Abstract
Context: An upper body/visceral fat distribution in obesity is closely linked with metabolic complications, whereas increased lower body fat is independently predictive of reduced cardiovascular risk.
Evidence Acquisition: The measured functions of different fat depots with regards to fatty acid storage and release in health and obesity were reviewed. The adverse effects of experimentally increasing free fatty acid (FFA) concentrations on liver, muscle, pancreatic β-cell, and endothelial function were noted.
Evidence Synthesis: The most dramatic abnormality in FFA metabolism is failure to suppress FFA concentrations/adipose tissue lipolysis normally in response to postprandial hyperinsulinemia. Upper body sc fat delivers the majority of FFA to the systemic circulation under postabsorptive and postprandial conditions. In upper body obesity, portal FFA concentrations resulting from both systemic and visceral adipose tissue lipolysis may be significantly greater than arterial FFA concentrations, exposing the liver to even greater amounts of FFA. Visceral fat also releases sufficient IL-6 to increase portal vein IL-6 concentrations, which can affect hepatic metabolism as well.
Conclusions: Lower body, upper body sc, and visceral fat depots have unique characteristics with regards to fatty acid metabolism. Selective dysregulation of these depots probably plays an important role with the metabolic complications of obesity.
Upper body fat distribution is associated with excess free fatty acid availability, which can, in turn, lead to metabolic abnormalities.
There is a wide range of body fat distribution in both lean and obese adults. The known, major environmental factors that affect body fat distribution include alcohol intake (1), cigarette smoking (2), and the timing of onset of childhood obesity (3). In addition, strong genetic factors seem to play a role in regional fat gain and loss (4,5). A predominantly upper body fat distribution, commonly associated with increased visceral fat, is associated with an abnormal metabolic profile over a wide range of body mass indexes (6,7).
There is little controversy that upper body/visceral obesity increases the risk for dyslipidemia (8), hypertension (9,10), type 2 diabetes (11,12), sleep apnea (13), etc. It is also recognized that increasing amounts of lower body fat are independently associated with a reduced risk of metabolic complications (14). Many (15,16,17), but not all (18), studies find that visceral fat mass is more strongly associated with an abnormal metabolic profile than upper body sc fat.
Defining Fat Depots
The various fat depots have unique characteristics. These range from the smaller, specific depots that track with visceral fat such as pericardial (19) and buccal (20) fat, to subdivisions of large depots like superficial and deep abdominal sc fat (21,22). Intra-abdominal fat includes omental and mesenteric (visceral) depots, both of which drain into the portal vein, along with perinephric fat, which drains into the systemic circulation. The lower body fat is commonly demarcated as all adipose tissue caudad to the inguinal ligament anteriorly and the ileac crest posteriorly. Subcutaneus lower body fat includes gluteal and leg depots, which may have differing characteristics (23), and adipose tissue in between the major muscle groups (so-called marbling) (24). Upper body sc fat includes superficial and deep truncal depots noted previously, upper extremity fat, and breast adipose tissue in women. One reasonably utilitarian approach is to characterize human body fat compartments as lower body fat, upper body sc fat, and intra-abdominal/visceral fat. An advantage of this approach is that the compartments can be measured readily using dual-energy x-ray absorptiometry and a single-slice computed tomography or magnetic resonance image of the abdomen (25,26). Those investigators with greater access to magnetic resonance imaging and considerable technical time to analyze the scans are able to define better the numerous specific fat depots (22).
Visceral Fat and Metabolic Complications–Cause and Effect?
Although the explanation for the strong association between visceral fat and metabolic abnormalities is not known, one hypothesis is that visceral fat produces and releases substances that cause metabolic abnormalities (27,28,29). More recently, an alternative hypothesis has been proposed. This concept is that visceral fat is another “ectopic fat depot” (30,31) not unlike pericardial fat (19), cheek fat (20), intramyocellular triglyceride (imTG) (32), and elevated hepatic triglyceride (33). In this paradigm, ectopic triglyceride accumulation is the result of energy imbalance wherein body fat stores exceed the functionally normal storage capacity of sc adipose tissue depots. The potential abnormal function(s) of sc fat includes a reduced ability to take up and store circulating triglyceride fatty acids along with excess free fatty acid (FFA) release under some conditions. This concept includes the abnormalities caused by greater infiltration of adipose tissue with inflammatory cells, excess release of potentially harmful cytokines, and reduced release of beneficial adipokines.
Normal Function of the Major Adipose Tissue Depots–Fatty Acid Metabolism
In vivo measures of systemic and regional adipose tissue FFA release have been performed in normal weight adult women and men under a variety of circumstances. Likewise, measures of dietary fatty acid storage and direct FFA storage in regional fat have been reported.
Under overnight postabsorptive conditions, upper body sc adipose tissue in both men and women is more lipolytically active than lower body adipose tissue as measured by FFA release per kg fat (34). In response to hyperinsulinemia (35) and meal ingestion (34,36), leg FFA release is much more readily suppressed than upper body sc fat. FFA release from the splanchnic bed, a surrogate measure of visceral adipose lipolysis, is relatively resistant to suppression by hyperinsulinemia and meal ingestion (34,35). The inability to measure FFA release directly into the portal vein in humans allows only indirect estimates of visceral adipose tissue lipolysis (37), and, thus, the rates of visceral adipose tissue lipolysis relative to visceral fat mass are not known.
Adipose tissue is also a major site of meal fatty acid storage. Normal weight men and women store roughly similar proportions of dietary fat in sc and visceral fat (38,39,40,41), but in men this is at the cost of greater triglyceridemia (40,41).
Paralleling the pattern of FFA release, the uptake of dietary fatty acids into upper body sc fat (milligram meal fat per gram of adipose tissue lipid) is more efficient than the uptake in lower body sc fat in normal weight men and women (38,39,40). Likewise, visceral fat stores more dietary fat (milligram meal fat per gram of adipose tissue lipid) than either upper body sc or lower body sc fat in both normal weight men (42) and women (38,43). This finding would argue that, at least in normal weight adults, visceral adipose tissue is more lipolytically active on a per unit weight basis than sc fat. However, the impact of visceral adipose tissue lipolysis on overnight postabsorptive hepatic FFA delivery appears to be very limited due to the typically small visceral fat mass in lean adults (37).
An unexpected recent finding is that systemic FFA can be restored back into sc and visceral fat without going through the very low-density lipoprotein (VLDL)-triglyceride pathway (44,45). Although the fraction of systemic FFA restored in whole body sc adipose tissue is relatively small (∼3% for men and 9% for women), this process may play a role in the shuttling of FFA from one depot to another in such a manner as to modify fat distribution.
Subcutaneous and visceral fat combined store approximately 50% of dietary fat (38,40). Thus, for nonobese adults consuming a typical U.S. diet containing 100 g fat and maintaining stable body composition, adipose tissue must also net release 50 g triglyceride/d as FFA for total and regional fat mass to remain stable. Understanding this balance it is possible to estimate the net FFA release from regional fat depots by measuring meal fatty acid storage. Adipose tissue also takes up VLDL-triglyceride (46,47) and FFA directly (44,45), and, thus, total regional adipose FFA release exceeds net FFA release when assessed relative to dietary fat storage. These concepts become relevant when considering the role of different fat depots in delivering FFA to the portal and systemic circulation in obesity.
Abnormalities in Fatty Acid Metabolism in Upper Body Obesity
Adipose tissue storage of fatty acids
Adipose tissue is considered a major site for clearance of triglyceride-rich lipoproteins. To the extent that this function might be impaired in obesity, especially upper body obesity, this could contribute to the hypertriglyceridemia associated with this condition. Recent studies have examined whether upper body sc fat in persons known to have disordered lipid metabolism has a reduced ability to take up triglycerides, which could contribute to postprandial hypertriglyceridemia. These studies failed to show an impaired ability of upper body sc fat to take up and store triglycerides (48). Similarly, the regional storage of dietary fat has been looked at in obese men and women (43,49). Increasing amounts of leg fat in women is associated with greater storage of dietary fat in lower body adipose tissue, whereas this is not the case for visceral fat (43). Upper body sc adipose tissue stored dietary fat in a manner different still from visceral or leg adipose tissue. Obese men store a much smaller proportion of dietary fat in sc fat than do obese women, with the difference being most marked between lower body obese women and obese men (49).
The adipose tissue clearance of VLDL-triglyceride fatty acids (46) and storage of systemic FFA (44,45) has been examined in obesity. Depending on how the data are expressed, there is some evidence for the reduced disappearance of VLDL-triglycerides across abdominal sc adipose tissue in obesity (46). Whether it is the lower body or visceral adipose tissue storage of VLDL-triglycerides that is altered in obesity, especially upper body obesity, is unknown, as is the fraction of VLDL-triglyceride fatty acids that are restored in adipose tissue.
Direct storage of systemic FFA back into adipose tissue in the postabsorptive state is remarkably different between men and women (45) and between different adipose tissue depots (44). Women with upper body obesity store a substantially greater portion of systemic FFA in lower body adipose tissue than do upper body obese men (45). However, direct FFA storage in upper body sc fat is similar in both upper body obese men and women. The greater clearance of FFA by lower body sc adipose tissue in women should theoretically limit the excess FFA available to muscle, liver, and other sites where FFA could reduce insulin sensitivity. Whether whole body and regional FFA storage is altered in lower body obesity is unknown, although women with lower body obesity tend to have normal FFA kinetics in any case (50,51,52).
Adipose tissue release of FFA
Whole body FFA release is increased in upper body obesity under postabsorptive (50,51) and postprandial (36,52) conditions. Except in metabolically uncontrolled obesity and type 2 diabetes, fasting FFA concentrations/flux in upper body obese is only about 30% greater than in lean or lower body obese adults (50). This is not a large difference, given that the day-to-day variability of FFA concentrations/kinetics is approximately 30% under uncontrolled dietary conditions (53) and approximately 15% with conditions of careful dietary control (54). These relatively small differences in overnight postabsorptive whole body lipolysis may not be the best explanation for the link between upper body/visceral obesity, FFA, and the metabolic complications of obesity. However, there are conditions under which FFAs are vastly and routinely different in upper body obesity.
The most consistent finding with regards to adipose tissue lipolysis is that of much greater FFA release during hyperinsulinemia in upper body/visceral obesity compared with the nonobese or lower body obese state (36,50,52). The excess postprandial FFA release results in elevated postprandial FFA concentrations [∼3- fold greater in upper body obese than lower body obese (36,52)]. By definition, this implies that adipocytes in upper body obesity are resistant to the antilipolytic effects of insulin. The explanation for this resistance is not clear. Some have argued that the enlarged abdominal or visceral adipocytes seen in upper body obesity are inherently resistant to insulin (55,56). Certainly, weight loss via diet and exercise, which reduce fat cell size, also improves insulin regulation of lipolysis (57), whereas surgical removal of abdominal sc fat via liposuction (no decrease in fat cell size) does not (58). On the other hand, surgical removal of omental fat during bariatric surgery was associated with significantly greater improvement in fasting plasma glucose and insulin concentrations along with a significantly greater decrease in body mass index than a control group that did not undergo resection of omentum (59). Unfortunately, we do not know whether regulation of adipose tissue lipolysis was affected by omentectomy or whether removal of visceral fat vs. greater relative weight loss accounted for the observed effects on insulin and glucose.
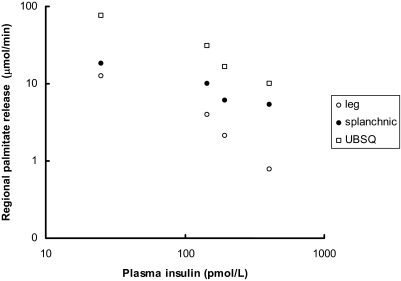
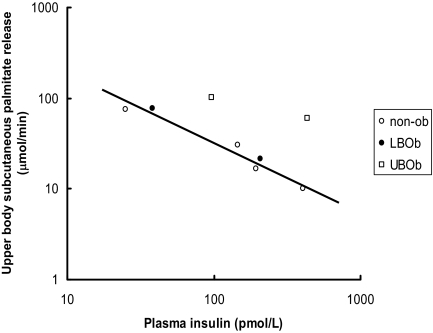
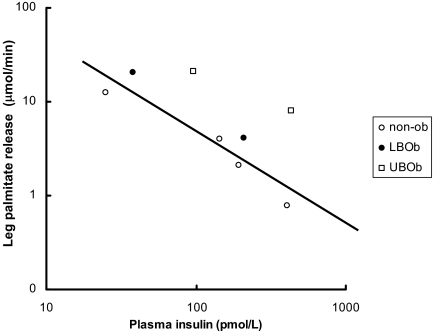
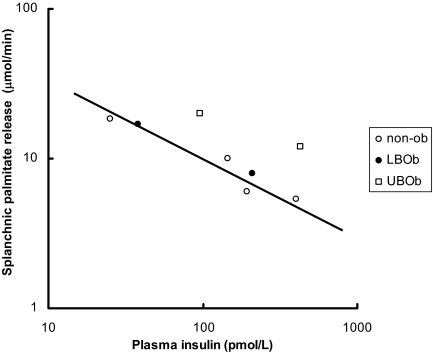
To provide a perspective on the relationship between plasma insulin concentrations vs. leg, splanchnic, and upper body sc adipose tissue FFA (palmitate) release, data from Meek (35) and Guo (36) et al. are plotted in Figs. 1–4 using a logarithmic display format (60). In insulin-sensitive nonobese adults, the relative suppression of FFA release from the splanchnic bed as a result of higher plasma insulin concentrations (35) seems blunted compared with leg and upper body sc fat (Fig. 1). Figures 2–4 depict the relationship between plasma insulin concentrations and palmitate release from upper body sc fat, leg fat, and the splanchnic bed in these same nonobese volunteers (35) compared with lower body obese and upper body obese women (36) studied before and during meal ingestion. Whereas the data points from nonobese and lower body obese (both more insulin sensitive) tend to display the same general relationship, the data points from upper body obese women (more insulin resistant) are displaced upwards, implying that each depot is to some degree insulin resistant. Because the total amount of FFA released from upper body sc fat is so much greater than that from the leg and splanchnic bed, however, insulin resistance in this depot appears to be quantitatively more important.
Figure 1.
Data from Meek et al. (35) obtained at baseline (n = 24) and during insulin clamp studies in nonobese adults are shown. The insulin doses were 0.25 (n = 6), 0.5 (n = 8), and 1.0 (n = 6) mU · kg−1 · min−1. The relationship between plasma insulin concentrations regional palmitate release are plotted on logarithmic axes. UBSQ, Upper body sc.
Figure 2.
Data from Meek et al. (35), who performed a series of insulin clamp studies in nonobese (non-ob) adults (see Fig. 1), and Guo et al. (36), who studied regional FFA release at baseline and during meal ingestion, are plotted. The relationship between plasma insulin concentrations and upper body sc palmitate release are plotted on logarithmic axes. The line shows the best fit between values from nonobese and lower body obese (LBOb) volunteers (n = 8). UBOb, Upper body obese (n = 8).
Figure 3.
Data from Meek et al. (35), who performed a series of insulin clamp studies in nonobese (non-ob) adults (see Fig. 1), and Guo et al. (36), who studied regional FFA release before and during meal ingestion, are plotted. The relationship between plasma insulin concentrations and leg palmitate release are plotted on logarithmic axes. The line shows the best fit between values from nonobese and lower body obese (LBOb) volunteers (n = 8). UBOb, Upper body obese (n = 8).
Figure 4.
Data from Meek et al. (35), who performed a series of insulin clamp studies in nonobese (non-ob) adults (see Fig. 1), and Guo et al. (36), who studied regional FFA release before and during meal ingestion, are plotted. The relationship between plasma insulin concentrations and splanchnic palmitate release are plotted on logarithmic axes. The line shows the best fit between values from nonobese and lower body obese (LBOb) volunteers (n = 8). UBOb, Upper body obese (n = 8).
The implications of excess adipose tissue lipolysis in upper body obesity deserve attention. Most of the studies examining the adverse effects of experimentally elevating plasma FFA concentrations have used the paradigm of assessing their effect on insulin-stimulated glucose disposal (61), insulin-suppressed glucose (62), and hepatic triglyceride production (63), as well as insulin-stimulated tissue blood flow (64). Thus, it is reasonable to consider that high postprandial FFA concentrations are particularly relevant to the metabolic abnormalities seen in obesity. Given the association between visceral fat metabolic complications of obesity (16,65) and the finding of greater splanchnic FFA release during hyperinsulinemia (35), it is tempting to blame visceral adipose tissue lipolysis for elevated postprandial FFA concentrations in upper body obesity. However, in vivo studies have shown this not to be the case.
In lean men and women, leg adipose tissue lipolysis contributes approximately 15–20% of basal, systemic FFA release (34,35). In obese men and women, the average was 28% of FFA release (36,37). Because leg adipose tissue lipolysis is so sensitive to insulin (35) and meal (34,36) suppression, it does not seem to contribute to the elevated postprandial FFA in obesity. Upper body sc adipose tissue FFA release accounts for the majority (>60%) of systemic FFA under basal (37,66) and insulin-suppressed conditions (35,36,67). The greater postprandial FFA concentrations in upper body obesity compared with lower body obesity could be entirely accounted for by excess FFA release from upper body sc fat (36), not visceral fat.
However, the net release of new FFA into the systemic circulation from the splanchnic bed does not fully reflect visceral adipose tissue lipolysis. The liver takes up a considerable fraction of FFA in the portal vein (68,69). In addition, some of the FFA entering the splanchnic bed via the arterial supply are taken up by nonhepatic tissues before they can enter the portal vein. Fasting FFA concentrations in the portal vein have not been found to be substantially greater than those typically seen in the arterial circulation (70). That said, the appearance of new FFA in the hepatic vein is probably a direct measure of the contribution of visceral adipose tissue lipolysis to systemic FFA availability and, thus, plasma FFA concentrations. We found that only 6–17% of systemic FFA come from the splanchnic bed under overnight postabsorptive conditions (34,35) but can increase to 40% during hyperinsulinemia (35). This might indicate either that visceral fat is very resistant to insulin’s antilipolytic effects when compared with sc fat [as noted in dogs (71)] or that the spillover of fatty acids from the hydrolysis of triglyceride-rich lipoproteins is a special issue in the splanchnic bed (72). In either case the liver is probably exposed to significantly greater FFA concentrations than the periphery during hyperinsulinemia, and this portal-systemic difference may well be exaggerated in upper body/visceral obesity.
Effects of increased FFA concentrations
FFA and the liver
The ability of insulin to suppress glucose production is thought to be through a combination of direct insulin action on the liver and an indirect action via suppression of FFA (73). The delivery of excess FFA to the liver from systemic and/or visceral adipose tissue lipolysis will prevent the normal insulin mediated suppression of glucose output by the liver. There is some controversy as to whether the effects of elevated FFA are on hepatic gluconeogenesis or a combination of gluconeogenesis and glycogenolysis. It is proposed that FFAs influence glucose output by creating surpluses of factors such as acetyl-coenzyme A, reduced nicotinamide adenine dinucleotide, ATP, and citrate, perhaps with intrahepatic triglyceride as the intermediate step. Elevated FFA also stimulate VLDL-triglyceride production in the face of hyperinsulinemia (63), and given the likelihood that portal FFA are quite substantially increased during hyperinsulinemia in visceral obesity, this could be an especially important effect of visceral fat (70).
FFA effect on the vasculature
Hypertension is one of the risk factors for cardiovascular disease, and is tightly associated with insulin resistance and visceral obesity. Many mechanisms are thought to fit hypertension into the circle. Systemic FFA, largely derived from sc adipose tissue lipolysis (not from visceral fat), may play a role in some abnormalities. These mechanisms include impaired: 1) insulin-mediated vasodilatation in vascular beds (74), 2) α-adrenergic stimulation (75), and 3) nitric oxide and endothelial dysfunction (64,76). Unfortunately, many of the studies have been conducted at levels of FFAs that are almost supraphysiological (64,76). Further studies to assess whether FFAs have similar effects at more physiologically elevated concentrations are required.
Elevated systemic FFA and β-cell dysfunction
It is established that fatty acids can have adverse effects on islet insulin content (77,78). One theory is that type 2 diabetes develops as part of a biphasic β-cell response to excess FFA (78,79). A number of mechanisms have been proposed to mediate the toxic effects of excess intracellular fatty acids, but species differences in how β-cells respond to fatty acids make it difficult to translate directly animal and cell model systems to human type 2 diabetes. Because the pancreas is not downstream of the portal vein, any adverse effects of FFA on human insulin secretion in visceral obesity would largely be an effect of abnormal regulation of sc adipose tissue lipolysis.
Muscle and FFA
The preferential oxidation of fatty acids by muscle mitochondria and the resultant competition with glucose (the Randle cycle) is a suggested mechanism for fatty acid-induced insulin resistance (80). FFA elevation has also interrupted muscle insulin receptor substrate and phosphatidylinositol 3-kinase insulin-mediated glucose uptake independent of their oxidative role, contributing to peripheral resistance in a novel pathway (81). imTG accumulation may provide a link between extracellular FFA and the intramyocellular environment because imTG correlates well with insulin resistance, and FFAs are a precursor of imTG (82,83). The precise mechanism(s) is not entirely known at present, but interesting possibilities include actions of diacylglycerols, ceramides, and long-chain acyl-coenzyme As, themselves as intracellular mediators of impaired insulin signaling. Again, muscle is exposed to systemic FFA concentrations, which predominantly originate from upper body sc fat. Thus, the adverse effects of FFA on muscle insulin action in obesity are largely an effect of abnormal regulation of sc adipose tissue lipolysis.
Adipokines in upper body obesity
A large number of substances are known to be produced by adipose tissue. Collectively, these have been termed adipokines, and their purported function and role in the metabolic complications of obesity are reviewed in a companion article (84). To date, the only adipokine documented to be uniquely overproduced by visceral fat is IL-6 (29). Adiponectin concentrations are reduced in adults with excess visceral fat, but strangely enough in obese women, concentrations correlate positively with leg fat mass (85). Whether this indicates that adiponectin is preferentially produced by leg fat or that women with greater amounts of leg fat are more insulin sensitive and, thus, have greater adiponectin concentrations, is unknown.
Summary
An upper body/visceral fat distribution in obesity is strongly linked with the metabolic complications of obesity. Evidence from studies that manipulate FFA concentrations suggests that a number of these metabolic abnormalities are caused by elevated FFAs. Because the most consistent abnormality in FFA metabolism is failure to normally suppress FFA in response to insulin/meal ingestion, we suggest that postprandial elevations of FFAs are especially problematic in obesity. FFAs released from visceral fat make a minor contribution to systemic FFA concentrations, which are the obligate concentrations affecting muscle, pancreatic β-cells, and vascular endothelium. In persons with visceral obesity, omental and mesenteric fat may play a special role in delivering both excess FFA and IL-6 to the liver. We do not yet understand why upper body sc fat, the source of the majority of systemic FFAs, is dysregulated in upper body obesity. Weight loss via diet/exercise is able to improve the regulation of FFA metabolism (57), whereas simply removing sc fat does not (58).
Footnotes
This work was supported by American Diabetes Association (ADA no. 7-06-DCS-03) and the United States National Institutes of Health Grants DK45343 and DK40484.
Disclosure Statement: The author has nothing to disclose.
Abbreviations: FFA, Free fatty acid; imTG, intramyocellular triglyceride; VLDL, very low-density lipoprotein.
References
- Wannamethee SG, Shaper AG, Whincup PH 2005 Alcohol and adiposity: effects of quantity and type of drink and time relation with meals. Int J Obes 29:1436–1444 [DOI] [PubMed] [Google Scholar]
- Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day NK, Khaw KT 2005 Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res 13:1466–1475 [DOI] [PubMed] [Google Scholar]
- Sachdev HS, Fall CH, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, Reddy KS, Barker DJ, Bhargava SK 2005 Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr 82:256–266 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Despres JP, Mauriege P 1993 Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 14:72–93 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G 1990 The response to long-term overfeeding in identical twins. N Engl J Med 322:1477–1482 [DOI] [PubMed] [Google Scholar]
- Björntorp P 1991 Metabolic implications of body fat distribution. Diabetes Care 14:1132–1143 [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Krakower GR 1994 Regional adiposity and morbidity. Physiol Rev 74:761–811 [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Alfarsi S, Adams PW, Wynn V 1976 Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 12:563–571 [DOI] [PubMed] [Google Scholar]
- Cassano PA, Segel MR, Vokonas PS, Weiss ST 1990 Body fat distribution, blood pressure, and hypertension. A Prospective cohort study of men in the normative aging study. Ann Epidemiol 1:33–48 [DOI] [PubMed] [Google Scholar]
- Seidell JC, Cigolini M, Deslypere J, Charzewska J, Ellsinger B, Cruz A 1991 Body fat distribution in relation to serum lipids and blood pressure in 38-year-old European men: the European fat distribution study. Atherosclerosis 86:251–260 [DOI] [PubMed] [Google Scholar]
- Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE 1997 Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 145:614–619 [DOI] [PubMed] [Google Scholar]
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC 1994 Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17:961–969 [DOI] [PubMed] [Google Scholar]
- Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK 2002 Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest 122:829–839 [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC, Hoorn study 2004 Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 27:372–377 [DOI] [PubMed] [Google Scholar]
- Cefalu WT, Wang ZQ, Webel S, Bell-Farrow A, Crouse JR, Hinson WH, Terry JG, Anderson R 1995 Contribution of visceral fat mass to the insulin resistance of aging. Metabolism 44:954–959 [DOI] [PubMed] [Google Scholar]
- Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R 1990 Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 39:897–901 [DOI] [PubMed] [Google Scholar]
- Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R 2006 Visceral fat is an independent predictor of all-cause mortality in men. Obesity 14:336–342 [DOI] [PubMed] [Google Scholar]
- Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM 1995 Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest 96:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS 2008 Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: a Framingham Heart Study. Circulation 117:605–613 [DOI] [PubMed] [Google Scholar]
- Levine JA 1998 Relation between chubby cheeks and visceral fat. N Engl J Med 339:1946–1947 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH 2000 Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278:E941–E948 [DOI] [PubMed] [Google Scholar]
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA 2001 Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 50:425–435 [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD 2008 Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 87:56–63 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE 2000 Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71:885–892 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Kanaley JA, Reed JE, Sheedy PF 1995 Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61:274–278 [DOI] [PubMed] [Google Scholar]
- Shadid S, Jensen MD 2003 Effects of pioglitazone vs diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care 26:3148–3152 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P 1990 “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 10:493–496 [PubMed] [Google Scholar]
- Kissebah AH, Peiris AN 1989 Biology of regional body fat distribution: relationship to non- insulin-dependent diabetes mellitus. Diabetes Metab Rev 5:83–109 [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S 2007 Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56:1010–1013 [DOI] [PubMed] [Google Scholar]
- Danforth Jr E 2000 Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 26:13 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Molavi B, Elbein SC, Kern PA 2007 Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab 9:1–10 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L 1999 Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606 [DOI] [PubMed] [Google Scholar]
- Korenblat KM, Fabbrini E, Mohammed BS, Klein S 2008 Liver, muscle and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134:1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD 1995 Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96:2297–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek S, Nair KS, Jensen MD 1999 Insulin regulation of regional free fatty acid metabolism. Diabetes 48:10–14 [DOI] [PubMed] [Google Scholar]
- Guo ZK, Hensrud DD, Johnson CM, Jensen MD 1999 Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48:1586–1592 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD 2004 Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA 2003 Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 285:E1282–E1288 [DOI] [PubMed] [Google Scholar]
- Marin P, Rebuffe-Scrive M, Bjorntorp P 1990 Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 20:158–165 [DOI] [PubMed] [Google Scholar]
- Romanski SA, Nelson R, Jensen MD 2000 Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 279:E455–E462 [DOI] [PubMed] [Google Scholar]
- Uranga AP, Levine J, Jensen M 2005 Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab 288:E547–E555 [DOI] [PubMed] [Google Scholar]
- Marin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjostrom L, Bjorntorp P 1992 The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 41:1242–1248 [DOI] [PubMed] [Google Scholar]
- Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD 2007 Meal fatty acid uptake in visceral fat in women. Diabetes 56 2589–2597 [DOI] [PubMed] [Google Scholar]
- Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD 2008 Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes 57:1186–1194 [DOI] [PubMed] [Google Scholar]
- Shadid S, Koutsari C, Jensen MD 2007 Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 56:1369–1375 [DOI] [PubMed] [Google Scholar]
- Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, Kirk ML, Potts JL, Hockaday TD 1992 Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 41:264–272 [DOI] [PubMed] [Google Scholar]
- Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL, Frayn KN 1990 Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 79:339–348 [DOI] [PubMed] [Google Scholar]
- Evans K, Burdge GC, Wootton SA, Collins JM, Clark ML, Tan GD, Karpe F, Frayn KN 2008 Tissue-specific stable isotope measurements of postprandial lipid metabolism in familial combined hyperlipidemia. Atherosclerosis 197:164–170 [DOI] [PubMed] [Google Scholar]
- Santosa S, Hensrud DD, Votruba SB, Jensen MD 2008 The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr, 88:1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM 1989 Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 83:1168–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaley JA, Cryer PE, Jensen MD 1993 Fatty acid kinetic responses to exercise. Effects of obesity, body fat distribution, and energy-restricted diet. J Clin Invest 92:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roust LR, Jensen MD 1993 Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42:1567–1573 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Caruso M, Heiling V, Miles JM 1989 Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38:1595–1601 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD 2003 Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson B, Smith U 1972 Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res 13:651–656 [PubMed] [Google Scholar]
- Goldrick RB, McLoughlin GM 1970 Lipolysis and lipogenesis from glucose in human fat cells of different sizes. Effects of insulin, epinephrine, and theophylline. J Clin Invest 49:1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadid S, Jensen MD 2006 Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia 49:149–157 [DOI] [PubMed] [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS 2004 Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 350:2549–2557 [DOI] [PubMed] [Google Scholar]
- Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P 2002 A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes 26:193–199 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Nielsen S 2007 Insulin dose response analysis of free fatty acid kinetics. Metabolism 56:68–76 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mokan M, Simoneau JA, Mandarino LJ 1993 Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, Barrett EJ, Bevilacqva S, DeFronzo RA 1983 Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G 1995 Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 95:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD 2000 Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49:1231–1238 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S 1987 Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 36:54–59 [DOI] [PubMed] [Google Scholar]
- Martin ML, Jensen MD 1991 Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 88:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD 2001 Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 280:E1000–E1006 [DOI] [PubMed] [Google Scholar]
- Basso LV, Havel RJ 1970 Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest 49:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Cardin S, Edgerton D, Cherrington A 2003 Splanchnic free fatty acid kinetics. Am J Physiol Endocrinol Metab 284:E1140–E1148 [DOI] [PubMed] [Google Scholar]
- Vogelberg KH, Gries FA, Moschinski D 1980 Hepatic production of VLDL-triglycerides. Dependence of portal substrate and insulin concentration. Horm Metab Res 12:688–694 [DOI] [PubMed] [Google Scholar]
- Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN 2002 Extreme insulin resistance of the central adipose depot in vivo. Diabetes 51:755–761 [DOI] [PubMed] [Google Scholar]
- Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM 2007 Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes 56:2878–2884 [DOI] [PubMed] [Google Scholar]
- Mittelman SD, Fu YY, Rebrin K, Steil G, Bergman RN 1997 Indirect effect of insulin to suppress endogenous glucose production is dominant, even with hyperglucagonemia. J Clin Invest 100:3121–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M, Edelman SV, Brechtel G, Baron AD 1990 Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. J Clin Invest 85:1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniakowski K, Sallee FR, Goodfriend TL, Zhang Z, Egan BM 1996 Fatty acids enhance neurovascular reflex responses by effects on α 1-adrenoceptors. Am J Physiol 270(6 Pt 2):R1340–R1346 [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD 1994 Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ 1998 Chronic exposure to free fatty acid reduces pancreatic β cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest 101:1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A 1999 Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes 48:524–530 [DOI] [PubMed] [Google Scholar]
- Paolisso G, Gambardella A, Amato L, Tortoriello R, D'Amore A, Varricchio M, D'Onofrio F 1995 Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia 38:1295–1299 [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA 1963 The glucose-fatty acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789 [DOI] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI 1999 Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Jensen MD 1998 Intramuscular fatty acid metabolism evaluated with stable isotopic tracers. J Appl Physiol 84:1674–1679 [DOI] [PubMed] [Google Scholar]
- Guo Z, Burguera B, Jensen MD 2000 Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol 89:2057–2064 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Kern P 2008 Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S64–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadid S, Stehouwer CDA, Jensen MD 2006 Diet/exercise versus pioglitazone: effects of insulin sensitization with decreasing or increasing fat mass on adipokines and inflammatory markers. J Clin Endocrinol Metab 91:3418–3425 [DOI] [PubMed] [Google Scholar]