Abstract
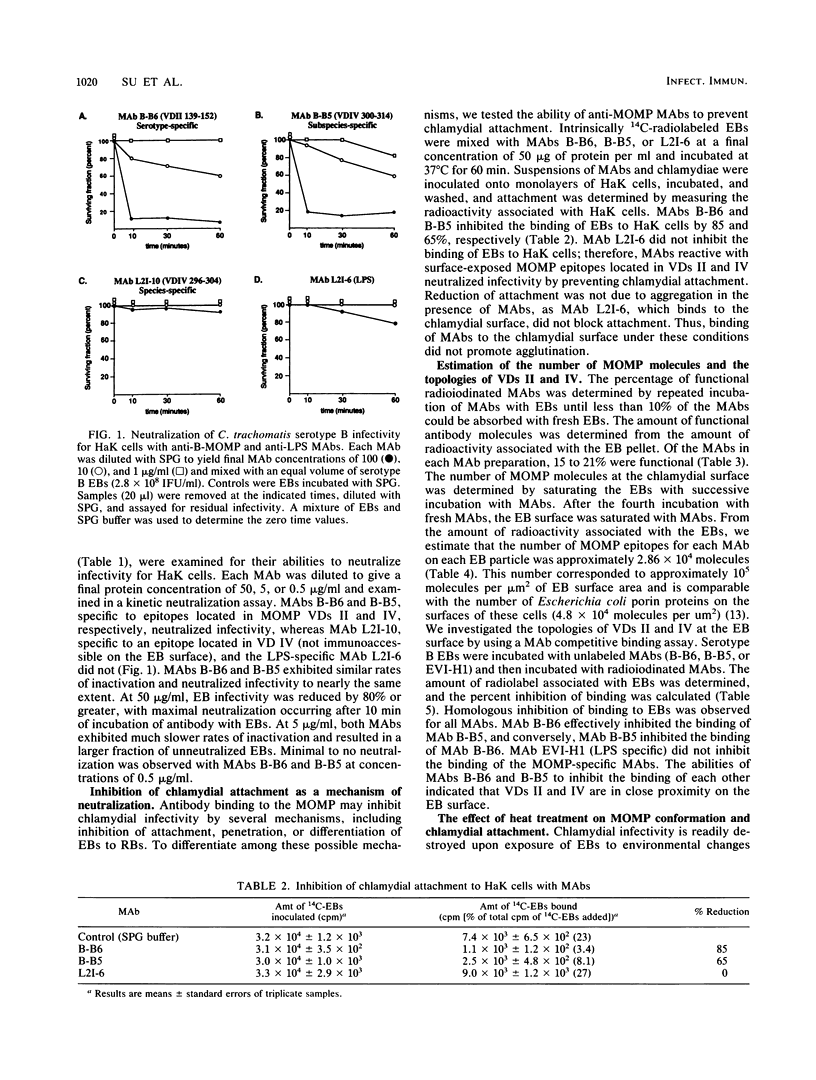
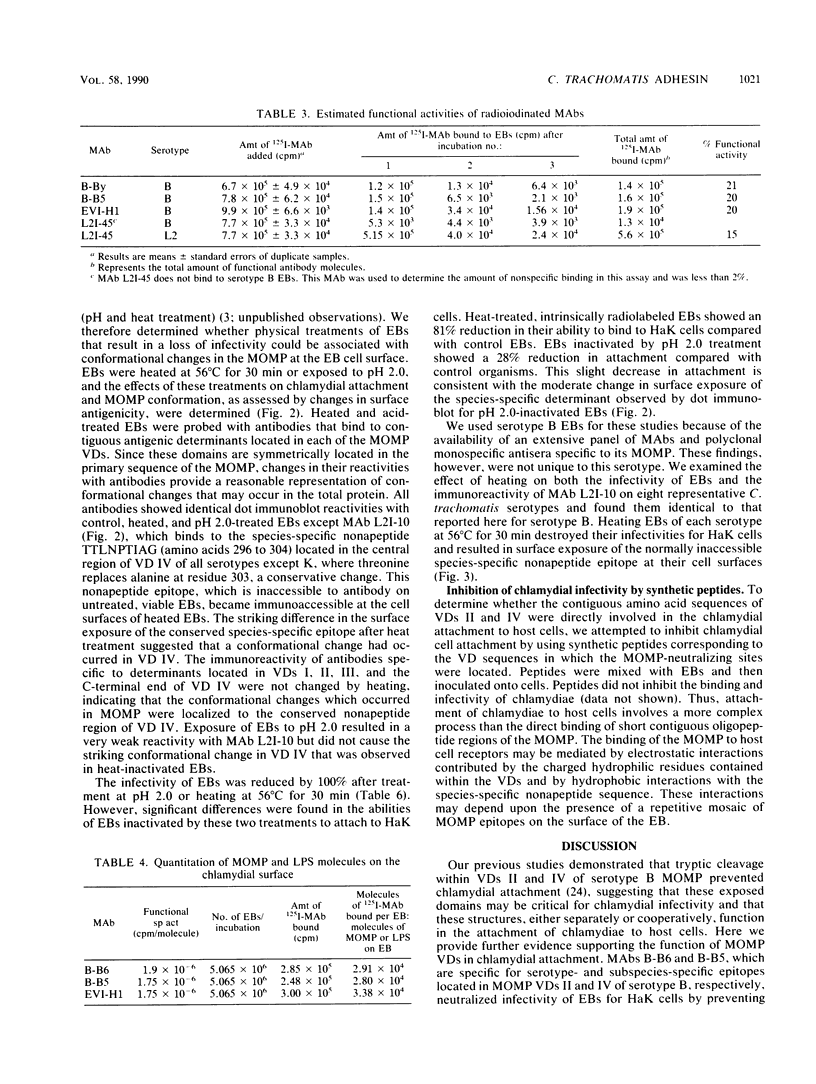
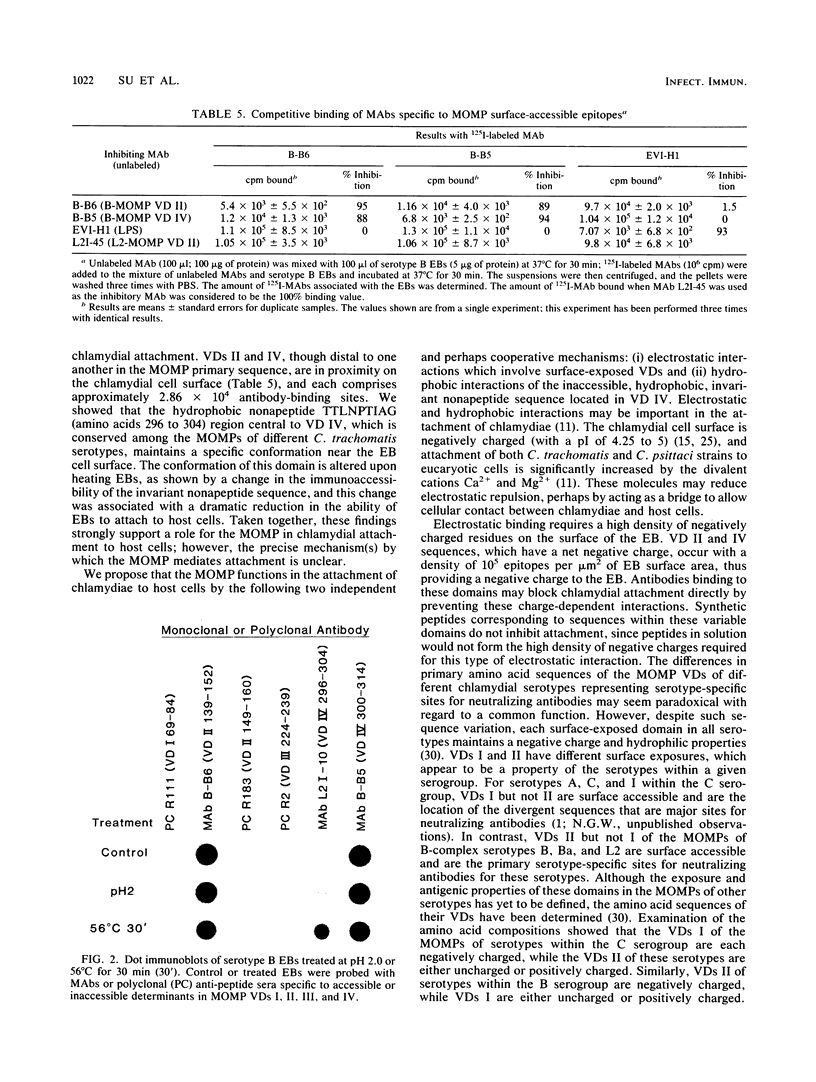
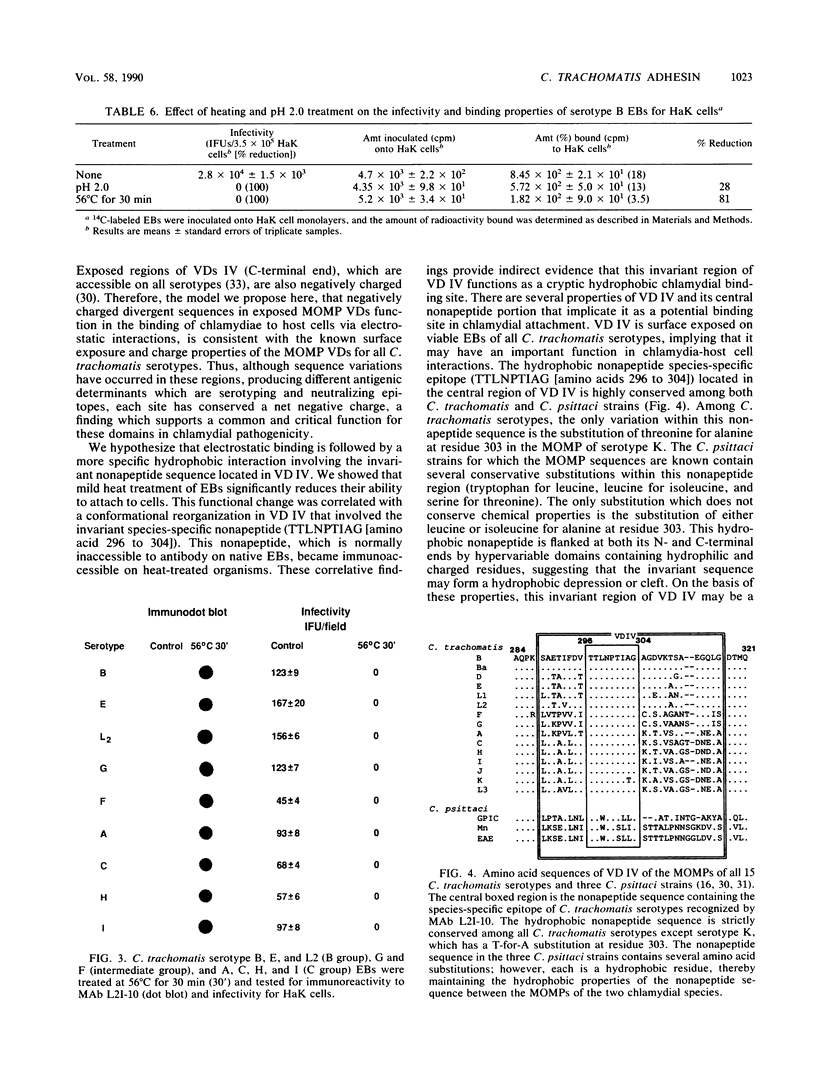
The major outer membrane protein (MOMP) of Chlamydia trachomatis is characterized by four symmetrically spaced variable domains (VDs I to IV) whose sequences vary among serotypes. The surface-exposed portions of these VDs contain contiguous sequences that are both serotyping determinants and in vivo target sites for neutralizing antibodies. Previous studies using surface proteolysis of C. trachomatis B implicated VDs II and IV of the MOMP of this serotype in the attachment of chlamydiae to host cells. In this study, we used monoclonal antibodies (MAbs) specific to antigenic determinants located in VDs II and IV of the MOMP of serotype B to further investigate the role of the MOMP in the attachment of chlamydiae to host cells. MABs specific to serotype- and subspecies-specific epitopes located in exposed VDs II and IV, respectively, neutralized chlamydial infectivity for hamster kidney cells by blocking chlamydial attachment. We radioiodinated these MAbs and used them to determine the number and topology of the surface-exposed VDs II and IV epitopes on chlamydial elementary bodies. VDs II and IV each comprised approximately 2.86 x 10(4) negatively charged sites and were in proximity on the chlamydial cell surface. These studies suggest that the MAbs blocked chlamydial attachment by inhibiting electrostatic interactions with host cells. We examined the effects of thermal inactivation on both chlamydial attachment and conformation of the MOMP. Heat-inactivated chlamydiae failed to attach to host cells and exhibited a conformational change in an inaccessible invariant hydrophobic nonapeptide sequence located within VD IV of the MOMPs of C. trachomatis serotypes. These findings suggest that in addition to electrostatic interactions, a common hydrophobic component of the MOMP also contributes to the binding of chlamydiae to host cells. Thus, we propose that the MOMP functions as a chlamydial adhesin by promoting nonspecific (electrostatic and hydrophobic) interactions with host cells. Surface-accessible negatively charged VDs appear to be important in electrostatic binding, while the invariant region of VD IV may provide a subsurface hydrophobic depression which further promotes binding of chlamydiae to host cells through hydrophobic interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Immunoassay for detecting Chlamydia trachomatis major outer membrane protein. J Clin Microbiol. 1983 Sep;18(3):539–545. doi: 10.1128/jcm.18.3.539-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Stewart S., Johnson S., Taylor H. Tear and serum antibody response to Chlamydia trachomatis antigens during acute chlamydial conjunctivitis in monkeys as determined by immunoblotting. Infect Immun. 1987 Jan;55(1):93–98. doi: 10.1128/iai.55.1.93-98.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Attachment of Chlamydia psittaci to formaldehyde-fixed and unfixed L cells. J Gen Microbiol. 1981 Aug;125(2):273–283. doi: 10.1099/00221287-125-2-273. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaipoel R. J., van Duin A. M. Isoelectric focusing of Chlamydia trachomatis. Infect Immun. 1979 Nov;26(2):775–778. doi: 10.1128/iai.26.2.775-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The evolution of RNA viruses. Bioessays. 1987 Sep;7(3):99–103. doi: 10.1002/bies.950070302. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Simmons J., Caldwell H. D. Enhancement of Chlamydia trachomatis infectious progeny by cultivation of HeLa 229 cells treated with DEAE-dextran and cycloheximide. J Clin Microbiol. 1984 Aug;20(2):217–222. doi: 10.1128/jcm.20.2.217-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Zhang Y. X., Barrera O., Watkins N. G., Caldwell H. D. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect Immun. 1988 Aug;56(8):2094–2100. doi: 10.1128/iai.56.8.2094-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. W., Jr, Hatch T. P. Surface properties of Chlamydia psittaci. Infect Immun. 1980 Jul;29(1):175–180. doi: 10.1128/iai.29.1.175-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]