by low pH or the addition of zinc as seen with other histidine-rich antimicrobial peptides (figure 2). Since SgII is present in a concentration of ∼16 μM in seminal plasma single fragments of SgII will be present in antimicrobial relevant concentrations in seminal plasma. The SgII peptide A was selected because of its high histidine-content and positive charge at neutral pH. This made it likely that the peptide would be antibacterial.
Figure 2.
A synthetic SgII-derived peptide has antibacterial activity. Antibacterial activity of a synthetic peptide derived from SgII (H-KQEGRDHDKSKGHFHMIVIHHKGGQAHHG-OH), SgII peptide A, was tested in CFU-assay against S. pyogenes and E. coli and shown as bacterial survival compared to buffer control. Average values from three independent experiments are shown.
Against S. pyogenes the peptide was only tested in neutral pH with no additional ions and against E. coli the peptides was tested in neutral pH, in pH 5.5 and with addition of ZnCl2 in neutral pH (final concentration 12.5 mM).
Not all histidine-rich fragments of semenogelins display these characteristics. We chose to test the antibacterial activity of another synthetic SgII fragment with histidines (HQDQEHGRKAHKISYPSSRTEERQLHHGE-OH), SgII peptide B. This peptide was very similar to SgII peptide A but had both a lower content of histidines and a lower positive charge at neutral pH. SgII peptide B did not display any significant antimicrobial activity even after addition of zinc or at low pH demonstrating that not all semenogelin fragments are antibacterial (data not shown). The demonstration of antibacterial activity of SgII indicated a major role of the semenogelins in the antibacterial activity of seminal plasma.
Antibacterial activity of semenogelins is dependent on their state of degradation
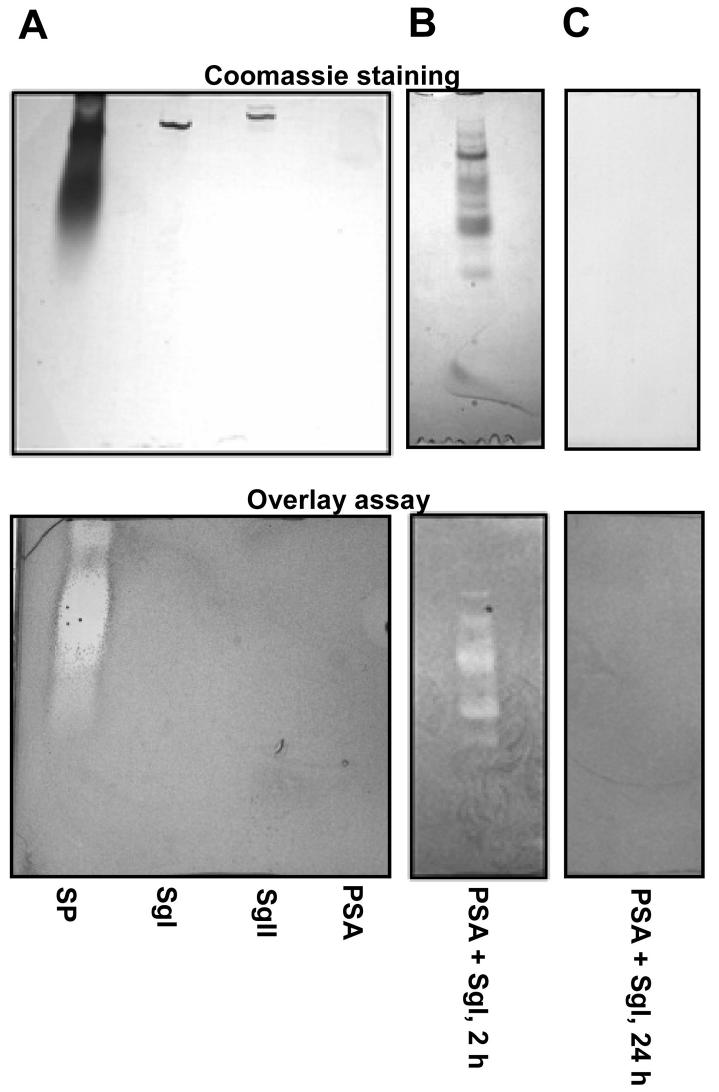
After ejaculation the semenogelins are continuously degraded to smaller and smaller fragments mainly by PSA-mediated proteolysis (21). Our method for identification of antibacterial (poly)peptides did not allow us to discriminate between the semenenogelin holoproteins or the degradation products. To determine if the semenogelin holoproteins or only the degradation products were antibacterial, we tested the antibacterial activity of purified SgI and II, PSA, and PSA-cleaved SgI in a gel overlay assay. We chose gel overlay assay since this allowed us both to analyze samples of semenogelin holoprotein stored in urea that prevents spontaneous degradation of the semenogelins and, importantly, to determine whether we had any degradation of the semenogelins. We did not find any antibacterial activity of either the semenogelin holoproteins (5 μg) or PSA (5 μg) (figure 3A), but semenogelin cleaved by PSA was antibacterial (figure 3B). Thus, only degradation products of the semenogelins were antibacterial and contributed to the antibacterial activity of seminal plasma.
Figure 3.
Antibacterial activity of SgI and SgII holoproteins requires cleavage by PSA. A. Seminal plasma (SP), purified SgI, purified SgII, and PSA were run on an AU-PAGE gel and analyzed by coomassie staining or by antibacterial gel overlay assay with E. coli.
B and C. SgI was cleaved by PSA for 4h (B) or for 24h (C) and run on an AU-PAGE gel and analyzed by coomassie staining or by antibacterial gel overlay assay with E. coli.
Bactericidal activity in seminal plasma from vasectomized patients and patients with dysfunctional seminal vesicles
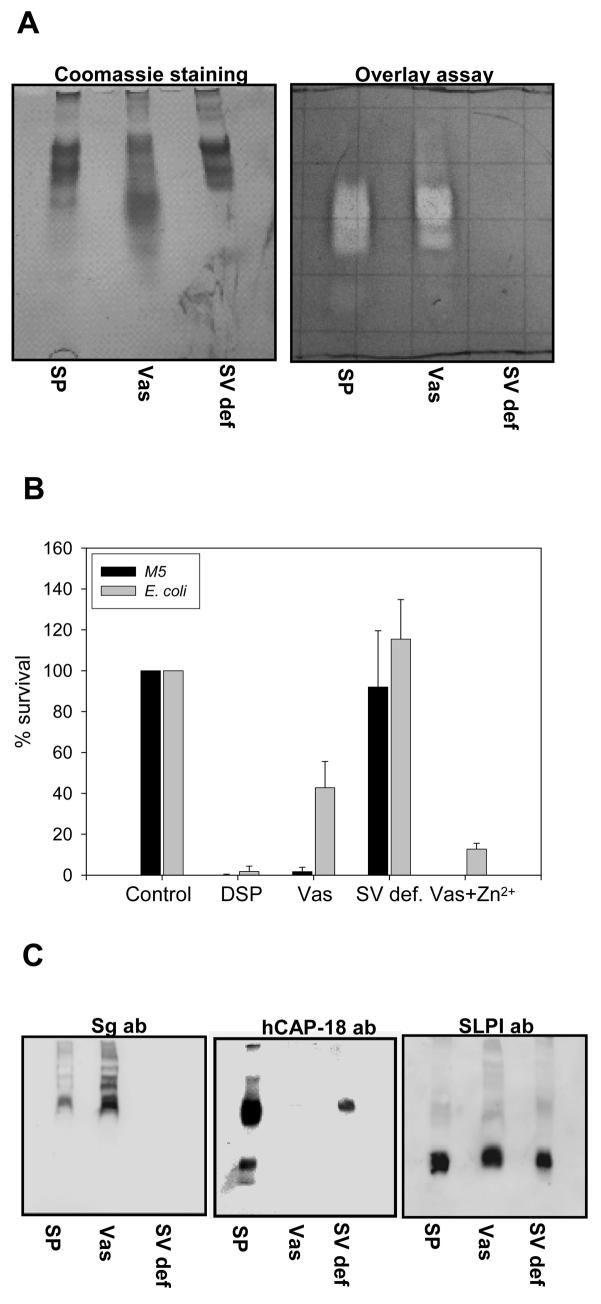
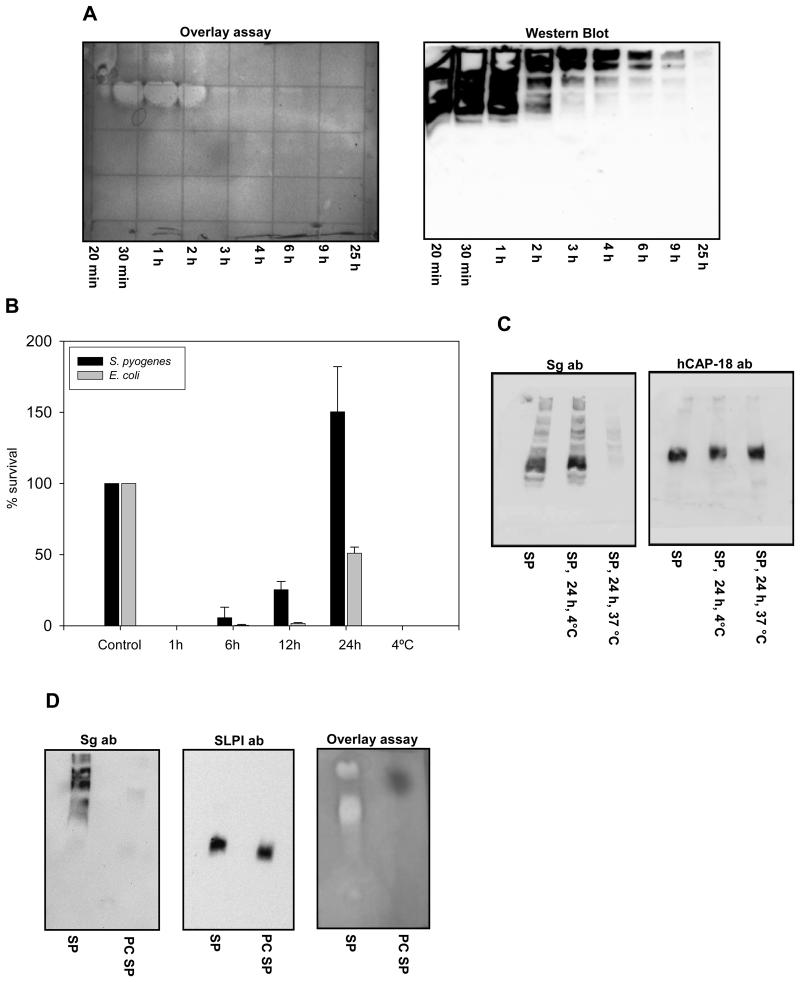
To determine the origin of the major bactericidal components in seminal plasma, we compared the antibacterial activity of normal seminal plasma with seminal plasma from two vasectomized patients (contains no material from testicles and epididymis) and seminal plasma from three patients with seminal vesicle dysfunction (contains no material from seminal vesicles). The seminal plasma samples were tested both in a CFU assay (after dialysis) and in a gel overlay assay. The seminal plasma from patients with dysfunctional seminal vesicles had no antibacterial activity (figure 4AB). Apart from a diminished activity against E.coli, the seminal plasma from vasectomized patients had similar antibacterial activity as compared to normal seminal plasma (figure 4B). Thus the major bactericidal activity in seminal plasma seemed to originate from the seminal vesicles.
Figure 4.
Antibacterial activity of seminal plasma from patients with dysfunctional seminal vesicles differs from that of normal donors and vasectomized patients. Normal seminal plasma (SP), seminal plasma from vasectomized patients (Vas) and seminal plasma from patients with dysfunctional seminal vesicles (SV def) were run on a AU-PAGE gel and either analyzed by a antibacterial gel overlay assay with E. coli and coomassie staining (A) or immunoblotting with anti-semenogelin antibodies, anti-hCAP-18-antibodies and anti-SLPI-antibodies was performed (C). After dialysis the antibacterial activity of normal seminal plasma, seminal plasma from vasectomized patients and seminal plasma from patients with dysfunctional seminal vesicles were tested in a CFU-assay against E. coli and S. pyogenes (B). The results are shown as bacterial survival compared to buffer control. Results are shown as average from three independent experiments.
2D-gel analysis and western blots of seminal plasma samples
To assess which of the identified (poly)peptides was responsible for the major antibacterial activity we analyzed normal seminal plasma, seminal plasma from vasectomized patients and seminal plasma from patients with dysfunctional seminal vesicles by 2D gel analysis and compared for protein content as previously described (23). Normal seminal plasma and plasma from vasectomized patients did not differ appreciably in protein content (figure 5 and table I). However, seminal plasma from patients with dysfunctionalk seminal vesicles had much lower protein content and was practically devoid of semenogelins. By spot matching, only one protein spot of SgI was identified in seminal plasma from patients with seminal vesicle dysfunction compared to 15 spots in both normal seminal plasma and seminal plasma from vasectomized patients. For SgII only one spot out of 6 was identified (table I). Since SgII is also expressed in the epididymis this was not surprising. These results were corroborated by Western blots that demonstrated that normal seminal plasma and seminal plasma from vasectomized patients contained semenogelin immunoreactivity whereas seminal plasma from patients with dysfunctional seminal vesicles did not (figure 4C). Furthermore immunoblotting was performed for two antimicrobial (poly)peptides not detected by the 2D gel analysis, hCAP-18 and SLPI. hCAP-18 is synthesized in the epididymis (15) and was found in normal seminal plasma and seminal plasma from patients with dysfunctional seminal vesicles but not in seminal plasma from vasectomized patients (figure 4C). SLPI is synthesized throughout the male genital tract (24) and was found in all samples (figure 4C). These data supported a major role for the semenogelins in the antibacterial activity of seminal plasma.
Figure 5.
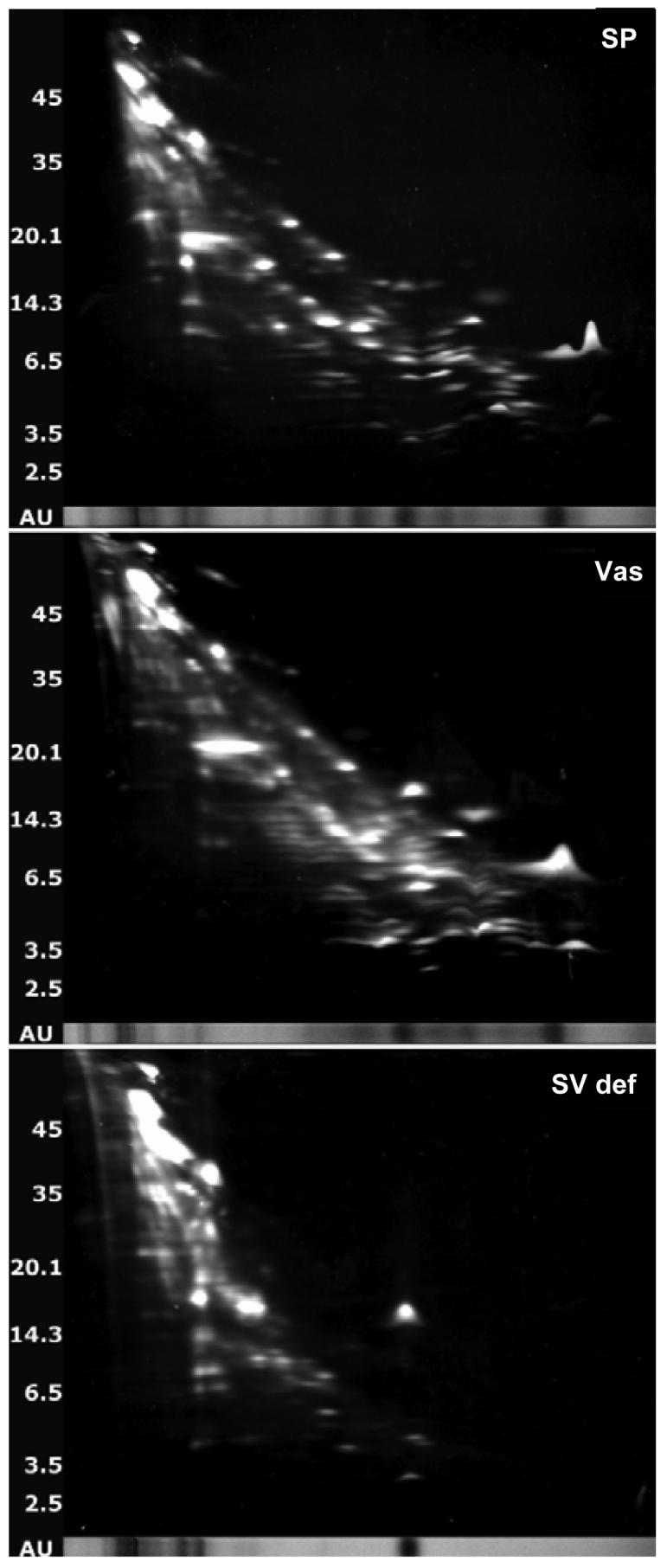
2D gel analysis of normal seminal plasma (SP), seminal plasma from a vasectomized patient (Vas), and seminal plasma from a patient with dysfunctional seminal vesicles (SV def.). 2-dimensional gel electrophoresis was performed. Protein spots were visualized by SYPRO Ruby gel stain (Bio-Rad), excised, and stored at 4°C in 1% acetic acid until analyzed by mass spectrometry.
Table I.
Analysis of protein content of seminal plasma samplesa
| Normal SP | Vas SP | SV def. SP | |
|---|---|---|---|
| Semenogelin I | 15 | 15 | 1 |
| Semenogelin II | 6 | 6 | 1 |
| Prolactin-induced protein | 4 | 3 | 0 |
| Prostatic acid phosphatase | 4 | 4 | 4 |
| Lactoferrin | 2 | 2 | 0 |
| Prostate specific antigen | 1 | 1 | 1 |
| Microseminoprotein beta | 1 | 1 | 1 |
| Miscellaneousb | 19 | 19 | 4 |
The seminal plasma were run on 2D gels and protein spots were identified in normal seminal plasma (normal SP) by mass spectrometry as described in (23) (A.M.C. and O.E.S, manuscript in preparation), and spot-matched to 2D gels from a vasectomized patient (Vas SP) and a patient with seminal vesicle dysfunction (SV def. SP).
Ig heavy chain region, keratin, ciliary rootlet coiled coil, DAP-2, clusterin, microtubule-actin crosslinking factor, intercellular adhesion molecule, growth differentiation factor 2.
Time dependent decrease in antibacterial activity of seminal plasma
Ejaculate was collected and samples were stored in urea at different time points to inhibit further degradation of the semenogelins. The samples were electrophoresed on AU-PAGE followed by either immunoblotting with anti-semenogelin antibodies or antibacterial gel overlay assay. The immunoreactive bands of semenogelins corresponded to the clearing zones on the overlay assay (figure 6A), and the immunoreactivity of the semenogelins was lost over time as the peptides were degraded. The loss of semenogelin immunoreactivity was paralleled by the disappearance of clearing zones on the overlay assay (figure 6A). These data were confirmed by a CFU assay whereby seminal plasma had lost antibacterial activity after incubation for 24 h at 37°C (figure 6B). The most prominent clearing zones were found after 30 min to 2 hours after ejaculation indicating the antibacterial activity is at a maximum after resolution of the seminal clot. In CFU assays the antibacterial activity of seminal plasma persisted for a longer period of time than in the gel overlay assay. This is most likely due to the facts that the CFU assay is much more sensitive and that in the CFU assay the antibacterial activity is a readout of all the active antibacterial (poly)peptides present in the sample while in overlay assay a single (poly)peptide must be present in antibacterial concentration for antibacterial activity to be found. In contrast to the semenogelins another antibacterial polypeptide susceptible to proteolytic processing, hCAP-18, was not degraded by incubation for 24 h at 37 °C (figure 6C). Incubation at 4°C for 24 h did neither cause loss of antibacterial activity (figure 6B) nor loss of the immunoactivity against semenogelins (fig. 6C). To validate these data antibacterial activity was examined by overlay assays with seminal plasma from six individual donors not contributing to the seminal plasma pools. For all six donors prominent bands with antibacterial were found corresponding to semenogelin immunoreactivity. After 48 hours incubation at 37 °C both the antibacterial activity and the semenogelin immunoreactivity were lost (data not shown).
Figure 6.
The antibacterial activity of seminal plasma decreases over time. A. The proteolytic activity in seminal plasma was stopped by urea at different time-points after ejaculation and the samples were run on an AU-PAGE gel and analyzed by antibacterial gel overlay assay with E. coli or subjected to immunoblotting with anti-semenogelin antibodies
B. Seminal plasma was incubated at 37°C for 1h, 6h, 12h and 24h and at 4°C for 24h. The samples were then dialyzed and tested in a CFU-assay against E. coli and S. pyogenes. The results are shown as bacterial survival compared to buffer control. Results are shown as average from three independent experiments.
C. Samples of seminal plasma (SP) and seminal plasma after incubation for 24 hours at 4 °C or 37 °C were analyzed by AU-PAGE followed by immunoblotting with anti-semenogelin and anti-hCAP-18 antibodies.
D. Sample of post coital seminal plasma (PC SP) collected from the vagina 10 hours after sexual intercourse were analyzed by AU-PAGE and subjected to either immunoblotting with anti-semenogelin antibodies and anti hCAP-18 antibodies or antibacterial overlay assay.
Between different pools of seminal plasma and different experiments we found the bands of semenogelins and the bacterial clearing zones had migrated differently. This is most likely due to the various degradations of the semenogelins in these experiments. To confirm that prolonged degradation of the semenogelins led to loss of antibacterial activity we incubated the holoprotein of SgI with PSA for 24 hours at 37 °C. This resulted in complete loss of antibacterial activity as well as a complete degradation of SgI (figure 3C).
To investigate whether the semenogelins are degraded post coitally in vivo, we examined a sample of seminal plasma collected from vagina 10 hours after sexual intercourse as previously described (14). Compared to seminal plasma, only small amounts of semenogelins were found in the post coital sample even after adjusting for a two-fold dilution (figure 6D). In contrast, SLPI was readily detected both in seminal plasma and in the post coital sample (figure 6D). Quantification of the intensity of the SLPI bands revealed only a loss of 20% in immunoreactivity in the post coital sample compared to the diluted seminal plasma. We have previously also detected large amounts of processed hCAP-18 in the post coital sample (14). Thus, the loss of semenogelin immunoreactivity seemed to be selective for the semenogelins. Furthermore, no antibacterial activity was found in the post coital sample by gel overlay assay (figure 6D), indicating the antibacterial activity of seminal plasma also after sexual intercourse is dependent on the semenogelins.
Zinc dependent antibacterial activity of seminal plasma
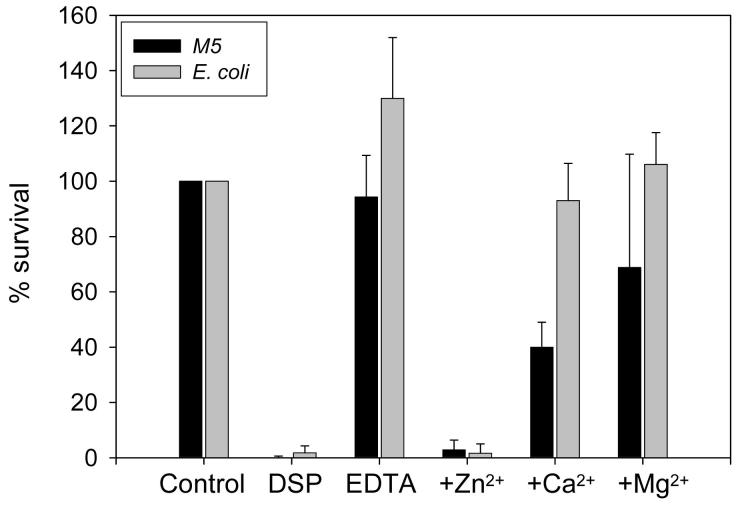
Semenogelins are histidine-rich proteins and bind zinc. Zinc is present in seminal plasma in a concentration of 2 mM and is bound to the semenogelins. The binding of zinc has implications for semenogelin-mediated inhibition of PSA activity (18) Since it is known that binding of zinc is important for the antimicrobial activity of other histidine-rich peptides (25), we wished to determine whether zinc was important for the antibacterial activity of seminal plasma. Seminal plasma was treated with EDTA to remove all divalent cations and then dialyzed in ammonium acetate to remove the EDTA. Seminal plasma depleted of divalent cations was not antibacterial in CFU assays (figure 7). The addition of zinc (unbound zinc removed by dialysis) fully restored the antibacterial activity. Addition of two other divalent cations, calcium or magnesium, did not fully restore the antibacterial activity of divalent cation-depleted seminal plasma (figure 7).
Figure 7.
The antibacterial activity of seminal plasma is zinc-dependent. EDTA was added to seminal plasma to remove divalent cations. The EDTA was removed by dialysis. Zinc, calcium or magnesium was added to the cation depleted seminal plasma and the unbound ions were removed by dialysis. Dialyzed normal seminal plasma (DSP), EDTA-treated (divalent cation-depleted) seminal plasma (EDTA) with and without addition of divalent cations were tested in a CFU-assay against E. coli and S. pyogenes. The results are shown as bacterial survival compared to buffer control. Results are shown as average from three independent experiments.
Some histidine-rich antimicrobial peptides, whose antibacterial activity is dependent on zinc at neutral pH, are capable of exerting antimicrobial activity at low pH without the presence of zinc (26). However, using a similar experimental setup we found no antibacterial activity of divalent cation-depleted seminal plasma in low pH demonstrating that binding of zinc is essential for the antibacterial activity of seminal plasma even at low pH (data not shown). Due to the importance of zinc, we tested whether the decrease in antibacterial activity of seminal plasma from vasectomized patients could be restored by addition of zinc. Indeed addition of zinc increased the antibacterial activity of seminal plasma from vasectomized patients against E. coli (figure 4B). No increase in antibacterial activity was seen when zinc was added to the seminal plasma from patients with dysfunctional seminal vesicles (data not shown).
Depletion of semenogelin from seminal plasma
In order to selectively remove semenogelin from seminal plasma, the seminal plasma was treated with EDTA, dialyzed, and incubated with nickel agarose. Flow-through representing the semenogelin-depleted seminal plasma was collected and the bound proteins were eluted. Western blots with semenogelin antibodies showed that semenogelin was depleted from the semenogelin-depleted seminal and present in the semenogelin eluate (figure 8A). SLPI was used as a control and found to be present in semenogelin-depleted seminal plasma but not in the eluate containing the semenogelins (figure 8A). Zinc was added to both the semenogelin-depleted seminal plasma and to the semenogelin containing eluate and the antibacterial activity was analyzed by gel overlay assay. The semenogelin-depleted seminal plasma was not antibacterial whereas the semenogelin-containing eluate demonstrated significant activity (figure 8B). The clearing zones on the antibacterial overlay assay corresponded to semenogelin immunoreactivity apart from the fastest migrating zone. As expected, the semenogelin-containing eluate was not antibacterial without addition of zinc (data not shown). To verify that the antimicrobial activity was due to semenogelins, we analyzed the semenogelin eluate by 2D electrophoresis and bands in the cationic antibacterial fraction were identified by spot matching (figure 8C, and table II). Of 24 spots, 11 were SgI and 6 were SgII. The majority of the other spots were also found in seminal plasma from the seminal vesicle deficient patient devoid of antimicrobial activity and none of the other proteins are known to have roles in host defense.
Figure 8.
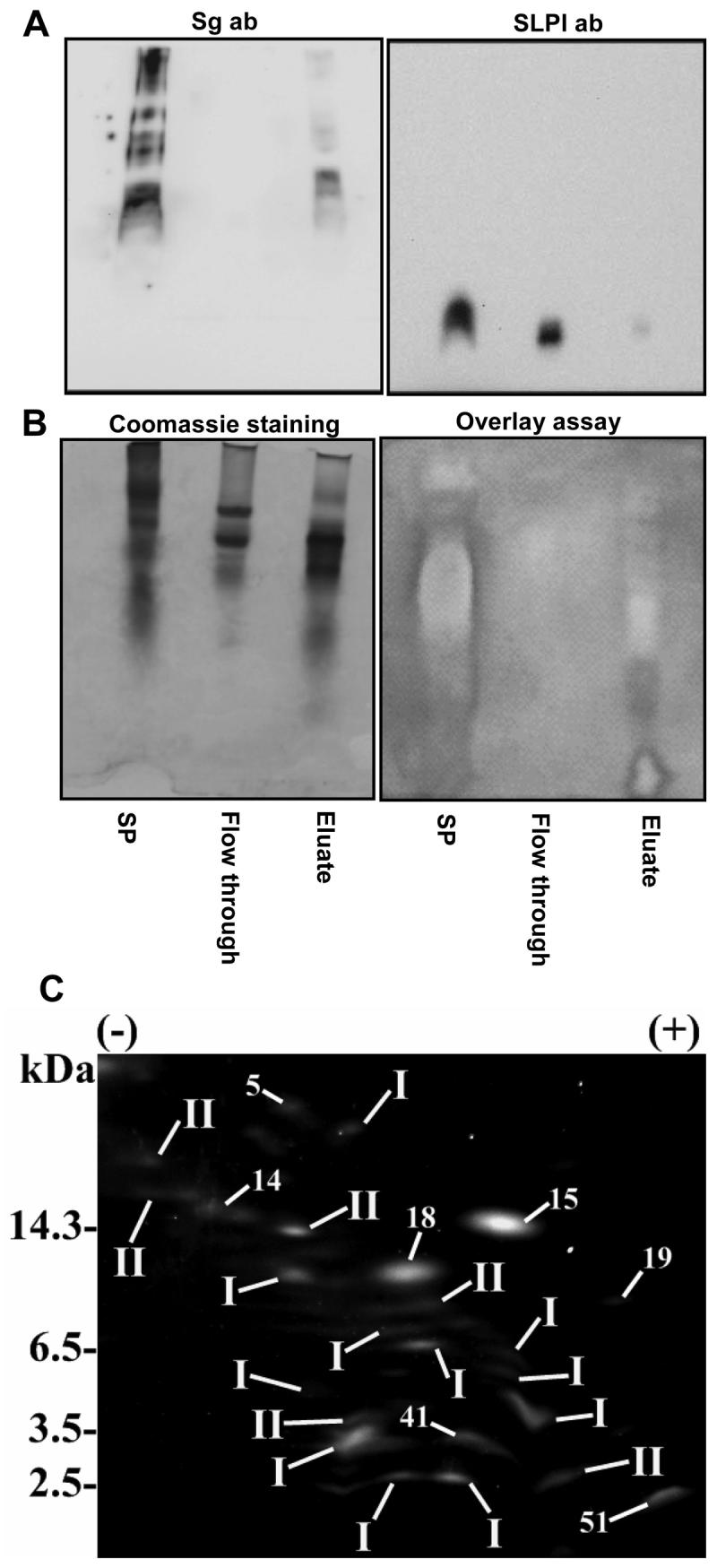
Antibacterial activity of semenogelin-depleted seminal plasma. Semenogelin was depleted from seminal plasma by applying divalent cation-depleted seminal plasma to nickel agarose and the semenogelin was eluted with imidazole. The imidazole was removed by dialysis and zinc was added both to the semenogelin depleted seminal plasma and to the semenogelin containing eluate. Normal seminal plasma (SP), flow through from the nickel agarose representing the semenogelin-depleted seminal plasma and the eluate representing the semenogelin-free seminal plasma were run on a AU-PAGE gel and subjected to immunoblotting with anti-semenogelin antibodies and anti-SLPI antibodies (A) or analyzed in a gel overlay assay against E. coli (B). 2D gel analysis of the cationic fraction of the semenogelin eluate (C). Proteins were identified by spot matching. I denotes SgI and II denotes SgII.
Table II.
Analysis of protein content of the cationic eluate from nickel-agarosea
| Spot # | Spot ID |
|---|---|
| 5 | Ig heavy chain V region |
| 14 | keratin 1, type II, cytoskeleton |
| 15 | microseminoprotein beta |
| 18 | Bullous pemphigoid antigen |
| 19 | protein product of HMFN1661 |
| 41 | SHQ1 homolog |
| 51 | microtubule-actin crosslinking factor |
The sample was run on 2D gels and protein spots were identified by spot matching to identified protein in seminal plasma.
Purified semenogelins are rapidly degraded if not stored in urea (21). Consequently, we found that the eluate from the nickel agarose column had to be stored in urea to avoid degradation of the semenogelins and preserve the antibacterial activity. Indeed, in absence of urea both the antibacterial activity and immunoreactivity towards the semenogelins were very rapidly and totally lost. The necessity of urea to preserve the antimicrobial activity of the semenogelin-containing eluate indicates that the fastest migrating clearing zone found by the antibacterial overlay assay not detected by the semenogelin antibody may be a semenogelin fragment not recognized by the monoclonal antibody used. Due to the presence of urea, which is antibacterial, antibacterial testing was not performed by CFU-assay.
In aggregate, our data demonstrate that the major bactericidal activity of seminal plasma was transient and generated as the semenogelins were degraded. As the semenogelins were further degraded they lost the bactericidal activity and the bactericidal activity of seminal plasma was consequently greatly reduced. Even though other antibacterial (poly)peptides like SLPI and lactoferrin are present in seminal plasma, we found that the contribution of these (poly)peptides to the bactericidal activity was minor compared to the activity of the semenogelin-derived peptides.
Discussion
Our data demonstrate that the holoproteins of the SgI and SgII that trap the spermatozoa after ejaculation are not bactericidal. However, the semenogelin-derived peptides formed by cleavage are the major bactericidal components in seminal plasma. When the seminal coagulum is liquefied the spermatozoa are exposed to a high concentration of semenogelin-derived antibacterial peptides. Accordingly, any microbes associated with the spermatozoa after ejaculation are trapped together with spermatozoa and are exposed to the semenogelin-derived peptides as the spermatozoa are released. It has previously been found that SgI associates with eppin, a cysteine-rich protein from testis and epidydimis, on the surface of spermatozoa and thereby provide the spermatozoa with antibacterial protection (13,27) even after the spermatozoa has left the vagina. Interestingly, even though seminal plasma displayed both rather broad and potent bactericidal activity, using the same assays seminal plasma was not bactericidal against N. gonorrhoeae, an important pathogen causing sexually transmitted disease.
We did not find any antibacterial activity of either SgI or SgII holoprotein in a gel overlay assay using 5 μg of both proteins. This is in contrast to a previous report by Bourgeon et al where the holoprotein of SgI was reported to be antibacterial in antibacterial radial diffusion assay (13). However, details of the purification were not provided nor was there any verification that the holoprotein used was intact. To keep the purified holoproteins of the semenogelins intact, the proteins must be stored in urea to prevent spontaneous aggregation and degradation (21). However, urea is antibacterial, and not compatible with the radial diffusion assay and consequently we used the antibacterial gel overlay assay. In this assay, samples are eletrophoresed in the presence of urea. Afterwards the urea is washed away and the gels are immediately put on a bacterial lawn, thus minimizing degradation. Furthermore, Coomassie staining of duplicate gels allowed verification that the intact holoproteins were used.
The antibacterial activity of seminal plasma greatly decreased over time and we found this to parallel further degradation of the semenogelin-derived peptides – but not other antibacterial (poly)peptides. As the semenogelins were degraded to smaller and smaller fragments, they lost their antibacterial activity. It is possible that prolonged antibacterial effect of the seminal plasma may disturb the normal vaginal flora. Accordingly, it is tempting to hypothesize that the transient effect of semenogelin-derived peptides in seminal plasma offers suitable antibacterial protection of the spermatozoa without imposing long term effects on the vaginal micro flora. Hence, after the spermatozoa have left the vagina the antibacterial activity of seminal plasma is gradually reduced as the semenogelins are further degraded.
The zinc concentration in seminal plasma is approximately 2 mM compared to 10-18 μM in plasma (1). Semenogelins are histidine-rich proteins, much like the histatins in saliva. Indeed the sequence of the PSA-generated semenogelin-fragments (21) resemble closely in charge and histidine content other histidine-rich peptides with known antimicrobial activity. Histidine-rich (poly)peptides are typically antimicrobial in a zinc-dependent manner (25). Free zinc is directly antibacterial at concentrations as low as 8 μM (unpublished data) but the zinc in seminal plasma is protein-bound mainly to the semenogelins (18) and we found that protein-free seminal fluid was devoid of antibacterial activity confirming that the most of zinc present in seminal plasma is protein-bound. Interestingly, we found that binding of zinc was necessary for the antibacterial activity of the semenogelin-derived peptides in seminal plasma. Other histidine-rich peptides lose the dependence on zinc for antimicrobial activity at low pH (26). However, using a similar experimental setup, we found that the antibacterial activity of seminal plasma was strictly dependent on zinc even at low pH. Thus, zinc is mandatory for the antibacterial activity of human seminal plasma even in the low pH found in the vagina.
In conclusion, we found that the major antibacterial activity of seminal plasma was transient and dependent on semenogelin-derived peptides in a zinc-dependent manner. We propose that the immediate protection of spermatozoa against microbes before entering the uterus as well as preventing ascension of bacterial pathogens along with the spermatozoa into the female upper genital tract is due to the semenogelin-derived peptides and that this antibacterial activity may be one of the most important physiological effects of these peptides. These findings ascribe a novel role for semen coagulation and liquefaction in the protection of spermatozoa and the female genital tract against bacteria.
Acknowledgments
The outstanding technical assistance of Maria Baumgarten and Malgorzata Berlikowski is greatly appreciated.
3Non-standard abbreviations
- AU
acid urea
- Sg
semenogelin
- PSA
prostate specific antigen
- SP
seminal plasma
- CF
cationic fraction
- Vas
seminal plasma from vasectomized patient
- SV def.
seminal plasma from patient with vesicular dysfunction
- PC
post coital
Footnotes
This work was supported by grants from the Novo Nordisk Foundation, The Swedish Research Council, The Alfred Österlund Foundation, Clas Groshinkys Memorial Foundation, The Åke Wiberg Foundation, The Royal Physiographic Society in Lund, The Swedish Medical Society, The Crafoord Foundation, The Harald Jeansson Foundation, and Harald and Greta Jeansson Foundation to O.E.S., and National Institutes of Health grants AI052017, AI060753 and AI065430 to A.M.C. O.E.S. is a recipient of a research fellowship from the Novo Nordisk Foundation.
References
- 1.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005;26:459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 2.De Lamirande E, Yoshida K, Yoshiike M, Iwamoto T, Gagnon C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J. Androl. 2001;22:672–679. [PubMed] [Google Scholar]
- 3.Lilja H, Lundwall A. Molecular-Cloning of Epididymal and Seminal Vesicular Transcripts Encoding A Semenogelin-Related Protein. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4559–4563. doi: 10.1073/pnas.89.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilja H, Laurell CB. The Predominant Protein in Human Seminal Coagulate. Scand. J. Clin. Lab. Invest. 1985;45:635–641. doi: 10.3109/00365518509155271. [DOI] [PubMed] [Google Scholar]
- 5.Malm J, Hellman J, Magnusson H, Laurell CB, Lilja H. Isolation and characterization of the major gel proteins in human semen, semenogelin I and semenogelin II. Eur. J. Biochem. 1996;238:48–53. doi: 10.1111/j.1432-1033.1996.0048q.x. [DOI] [PubMed] [Google Scholar]
- 6.Lilja H, Laurell CB. Liquefaction of Coagulated Human-Semen. Scand. J. Clin. Lab. Invest. 1984;44:447–452. doi: 10.3109/00365518409083836. [DOI] [PubMed] [Google Scholar]
- 7.Lee C, Keefer M, Zhao ZW, Kroes R, Berg L, Liu XX, Sensibar J. Demonstration of the role of prostate-specific antigen in semen liquefaction by two-dimensional electrophoresis. J. Androl. 1989;10:432–438. doi: 10.1002/j.1939-4640.1989.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 8.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 9.Mardh PA, Colleen S. Lysozyme in Seminal Fluid of Healthy Males and Patients with Prostatitis and in Tissues of Male Urogenital Tract. Scand. J. Urol. Nephrol. 1974;8:179–183. doi: 10.3109/00365597409132126. [DOI] [PubMed] [Google Scholar]
- 10.Wichmann L, Vaalasti A, Vaalasti T, Tuohimaa P. Localization of Lactoferrin in the Male Reproductive-Tract. Int. J. Androl. 1989;12:179–186. doi: 10.1111/j.1365-2605.1989.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 11.Nevalainen TJ, Meri KM, Niemi M. Synovial-Type (Group-Ii) Phospholipase-A2 Human Seminal Plasma. Andrologia. 1993;25:355–358. doi: 10.1111/j.1439-0272.1993.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 12.Schiessler H, Arnhold M, Ohlsson K, Fritz H. Inhibitors of Acrosin and Granulocyte Proteinases from Human Genital-Tract Secretions. Hoppe-Seylers Z. Physiol. Chem. 1976;357:1251–1260. doi: 10.1515/bchm2.1976.357.2.1251. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeon F, Evrard B, Brillard-Bourdet M, Colleu D, Jegou B, Pineau C. Involvement of semenogelin-derived peptides in the antibacterial activity of human seminal plasma. Biol. Reprod. 2004;70:768–774. doi: 10.1095/biolreprod.103.022533. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen OE, Gram L, Johnsen AH, Andersson E, Bangsboll S, Tjabringa GS, Hiemstra PS, Malm J, Egesten A, Borregaard N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin - A novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003;278:28540–28546. doi: 10.1074/jbc.M301608200. [DOI] [PubMed] [Google Scholar]
- 15.Malm J, Sørensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Stahle-Backdahl M, Borregaard N, Egesten A. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 2000;68:4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szecsi PB, Dalgaard D, Stakemann G, Wagner G, Foltmann B. The Concentration of Pepsinogen-C in Human-Semen and the Physiological Activation of Zymogen in the Vagina. Biol. Reprod. 1989;40:653–659. doi: 10.1095/biolreprod40.3.653. [DOI] [PubMed] [Google Scholar]
- 17.Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum. Reprod. Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson M, Linse S, Frohm B, Lundwall A, Malm J. Semenogelins I and II bind zinc and regulate the activity of prostate-specific antigen. Biochem. J. 2005;387:447–453. doi: 10.1042/BJ20041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjartell A, Malm J, Moller C, Gunnarsson M, Lundwall A, Lilja H. Distribution and tissue expression of semenogelin I and II in man as demonstrated by in situ hybridization and immunocytochemistry. J. Androl. 1996;17:17–26. [PubMed] [Google Scholar]
- 20.Sørensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 21.Malm J, Hellman J, Hogg P, Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): Substrate specificity and regulation by Zn2+, a tight-binding inhibitor. Prostate. 2000;45:132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer RI, Rosenman M, Harwig SSSL, Jackson R, Eisenhauer P. Ultrasensitive Assays for Endogenous Antimicrobial Polypeptides. J. Immunol. Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 23.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J. Immunol. 2005;175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsson K, Bjartell A, Lilja H. Secretory Leukocyte Protease Inhibitor in the Male Genital-Tract - Psa-Induced Proteolytic Processing in Human Semen and Tissue Localization. J. Androl. 1995;16:64–74. [PubMed] [Google Scholar]
- 25.Rydengard V, Nordahl EA, Schmidtchen A. Zinc potentiates the antibacterial effects of histidine-rich peptides against Enterococcus faecalis. Febs. J. 2006;273:2399–2406. doi: 10.1111/j.1742-4658.2006.05246.x. [DOI] [PubMed] [Google Scholar]
- 26.Kacprzyk L, Rydengard V, Morgelin M, Davoudi M, Pasupuleti M, Malmsten M, Schmidtchen A. Antimicrobial activity of histidine-rich peptides is dependent on acidic conditions. Biochim. Biophys. Acta. 2007;1768:2667–2680. doi: 10.1016/j.bbamem.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Yenugu S, Richardson RT, Sivashanmugam P, Wang ZJ, O'Rand MG, French FS, Hall SH. Antimicrobial activity of human EPPIN, an androgen-regulated, sperm-bound protein with a whey acidic protein motif. Biol. Reprod. 2004;71:1484–1490. doi: 10.1095/biolreprod.104.031567. [DOI] [PubMed] [Google Scholar]