Abstract
Context: Adipose tissue is increasingly recognized as an active endocrine organ with many secretory products and part of the innate immune system. With obesity, macrophages infiltrate adipose tissue, and numerous adipocytokines are released by both macrophages and adipocytes. Adipocytokines play important roles in the pathogenesis of insulin resistance and associated metabolic complications such as dyslipidemia, hypertension, and premature heart disease.
Evidence Acquisition: Published literature was analyzed with the intent of addressing the role of the major adipose secretory proteins in human obesity, insulin resistance, and type 2 diabetes.
Evidence Synthesis: This review analyzes the characteristics of different adipocytokines, including leptin, adiponectin, pro-inflammatory cytokines, resistin, retinol binding protein 4, visfatin, and others, and their roles in the pathogenesis of insulin resistance.
Conclusions: Inflamed fat in obesity secretes an array of proteins implicated in the impairment of insulin signaling. Further studies are needed to understand the triggers that initiate inflammation in adipose tissue and the role of each adipokine in the pathogenesis of insulin resistance.
The relationships between adipocytokines and inflammation are reviewed, particularly adipocytokines that have been studied in humans, with the clinical implications for obesity and insulin resistance discussed.
Many recent epidemiological studies have documented the rapid increase in the prevalence of obesity. According to data from the Center for Disease Control Behavioral Risk Factor Surveillance System, 22 states in the United States have an obesity [body mass index (BMI) >30 kg/m2] prevalence of over 30% in 2006, whereas only 10 yr earlier, no state had an obesity prevalence of more than 20%. Along with the increase in obesity is a parallel increase in the prevalence of type 2 diabetes, impaired glucose tolerance (1,2), and other complications of obesity, such as hypertension, sleep apnea, and arthritis. Whether or not the obesity epidemic leads to an increase in the incidence of new obesity related malignancies remains to be determined (3,4). A recent study suggested that future life expectancy may decrease for the first time due to the increase in obesity (5).
The metabolic complications of obesity, often referred to as the metabolic syndrome, consist of insulin resistance, often culminating in β-cell failure, impaired glucose tolerance and type 2 diabetes, dyslipidemia, hypertension, and premature heart disease. Abdominal obesity, ectopic lipid accumulation, hepatic steatosis, and sleep apnea can also be included in the metabolic complications of obesity (6).
This paper is intended to provide an overview of the pathogenesis of the metabolic complications of obesity, with particular emphasis on the role of inflammation and adipose tissue-derived proteins. There are many adipokines, and space limitations do not permit a thorough discussion of all of them. Therefore, this review will discuss a number of the major adipokines, and will focus on adipokines related to inflammation, and in particular adipokines that have been the subject of studies in humans, and where there are clinical implications for obesity and insulin resistance.
Obesity Is Associated with Inflammation
The role of adipose tissue in metabolic syndrome has continued to evolve with the description of numerous secretory products from adipocytes. These “adipokines” are important determinants of insulin resistance, either through a traditional (circulating) hormonal effect, or through local effects on the adipocyte.
In the mid-1990s, the expression of TNFα by adipose tissue of obese rodents and humans was first described (7,8). Subsequently, other adipose tissue-derived proteins were described, and many of these adipokines have been implicated in the pathogenesis of the chronic inflammation and insulin resistance associated with obesity. In addition to the production of pro-inflammatory cytokines that promote metabolic complications, adipose tissue is the sole source of adiponectin, which is antiinflammatory and associated with protection from atherosclerosis (9,10).
The study of adipose tissue inflammation was considerably impacted by the demonstration of resident macrophages in adipose tissue (11,12). The adipose tissue of obese rodents and humans contains increased numbers of macrophages, and once activated, macrophages secrete a host of cytokines such as TNFα, IL-6, and IL-1 (13), and the adipose tissue resident macrophages were responsible for the expression of most of the tissue TNFα and IL-6. The expression of macrophage markers in human adipose tissue was high in subjects with obesity and insulin resistance, and was also correlated with the expression of TNFα and IL-6 (12,14).
There are a number of possible mechanisms underlying the infiltration of macrophages into adipose tissue. One possibility is the elaboration of chemokines by adipocytes, which would then attract resident macrophages. Adipocytes express low levels of monocyte chemoattractant protein (MCP)-1, and increased expression is found in obese subjects (14). From an evolutionary perspective, adipose macrophages may have represented an important part of the host defense against injury or infection. On the other hand, recent studies have suggested that macrophages infiltrate adipose tissue as part of a scavenger function in response to adipocyte necrosis. Careful immunohistological studies of mouse and human adipose tissue demonstrated that most of the macrophages in adipose tissue of obese mice were surrounding dead adipocytes and formed a syncytium, often referred to as a “crown-like structure” (15). With the rapid development of obesity in both diet and genetically obese rodent models, the number of crown-like structures in adipose tissue increases rapidly, and the macrophage burden surrounding necrotic adipocytes becomes considerable (16).
If adipocyte necrosis is indeed the initiating event in the process of macrophage infiltration, there are a number of possible causes. Hypoxia has been proposed to be an inciting etiology of necrosis (17). With obesity and progressive adipocyte enlargement, the blood supply to adipocytes may be reduced (18), and the induction of adipocyte hypoxia in vitro results in the expression of a number of inflammatory cytokines (19,20,21). Indeed, an increased prevalence of insulin resistance in patients with sleep apnea independent of obesity has been reported, which is perhaps due to intermittent hypoxia, inflammation, and oxidative stress (22).
Another body of thought suggests that unbridled adipocyte expansion and triglyceride accumulation in adipose tissue are ultimately a benign phenomenon, and perhaps even beneficial to relieve lipotoxicity in liver, skeletal muscle, and other ectopic sites (23). Adipose tissue inflammation may occur when adipocyte expansion is limited, either due to impaired adipocyte development, decreased lipid synthesis, or matrix factors that prevent cell enlargement. One example of limited adipocyte development is lipodystrophy. Humans and rodents with extreme forms of lipodystrophy have little or no adipose tissue and extreme ectopic fat deposition, leading to lipotoxicity and insulin resistance (24). However, lesser degrees of lipodystrophy occur in patients with HIV lipodystrophy, who demonstrate features of the metabolic syndrome. The adipose tissue of HIV-infected patients demonstrated increased inflammation and lower levels of expression of lipin-β (25,26,27,28). Lipin is a phosphatidate phosphatase involved in lipid synthesis, and animals with lipin deficiency are lipodystrophic (29,30). In addition, lower levels of adipose lipin expression are found in non-HIV infected subjects with insulin resistance, and peroxisome proliferator activated receptor (PPAR)-γ agonists increase the expression of the β-isoform of lipin (31). Other genes involved in adipocyte differentiation or lipogenesis may also be associated with adipose tissue inflammation. Therefore, the association of obesity with insulin resistance and inflammation is well established. The concept of limited adipocyte expansion, leading to inflammation and many of the metabolic consequences of obesity, is somewhat counterintuitive, but enjoys some support in the literature. This concept clearly needs further development and clarification with future research.
Adipokines Expressed by Adipocytes
Leptin
Leptin (Greek, leptos, thin), is a 167-amino acid hormone secreted largely by adipose tissue that controls food intake and energy expenditure (32). Circulating levels of leptin parallel fat cell stores, increasing with overfeeding and decreasing with starvation. The absence of leptin or a mutation in leptin receptor genes induces a massive hyperphagia and obesity in animal models (33), and humans (34,35), however, the prevalence of these mutations in obese humans is rare.
The effects of leptin are mediated by receptors, mainly located in the central nervous system, and in other tissues, including adipocytes and endothelial cells. Leptin receptor belongs to the class I family of cytokine receptors, and it engages both the signal transducer and activator of transcription-3 (STAT3) pathway and the insulin receptor substrate phosphoinositide-3 kinase pathway, among others (36). It has been shown that STAT3 is essential for mediating food intake, liver glucose production, and gonadotropin secretion (36), however, the control of adipose tissue metabolism by leptin is STAT3 independent (37). Recently, Buettner et al. (37) showed that the infusion of leptin in hypothalamus led to the suppression of lipogenesis in adipose tissue through activation of the phosphoinositide-3 kinase pathway, sympathic nervous system, and the engagement of adipose tissue endocannabinoid system.
Other potential physiological roles for leptin have been described. Leptin modulates the T-cell immune response, stimulates proliferation of T-helper cells, and increases production of pro-inflammatory cytokines by regulating different immune cells (38,39). Leptin is also important in regulating the reproductive system and the onset of puberty, and leptin deficiency is associated with hypogonadism (40).
The increased risk of cardiovascular disease with obesity makes adipokines, including leptin, an attractive instigator of atherosclerosis. In a large prospective study, leptin was independently associated with an increased risk of coronary artery disease (41). However, the question whether leptin directly causes atherosclerosis in obese individuals is still unresolved. In in vitro or animal studies, different atherogenic properties, including increased oxidative stress, impairment of vasorelaxation, and increased thrombosis, have been described for leptin (42,43).
Potential use of leptin as a drug
Treatment with recombinant human leptin reverses hyperphagia, obesity, hypogonadism, and impaired T-cell-mediated immunity associated with congenital leptin deficiency (44,45). In addition, leptin replacement is a very promising therapeutic approach for the management of the complications of lipodystrophy (46). In contrast, leptin treatment for the reversal of typical obesity and obesity related metabolic disorders has not proven to be successful (47). Obese individuals, for unknown reasons, become resistant to the satiety and weight-reducing effect of leptin. A recent study reported a synergistic effect for weight loss with leptin and amylin coadministration in diet-induced obese rats by restoring hypothalamic sensitivity to leptin (48). If confirmed in clinical research studies, the restoration of leptin sensitivity might change the neurohormonal approaches to obesity pharmacotherapy.
Adiponectin
Adiponectin is a 30-kDa protein secreted from adipocytes (9), and its circulating levels are decreased in obesity induced insulin resistance (49,50). Paradoxically, in rare cases of severe insulin resistance with proximal defect in insulin action, elevated levels of adiponectin have been reported (51). Mice lacking adiponectin have reduced insulin sensitivity (52,53,54); in contrast, adiponectin overexpression in ob/ob mice, confers dramatic metabolic improvements (23).
Once adiponectin is synthesized, it undergoes several posttranslational modifications, including hydroxylation and glycosylation (55), and some of these modifications are necessary for its bioactivity (56). Circulating adiponectin is found in several different isoforms, including trimer, low-molecular weight (-hexamers), and high-molecular weight (HMW) (18mers) forms (57,58). Different adiponectin oligomers hold distinct biological functions. Most insulin-sensitizing effects of adiponectin have been linked to the HMW isoform, whereas the central effects of adiponectin have been contributed to hexamer and trimer isoforms (55).
The distribution of adiponectin oligomers in the circulation is primarily controlled at the level of secretion from adipocytes. Molecular chaperones in the endoplasmic reticulum (ER), including ER protein of 44 kDa and ER oxidoreductase 1-Lα, play an important role in the secretion of adiponectin (55,63).
Several studies have linked hypoadiponectinemia to diabetes (50), hypertension (59), atherosclerosis, and endothelial dysfunction (60). More recent studies have shown that the HMW oligomer is inversely associated with the risk for diabetes independent of total adiponectin (61), and the HMW oligomer is responsible for the association of adiponectin with traits of metabolic syndrome (62,63).
Adiponectin inserts its effects through two transmembrane receptors (AdipoR1 and AdipoR2) that are ubiquitously expressed. AdipoR1 is predominantly expressed in skeletal muscle with a preference for binding to globular adiponectin, whereas AdipoR2 is most abundant in the liver with a preference for binding to full-length adiponectin (64). Adiponectin improves insulin sensitivity by increasing energy expenditure and fatty acid oxidation through activation of AMP-activated protein kinase (AMPK), and by increasing the expression of PPARα target genes such as CD36, acyl-coenzyme oxidase, and uncoupling protein 2 (60). Alternatively, adiponectin may lead to an improved metabolic profile by the expansion of sc adipose tissue with decreased levels of macrophage infiltration (23), similar to the actions of PPARγ agonists. Thiazolidinediones (TZDs) are known to increase circulating levels of adiponectin, mostly the HMW form, by 2- to 3-fold (65,66,67), and improve insulin resistance by diversion of fat from ectopic sites to sc adipose tissue (68). Interestingly, insulin-sensitizing effects of TZDs are significantly diminished in the absence of adiponectin (54), suggesting an important role of adiponectin in reduction of lipotoxicity and inflammation associated with obesity.
Adiponectin has also had vasculoprotective effects mediated via an increase in endothelial nitric oxide production, or modulation of expression of adhesion molecules and scavenger receptors (60,69).
In addition to peripheral actions, it has been suggested that adiponectin has central effects in the regulation of energy homeostasis (70). Adiponectin was present in cerebrospinal fluid largely in the form of trimer and hexamer, in contrast to the distribution of adiponectin in serum, which consists of higher molecular masses (71). It has been proposed that adiponectin increases food intake by enhancing hypothalamic AMPK activity in fasting conditions (72).
Resistin
Resistin is a 12-kDa peptide that was originally discovered as a result of examining differential gene expression of mouse adipose tissue after TZD treatment (73). Resistin is part of a gene family of “Resistin-like molecules,” and is increased along with PPARγ during the differentiation of 3T3-L1 adipocytes (74). Resistin was decreased by TZD treatment of mice and was increased in insulin-resistant mice. Furthermore, treatment with antiresistin antibody improved insulin sensitivity and glucose transport in mice and mouse adipocytes, respectively (73). Additional studies in mice suggest that an important site of action of resistin is on hepatic glucose production (75). Although these data in mice are exciting, the role of resistin in human insulin resistance is less clear. Resistin is expressed by adipocytes in mice but is expressed by the macrophages of humans (76). A number of studies have examined plasma resistin levels or adipose resistin expression, and have found variable associations with insulin resistance (77,78,79,80). A recent large study involving the Framingham offspring cohort found a significant relationship between insulin resistance and resistin, however, this relationship was considerably weaker than the relationship with adiponectin, and was lost after adjustment for BMI (81). Resistin decreases after TZD treatment of humans, although resistin was also decreased by metformin treatment (65,82). Therefore, resistin is clearly an important adipokine that likely plays a role in the development of insulin resistance; however, it appears to be quantitatively less important in humans than other adipokines.
Retinol binding protein 4 (RBP4)
One interesting rodent model of insulin resistance is the adipose tissue-specific glucose transporter 4 (Glut4) knockout mouse (83), in which the defect in adipose tissue glucose transport yielded peripheral insulin resistance, apparently due to a circulating factor. RBP4 was identified as a highly expressed circulating adipokine in this model and caused insulin resistance when overexpressed or injected into mice (84). Since that time, a number of human studies have been performed that examined RBP4 protein levels in circulation and/or its gene expression in adipose tissue in subjects with varying degrees of obesity, insulin resistance, or type 2 diabetes. Some papers demonstrated a positive association between RBP4 and insulin resistance or obesity (84,85,86,87,88,89,90,91,92,93,94), sometimes with strikingly strong correlations, whereas others have not found such a relationship (95,96,97,98,99,100). One study found no relationship between RBP4 and insulin sensitivity in older subjects, but a weak relationship in young subjects, suggesting an age-related difference (101). Another study found no significant relationship between RBP4 and insulin sensitivity, but RBP4 was associated with adipose tissue macrophage markers, suggesting a possible role of RBP4 in inflammation (95). The response to TZDs has been examined in fewer studies, and again the response was inconsistent. If RBP4 is associated with insulin resistance, one would expect a decrease after treatment with rosi- or pioglitazone. Such a response was found in Glut4 knockout mice (84) and in some human studies (90,91,102,103). However, in other studies, human subjects treated with TZDs demonstrated no change or an increase in RBP4 mRNA (94,95), and the addition of pioglitazone to adipocytes in vitro also resulted in increased RBP4 mRNA (95). RBP4 circulates bound to transthyretin, which decreases RBP4 renal clearance, and transthyretin plasma levels were increased 4-fold in ob/ob mice compared with lean mice or diet-induced obese mice (104). Although RBP4-transthyretin binding may be important physiologically, this area needs further study. Thus, the data are currently conflicting on the role of RBP4 in insulin resistance and the metabolic complications of obesity. Because of the association with Glut4, RBP4 is presumed to play a role in fuel sensing in the adipocyte, but in other respects, a possible mechanism for causing insulin resistance is not clear.
Visfatin
Visfatin is expressed in many cells and tissues, and was previously identified as a protein involved in B-cell maturation (pre-B colony enhancing factor) (105,106). More recently, visfatin was described to be a highly expressed protein with insulin-like functions, and was predominantly found in visceral adipose tissue, from which the name visfatin was derived (107). Injection of visfatin in mice lowered blood glucose, and mice with a mutation in visfatin had higher glucose levels.
Although these initial studies were promising, subsequent studies of visfatin in humans have generally not confirmed the initial study, which was, in part, retracted (108). A subsequent study did not confirm the insulin mimetic action of visfatin but instead demonstrated that visfatin has nicotinamide adenine dinucleotide (NAD) biosynthetic activity, which is essential for B-cell function (109). In human studies, a positive correlation between visceral adipose tissue visfatin gene expression and BMI was noted, along with a negative correlation between BMI and sc fat visfatin (110,111), suggesting that visfatin regulation in these different depots is different, and adipose depot ratios are highly dependent on the obesity of the subjects. No difference in visfatin expression between fat depots of humans was noted (110,111), and visfatin was expressed predominantly by nonmacrophage cells in the adipose tissue stroma (111). Plasma visfatin was positively associated with BMI in one study (110), but not in others (111,112). Variable results were obtained regarding the relationship between visfatin and diabetes or insulin resistance (111,112,113,114), and visfatin was not responsive to PPARγ agonists and was not correlated with macrophage markers (111). Therefore, there are a number of inconsistencies among the different studies of visfatin, and the role of this adipokine in obesity and insulin resistance is not clear.
Inflammatory Cytokines Produced by Macrophages
Obesity is characterized by increased fat mass frequently associated with chronic inflammation. Yet, the mechanisms triggering the inflammatory pathway in obesity are to be determined, and discussed previously. An increased number of macrophages resident in human adipose tissue has been reported in obesity (14) that may contribute to the inflammatory process by secreting pro-inflammatory cytokines such as TNFα, IL6, and MCP-1. In addition to increased infiltration of macrophages in adipose tissue, obesity is associated with changes in the phenotype of macrophages from alternatively activated toward a more classical and pro-inflammatory cell (115) as the source of pro-inflammatory mediators. Inactivation of the nuclear factor-κB pathway, which induces inflammatory mediators, has led to the protection against insulin resistance (116).
TNFα
Of the pro-inflammatory cytokines, TNFα is well described to disturb insulin signaling. Mice lacking TNFα or TNFα receptors are resistant to the development of obesity induced insulin resistance (117,118). In adipose tissue, TNFα is mostly secreted by macrophages in the stromal vascular fraction. Circulating TNFα and adipose tissue TNFα gene expression are increased in insulin resistance (119), and acute infusion of TNFα inhibited insulin-induced glucose uptake in healthy subjects (120). Neutralization of TNFα in rodents has improved insulin resistance (7), whereas attempts to neutralize TNFα in humans to improve insulin resistance have generally not been successful (121), although more recent studies have shown slight improvement in insulin resistance with TNFα inhibition (122,123,124). Limited effects of TNFα blockade on insulin resistance could be explained by the paracrine actions of TNFα. Further investigations on the mechanisms involved in TNFα overexpression associated with obesity and molecular signals underlying TNFα-induced metabolic dysregulation are warranted.
IL-6
IL-6 is another cytokine similar to TNFα that is overexpressed in the adipose tissue of obesity (119). The role of IL-6 in metabolic changes associated with obesity is unclear. There are some reports of IL-6 causing impaired insulin signaling in the liver and adipocytes by inducing ubiquitin-mediated degradation of insulin receptor substrate through suppressor of cytokine signaling (SOCS) 1 and 3 (125,126). However, effects of IL-6 on insulin sensitivity in skeletal muscle is controversial (126). Exercise that is associated with increased insulin action in skeletal muscle increases circulating IL-6 levels dramatically (127), suggesting possible antiinflammatory roles for IL-6 in skeletal muscle. The data on the increased onset of obesity and diabetes in mice lacking IL-6 are conflicting (128,129).
MCPs
As discussed previously, infiltration of macrophages into adipose tissue is an important contributor of the increased inflammatory process in obesity. Adipocytes secrete various chemoattractants that draw monocytes from circulation into adipose tissue. MCP-1, also known as chemokine (C-C motif) ligand 2 (CCL-2), is one the chemoattractants that plays an important role in the recruitment of macrophages. Moreover, obesity is associated with increased plasma levels of MCP-1 and overexpression in adipose tissue (14,130). Mice lacking MCP-1 receptor (CCR-2) have decreased adipose tissue macrophage infiltration and improved metabolic function (12). Similarly, it has been demonstrated that mice lacking MCP-1 have reduced adipose tissue macrophage infiltration (131), however, a more recent study did not confirm this finding (132). This suggests that there are other candidates that might play a role in the recruitment of macrophages into the adipose tissue, such as macrophage inflammatory protein-1α (11) or osteopontin (133,134). Osteopontin is an extracellular matrix protein that promotes monocyte chemotaxis, and the lack of osteopontin in mice caused improved insulin sensitivity and decreased macrophage infiltration into adipose tissue (134).
Adipokines Involved with Thrombosis: Thrombospondin (TSP) and Plasminogen Activator Inhibitor 1 (PAI-1)
PAI-1 is elevated in subjects with metabolic complications of obesity, and is expressed in the stromal fraction of adipose tissue, including endothelial cells (135,136,137,138,139). PAI-1 inhibits both tissue-type plasminogen activator and urokinase-type plasminogen activator through its serine protease inhibitor function, and this inhibition of fibrinolysis may contribute to a pro-thrombotic state (140).
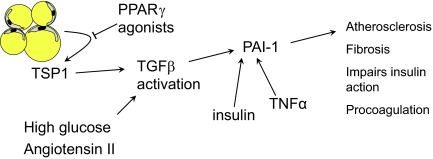
PAI-1 gene expression is controlled by TGF-β, which combines with phosphorylated SMAD and binds to the PAI-1 promoter (141). Another important link in PAI-1 activation was the recent demonstration of TSP1 expression in adipocytes (142). TSP1 is expressed by many tissues, and has many different activities, including inhibition of angiogenesis, cell proliferation, and wound healing (143,144). TSP1 is a major activator of TGF-β (145), and PAI-1 activation by TSP1 has been described (146) (Fig. 1).
Figure 1.
Role of TSP1, TGF-β, and PAI-1 in adipose tissue. TSP1 is expressed by adipose tissue, and activates TGF-β, which in turn activates PAI-1, which is a procoagulant. TGF-β is also activated by high glucose and angiotensin II. TSP1 expression is inhibited by PPARγ agonists, which may explain some of the beneficial effects of these drugs.
A recent study demonstrated TSP1 expression largely by adipocytes compared with the stromal vascular fraction of adipose tissue, suggesting that TSP1 is a true adipokine (142). TSP1 expression was increased in obese, insulin-resistant subjects, was associated with plasma PAI-1 levels, and was positively associated with adipose tissue macrophage markers. In addition, TSP1 expression was decreased by treatment of subjects or adipocytes with the PPARγ agonist, pioglitazone. TSP1 has chemotactic properties (143) that provide a link between TSP1 and macrophage-mediated adipocyte inflammation. In addition, adipocyte-macrophage coculture experiments demonstrated TSP1 gene and protein up-regulation by both cells, suggesting a feed-forward inflammatory mechanism in adipose tissue (142). TSP1 may be an important component of inflammation and coagulation in the metabolic complications of obesity.
Summary
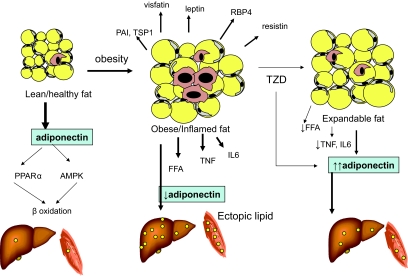
Adipose tissue was once recognized simply as an inert storage organ, but now is appreciated increasingly as an endocrine organ and part of an innate immune system. Factors secreted from adipose tissue contribute considerably to the regulation of metabolism and inflammatory responses. The adipose tissue of insulin-sensitive humans secretes adiponectin abundantly, which is associated with a favorable metabolic condition. However, with adiposity, adiponectin secretion decreases significantly, and multiple adipocyte-derived factors induce activation and infiltration of macrophage into adipose tissue. Activated macrophages secrete cytokines that can contribute to more macrophage infiltration. As shown in Fig. 2, inflamed fat in obesity secretes an array of proteins implicated in the impairment of insulin signaling. In addition, in a hypothetical model, inflamed fat releases more free fatty acids that contribute to fat accumulation in ectopic sites, including liver and muscle. Increased lipid content in liver and muscle has been associated with insulin resistance. PPARγ ligands, such as TZDs, improve insulin resistance through multiple potential mechanisms. PPARγ agonists enhance adipogenesis, increase adiponectin, and exert antiinflammatory effects on macrophages resident in adipose tissue. Future studies are needed to focus on the factors initiating the inflammatory process in adipose tissue and the regulation of adipocyte secretory products.
Figure 2.
Changes in adipose tissue, liver, and muscle with obesity and insulin resistance. The adipose tissue of lean subjects contains few macrophages, and secretes relatively high levels of adiponectin, and low levels of inflammatory cytokines. β-Oxidation of lipids in muscle is high, and there is little ectopic fat in the muscle and liver. With obesity and insulin resistance, adipose tissue contains many macrophages, and the tissue secretes high levels of many adipokines, and low levels of adiponectin. This adipose tissue may be limited in its lipid storage capacity, and this feature, along with the pro-inflammatory state, promotes ectopic lipid accumulation. The adipose tissue in some subjects can be characterized as expandable, meaning the tissue can accommodate more lipid. This may result from treatment with a TZD. Such adipose tissue may be less inflamed, and because this adipose tissue can accumulate more lipid, there is less ectopic fat.
Footnotes
This work was supported by Merit Review Grant from the Veterans Administration (to N.R.), and DK71277 and DK080327 from the National Institutes of Health (to P.A.K.).
Disclosure Statement: P.A.K. and N.R. have received honoraria for speaking from Takeda Pharmaceuticals.
Abbreviations: AMPK, AMP-activated protein kinase; BMI, body mass index; ER, endoplasmic reticulum; Glut4, glucose transporter 4; HMM, high-molecular mass; MCP, monocyte chemoattractant protein; PAI-1, plasminogen activator inhibitor 1; PPAR, peroxisome proliferator activated receptor; RBP4, retinol binding protein 4; STAT3, signal transducer and activator of transcription-3; TSP, thrombospondin; TZD, thiazolidinedione.
References
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD 1998 Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21:518–524 [DOI] [PubMed] [Google Scholar]
- Harris MI 1998 Diabetes in America: epidemiology and scope of the problem. Diabetes Care 21(Suppl 3):C11–C14 [DOI] [PubMed] [Google Scholar]
- Rose DP, Haffner SM, Baillargeon J 2007 Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 28:763–777 [DOI] [PubMed] [Google Scholar]
- Bray GA 2004 Medical consequences of obesity. J Clin Endocrinol Metab 89:2583–2589 [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS 2005 A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352:1138–1145 [DOI] [PubMed] [Google Scholar]
- Parati G, Lombardi C, Narkiewicz K 2007 Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol 293:R1671–R1683 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM 1993 Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB 1995 The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 95:2111–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF 1995 A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749 [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y, Osaka CAD Study Group, Coronary artery disease 2003 Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23:85–89 [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H 2003 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS 2003 Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA 2005 Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54:2305–2313 [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Greenberg AS, Obin MS 2005 Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355 [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, Greenberg AS, Obin MS 2007 Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS 2004 Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355 [DOI] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV 2008 Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32:451–463 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wang B, Wood IS 2008 Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100:227–235 [DOI] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I 2007 Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911 [DOI] [PubMed] [Google Scholar]
- Wang B, Wood IS, Trayhurn P 2007 Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455:479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JC, Ip MS 2007 An update on obstructive sleep apnea and the metabolic syndrome. Curr Opin Pulm Med 13:484–489 [DOI] [PubMed] [Google Scholar]
- Kim JY, van de WE, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE 2007 Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal AK, Garg A 2006 Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet 7:175–199 [DOI] [PubMed] [Google Scholar]
- Giralt M, Domingo P, Guallar JP, Rodriguez de la Concepcion ML, Alegre M, Domingo JC, Villarroya F 2006 HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV-/HAART-associated lipodystrophy. Antivir Ther 11:729–740 [PubMed] [Google Scholar]
- Johnson JA, Albu JB, Engelson ES, Fried SK, Inada Y, Ionescu G, Kotler DP 2004 Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am J Physiol Endocrinol Metab 286:E261–E271 [DOI] [PubMed] [Google Scholar]
- Lagathu C, Kim M, Maachi M, Vigouroux C, Cervera P, Capeau J, Caron M, Bastard JP 2005 HIV antiretroviral treatment alters adipokine expression and insulin sensitivity of adipose tissue in vitro and in vivo. Biochimie 87:65–71 [DOI] [PubMed] [Google Scholar]
- Lindegaard B, Larsen LF, Hansen AB, Gerstoft J, Pedersen BK, Reue K 2007 Adipose tissue lipin expression levels distinguish HIV patients with and without lipodystrophy. Int J Obes (Lond) 31:449–456 [DOI] [PubMed] [Google Scholar]
- Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K 2007 Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem 282:3450–3457 [DOI] [PubMed] [Google Scholar]
- Phan J, Reue K 2005 Lipin, a lipodystrophy and obesity gene. Cell Metab 1:73–83 [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Rasouli N, Varma V, Miles LM, Phanavanh B, Starks TN, Phan J, Spencer III HJ, McGehee Jr RE, Reue K, Kern PA 2006 Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor γ activation. Diabetes 55:2811–2818 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL 1998 Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S 2007 Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903–908 [DOI] [PubMed] [Google Scholar]
- Buettner C, Pocai A, Muse ED, Etgen AM, Myers Jr MG, Rossetti L 2006 Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C 2008 Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI 1998 Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901 [DOI] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM 1998 Leptin regulates proinflammatory immune responses. FASEB J 12:57–65 [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD 1950 Obese, a new mutation in the house mouse. J Hered 41:317–318 [DOI] [PubMed] [Google Scholar]
- Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N 2001 Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 104:3052–3056 [DOI] [PubMed] [Google Scholar]
- Koh KK, Park SM, Quon MJ 2008 Leptin and cardiovascular disease: response to therapeutic interventions. Circulation 117:3238–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey L, Hesong Z 2006 Role of leptin in atherogenesis. Exp Clin Cardol 11:269–275 [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S 2002 Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S 1999 Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A 2002 Leptin-replacement therapy for lipodystrophy. N Engl J Med 346:570–578 [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M 1999 Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282:1568–1575 [DOI] [PubMed] [Google Scholar]
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD 2008 Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105:7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G 2003 Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-α expression. Diabetes 52:1779–1785 [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA 2001 Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- Semple RK, Halberg NH, Burling K, Soos MA, Schraw T, Luan J, Cochran EK, Dunger DB, Wareham NJ, Scherer PE, Gorden P, O'Rahilly S 2007 Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes 56:1712–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T 2002 Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866 [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y 2002 Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8:731–737 [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE 2006 Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to PPARγ-agonists. J Biol Chem 281:2654–2660 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A 2008 Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 409:623–633 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A 2006 Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 281:16391–16400 [DOI] [PubMed] [Google Scholar]
- Banga A, Bodles AM, Rasouli N, Ranganathan G, Kern PA, Owens RJ 2008 Calcium is involved in formation of high molecular weight adiponectin. Metab Syndr Relat Disord 6:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE 2003 Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 278:9073–9085 [DOI] [PubMed] [Google Scholar]
- Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, Ong LH, Tam S, Tan KC, Janus ED, Lam TH, Lam KS 2007 Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension 49:1455–1461 [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T 2005 Adiponectin and adiponectin receptors. Endocr Rev 26:439–451 [DOI] [PubMed] [Google Scholar]
- Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB 2008 Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT 2006 Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 55:249–259 [PubMed] [Google Scholar]
- Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE 2007 Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 27:3716–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K 2006 Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA 2006 Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab 290:E42–E46 [DOI] [PubMed] [Google Scholar]
- Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ 2006 Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab 291:E1100–E1105 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE 2004 Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 274:12152–12162 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA 2005 Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab 288:E930–E934 [DOI] [PubMed] [Google Scholar]
- Zhu W, Cheng KKY, Vanhoutte PM, Lam KSL, Xu A 2008 Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 114:361–374 [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N 2008 The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582:74–80 [DOI] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Schraw T, Kos K, O'hare JP, Ahima R, Kumar S, Scherer PE 2007 Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50:634–642 [DOI] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T 2007 Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA 2001 A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA 98:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala MW, Obici S, Scherer PE, Rossetti L 2003 Adipose-derived resistin and gut-derived resistin-like molecule-β selectively impair insulin action on glucose production. J Clin Invest 111:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A 2006 Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49:744–747 [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV 2003 Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab 88:5452–5455 [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, Smith SR 2004 Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab 89:1844–1848 [DOI] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS 2003 Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 88:4848–4856 [DOI] [PubMed] [Google Scholar]
- Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA 2004 High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes [Erratum (2004) 53:2518] 53:1279–1284 [DOI] [PubMed] [Google Scholar]
- Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino Sr RB, Wilson PW, Meigs JB 2008 Associations of adiponectin, resistin, and tumor necrosis factor-α with insulin resistance. J Clin Endocrinol Metab 93:3165–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin D, Hadigan C, Lehrke M, Mazza S, Lazar MA, Grinspoon S 2005 Resistin levels in human immunodeficiency virus-infected patients with lipoatrophy decrease in response to rosiglitazone. J Clin Endocrinol Metab 90:3423–3426 [DOI] [PubMed] [Google Scholar]
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB 2001 Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409:729–733 [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB 2005 Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–362 [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB 2006 Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354:2552–2563 [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee HR, Shim JY, Im JA, Lee DC 21 May 2008 Abdominal visceral fat reduction is associated with favorable changes of serum retinol binding protein-4 in nondiabetic subjects. Endocr J [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Reinehr T, Stoffel-Wagner B, Roth CL 2008 Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab 93:2287–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeberli I, Biebinger R, Lehmann R, L'allemand D, Spinas GA, Zimmermann MB 2007 Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin Endocrinol Metab 92:4359–4365 [DOI] [PubMed] [Google Scholar]
- Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, Schmidt M, Mann K, Janssen OE 2007 Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. Eur J Endocrinol 157:201–207 [DOI] [PubMed] [Google Scholar]
- Haider DG, Schindler K, Mittermayer F, Muller M, Nowotny P, Rieger A, Luger A, Ludvik B, Wolzt M 2007 Effect of rosiglitazone on visfatin and retinol-binding protein-4 plasma concentrations in HIV-positive patients. Clin Pharmacol Ther 81:580–585 [DOI] [PubMed] [Google Scholar]
- Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K 2007 Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab 92:3224–3229 [DOI] [PubMed] [Google Scholar]
- Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, Bluher M, Kahn BB 2007 Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 6:79–87 [DOI] [PubMed] [Google Scholar]
- Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Haring HU 2007 High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care 30:1173–1178 [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T 2007 Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab 92:2712–2719 [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer III HJ, Rashidi AA, McGehee Jr RE, Fried SK, Kern PA 2007 Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 92:2590–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J 2006 Retinol-binding protein 4 in human obesity. Diabetes 55:2805–2810 [DOI] [PubMed] [Google Scholar]
- Hogstrom M, Nordstrom A, Nordstrom P 2008 Retinol, retinol-binding protein 4, abdominal fat mass, peak bone mineral density, and markers of bone metabolism in men: the Northern Osteoporosis and Obesity (NO2) Study. Eur J Endocrinol 158:765–770 [DOI] [PubMed] [Google Scholar]
- Ueland T, Dalsoren T, Voldner N, Godang K, Henriksen T, Bollerslev J 2008 Retinol-binding protein-4 is not strongly associated with insulin sensitivity in normal pregnancies. Eur J Endocrinol 159:49–54 [DOI] [PubMed] [Google Scholar]
- von Eynatten M, Humpert PM 2008 Retinol-binding protein-4 in experimental and clinical metabolic disease. Expert Rev Mol Diagn 8:289–299 [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Rodriguez A, Catalan V, Ramirez B, Silva C, Rotellar F, Gil MJ, Salvador J, Fruhbeck G 2008 Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clin Endocrinol (Oxf) 69:208–215 [DOI] [PubMed] [Google Scholar]
- Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, McNurlan MA, Gelato MC 2008 Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 16:893–895 [DOI] [PubMed] [Google Scholar]
- Hammarstedt A, Pihlajamaki J, Graham TE, Kainulainen S, Kahn BB, Laakso M, Smith U 2008 High circulating levels of RBP4 and mRNA levels of aP2, PGC-1α and UCP-2 predict improvement in insulin sensitivity following pioglitazone treatment of drug-naive type 2 diabetic subjects. J Intern Med 263:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KD, Chang YH, Wang CL, Yang YH, Hsiao PJ, Li TH, Shin SJ 2008 Thiazolidinedione addition reduces the serum retinol-binding protein 4 in type 2 diabetic patients treated with metformin and sulfonylurea. Transl Res 151:309–314 [DOI] [PubMed] [Google Scholar]
- Mody N, Graham TE, Tsuji Y, Yang Q, Kahn BB 2008 Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am J Physiol Endocrinol Metab 294:E785–E793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani T, Okuno S, Fujisawa H 2003 Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett 544:74–78 [DOI] [PubMed] [Google Scholar]
- Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I 1994 Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol 14:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I 2005 Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426–430 [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I 2007 Retraction. Science 318:565 [DOI] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S 2007 Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab 6:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M 2005 Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 54:2911–2916 [DOI] [PubMed] [Google Scholar]
- Varma V, Yao-Borengasser A, Rasouli N, Bodles AM, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer III HJ, McGehee Jr RE, Fried SK, Kern PA 2007 Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipid and inflammation. J Clin Endocrinol Metab 92:666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ 2006 Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 91:295–299 [DOI] [PubMed] [Google Scholar]
- Hammarstedt A, Pihlajamaki J, Sopasakis VR, Gogg S, Jansson PA, Laakso M, Smith U 2006 Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab 91:1181–1184 [DOI] [PubMed] [Google Scholar]
- Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B 2006 Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab 91:1578–1581 [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR 2007 Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M 2005 IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198 [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS 1997 Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610–614 [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Hotamisligil GS 1998 Functional analysis of tumor necrosis factor (TNF) receptors in TNF-α-mediated insulin resistance in genetic obesity. Endocrinology 139:4832–4838 [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G 2001 Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:E745–E751 [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK 2005 Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54:2939–2945 [DOI] [PubMed] [Google Scholar]
- Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R 1996 Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45:881–885 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J 2006 Anti-tumor necrosis factor-α blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol 24:83–86 [PubMed] [Google Scholar]
- Seriolo B, Ferrone C, Cutolo M 2008 Longterm anti-tumor necrosis factor-α treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol 35:355–357 [PubMed] [Google Scholar]
- Tam LS, Tomlinson B, Chu TT, Li TK, Li EK 2007 Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol 26:1495–1498 [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E 2000 SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275:15985–15991 [DOI] [PubMed] [Google Scholar]
- Kristiansen OP, Mandrup-Poulsen T 2005 Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54(Suppl 2):S114–S124 [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M 2006 Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55(Suppl 2):S48–S54 [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA 2004 Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab 287:E182–E187 [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO 2002 Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8:75–79 [DOI] [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ 2003 Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 100:7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M 2006 MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS 2007 Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56:2242–2250 [DOI] [PubMed] [Google Scholar]
- Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, Aszmann O, Ludvik B, Silberhumer GR, Prager G, Stulnig TM 2008 Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 149:1350–1357 [DOI] [PubMed] [Google Scholar]
- Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D 2007 Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 117:2877–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhanvague I 1997 Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 46:860–867 [DOI] [PubMed] [Google Scholar]
- Cigolini M, Tonoli M, Borgato L, Frigotto L, Manzato F, Zeminian S, Cardinale C, Camin M, Chiaramonte E, De Sandre G, Lunardi C 1999 Expression of plasminogen activator inhibitor-1 in human adipose tissue: a role for TNF-α? Atherosclerosis 143:81–90 [DOI] [PubMed] [Google Scholar]
- Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I 2000 Plasminogen activator inhibitor 1, transforming growth factor-β1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 49:1374–1380 [DOI] [PubMed] [Google Scholar]
- Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF 2006 Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 30:1308–1314 [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Wassel FC, Vittinghoff E, Harris TB, Park SW, Goodpaster BH, Tylavsky F, Cummings SR 2006 Adipocytokines and incident diabetes mellitus in older adults: the independent effect of plasminogen activator inhibitor 1. Arch Intern Med 166:350–356 [DOI] [PubMed] [Google Scholar]
- Sprengers ED, Kluft C 1987 Plasminogen activator inhibitors. Blood 69:381–387 [PubMed] [Google Scholar]
- Song CZ, Siok TE, Gelehrter TD 1998 Smad4/DPC4 and Smad3 mediate transforming growth factor-β (TGF-β) signaling through direct binding to a novel TGF-β-responsive element in the human plasminogen activator inhibitor-1 promoter. J Biol Chem 273:29287–29290 [DOI] [PubMed] [Google Scholar]
- Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer III HJ, Lee MJ, Fried SK, McGehee Jr RE, Peterson CA, Kern PA 2008 Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P 2001 Thrombospondins as matricellular modulators of cell function. J Clin Invest 107:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, Gahtan V 2004 The role of thrombospondin-1 in human disease. J Surg Res 122:135–142 [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N 1998 Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93:1159–1170 [DOI] [PubMed] [Google Scholar]
- Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S 1996 Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 93:106–110 [DOI] [PubMed] [Google Scholar]