Abstract
Context: Although the prevalence rates of childhood obesity have seemingly been stable over the past few years, far too many children and adolescents are still obese. Childhood obesity, and its associated metabolic complications, is rapidly emerging as one of the greatest global challenges of the 21st century. About 110 million children are now classified as overweight or obese.
Evidence Acquisition: In this review we first describe the most recent data on the prevalence, severity, and racial/ethnic differences in childhood obesity. Obesity is associated with significant health problems in the pediatric age group and is an important early risk factor for much of adult morbidity and mortality.
Evidence Synthesis: We review the metabolic complications associated with childhood obesity. Particular emphasis is given to the description of studies regarding the impact of varying degrees of obesity on the cardiometabolic risk factors in youth. We further describe studies in obese adolescents that have examined the importance of ectopic lipid deposition in the visceral abdominal depot and in insulin sensitive tissues in relation to the presence of insulin resistance. We end by describing studies that have examined β-cell function in obese adolescents with normal glucose tolerance.
Conclusions: The growing number of obese children and adolescents worldwide is of great concern. Many obese children and adolescents already manifest some metabolic complications, and these children are at high risk for the development of early morbidity. Understanding the underlying pathogenesis of this peculiar phenotype is of critical importance.
The most recent data on the prevalence, severity and racial/ethnic differences in childhood obesity are reviewed. Obesity is associated with significant health issues in this pediatric age group and is an important early risk factor for much of adult morbidity and mortality.
During the past 3 decades, prevalence rates of childhood and adolescent obesity [defined as body mass index (BMI) above the 95th percentile for age and sex] have more than doubled in the United States (1,2). Recently, however, there is emerging evidence that the prevalence rates of childhood obesity may have reached a plateau. It is noteworthy that in their last report Ogden et al. (3) found that 11.3% of children and adolescents were at or above the 97th percentile of BMI for age from the 2000 Centers for Disease Control and Prevention growth charts; 16.3% had a BMI for age at or above the 95th and 31.9% were at or above the 85th percentile. Although Ogden et al. (3) found no increase in prevalence between the past National Health and Nutrition Examination Survey (NHANES) surveys, it is too early to know whether the data do reflect a true plateau.
Comparison between racial/ethnic groups
Obesity has increased among both genders and among all racial, ethnic, and socioeconomic groups; however, the prevalence of obesity is disproportionately higher among African-Americans, Mexican-Americans, and Native Americans than other ethnic groups (1,2).
The Centers for Disease Control and Prevention reported in 2000 that the prevalence of obesity among 12- to 19-yr-old non-Hispanic blacks was 23.6% and in Mexican-Americans, 23.4% compared with 12% of non-Hispanic white children. The racial/ethnic differences in the prevalence in this age group are dramatic, an increase in more than 10 percentage points between 1988–1994 and 1999–2000. In contrast to these significant changes in obesity rates in both African-American and Mexican adolescent boys and girls, the prevalence rates remained relatively stable at about 10% in non-Hispanics during NHANES 1988–1994 to NHANES 1999–2000 (2).
International trends
Obesity represents one of the most important public health issues (World Health Organization-Food and Agriculture Organization, 2002) and excess body weight is the sixth most important risk factor contributing to the overall burden of disease worldwide; about 110 million children are now classified as overweight or obese (4). Developing countries, like Latin America, undergoing a rapid nutritional transition, are reporting increasing trends in childhood obesity. Ironically, in the developing countries in which underweight and poor growth were previously the main health concerns in children, overweight and obesity are now becoming significantly prevalent as a consequence of an environment characterized by easily available, cheap, high-caloric foods combined with sedentary lifestyles (5).
Wang and Lobstein (6) reviewed the worldwide trends in childhood obesity in 25 countries for school-age populations and in 42 countries for preschool age populations. They observed that the prevalence of childhood overweight has increased in almost all countries for which data were available, and obesity and overweight has increased more dramatically in economically developed countries and in urbanized populations (7).
Impact of the degree of overall obesity on cardiometabolic risk factors in children and adolescents
As the prevalence of childhood obesity increases, its health implications are becoming more evident (8,9). Obesity is associated with significant health problems in the pediatric age group and is an important early risk factor for much of adult morbidity and mortality (9,10,11,12). Childhood obesity frequently persists into adulthood, with up to 80% of obese children reported to become obese adults (13). Many of the metabolic and cardiovascular complications of obesity are already present during childhood and are closely related to the presence of insulin resistance/hyperinsulinemia, the most common abnormality of obesity (14). Although there is no accepted definition of insulin resistance in both adults and pediatrics, here insulin resistance is defined using surrogate markers such as homeostasis model assessment insulin resistance index or whole-body insulin sensitivity index (WBISI; or Matsuda Index). Using age and gender BMI z-score as a measure of adiposity in children, recent studies have been able to examine the relationships between increasing BMI z-score and complications of overweight in both children and adolescents (15). Bell et al. (15) reported a continuous relationship with increasing BMI z-score and components of the metabolic syndrome such as blood pressure, fasting insulin levels, presence of acanthosis nigricans, and elevated aminotransferase levels. The relationships with both high-density lipoprotein (HDL)-cholesterol and triglyceride levels with BMI z-score was found to be curvilinear, suggesting that a change in BMI z-score at the high end of the spectrum (>2.00) have a greater impact on unfavorable lipid profiles.
At the current time, little is known about the epidemiology and pathophysiology of the metabolic syndrome in children, in contrast to the more extensive understanding in adults (16,17). This is due in part to the lack of consensus for a pediatric definition of metabolic syndrome and also to the controversial issue surrounding its existence in pediatrics. To begin assessing the impact of varying degrees of obesity on the prevalence of the cardiometabolic risk factors in children and adolescents, we completed a cross-sectional analysis of the initial metabolic syndrome assessments in our cohort of obese youth (10). As in adults, subjects were classified as having the metabolic syndrome if they met three or more of the following criteria for age and gender: 1) BMI greater than the 97th percentile (BMI z-score above 2); 2) triglycerides above the 95th percentile; 3) HDL-cholesterol under the fifth percentile; 4) systolic and/or diastolic blood pressure above the 95th percentile; and 5) impaired glucose tolerance.
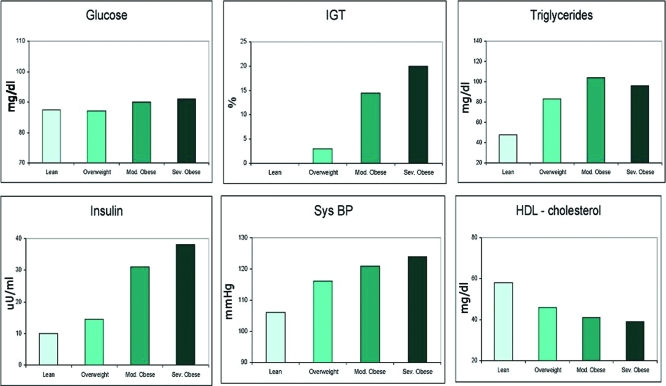
The impact of increasing weight on cardiometabolic parameters is illustrated in Fig. 1. Fasting glucose levels changed minimally with increasing weight in this cohort. In contrast, the prevalence of impaired glucose tolerance greatly increased in the children and adolescents with moderate and severe obesity. A similar pattern was also observed for plasma insulin, triglycerides levels, and systolic blood pressure, whereas a significant decrease in HDL-cholesterol was seen with varying degrees of obesity. The degree of obesity and prevalence of the metabolic syndrome were strongly associated, after adjustment for race (P = 0.009) and race and gender (P = 0.027). Overall, prevalence of the metabolic syndrome was 38.7% in moderately obese and 49.7% percent in severely obese subjects; no overweight or nonobese subject met the criteria. The main findings of the study are that the prevalence of metabolic syndrome appears far more common than previously reported and increases directly with the degree of obesity. Moreover, each element of the syndrome worsens across a spectrum of degrees of obesity, independent of age, gender, and pubertal status. Our results demonstrate a significant adverse effect of worsening obesity on each component of metabolic syndrome, further emphasizing the deleterious impact of increasing weight in this age group. Landmark studies from the Bogalusa Heart Study demonstrated that cardiovascular risk factors present in childhood are predictive of coronary artery disease in adulthood (18). Among these risk factors, low-density lipoprotein-cholesterol and BMI measured in childhood were found to predict carotid intima-media thickness in young adults (19,20). There is now substantial evidence that obesity in childhood creates the metabolic platform for adult cardiovascular disease (21,22,23,24).
Figure 1.
Impact of degree of overweight on cardiometabolic risk factors in children and adolescents. Sys BP, Systolic blood pressure.
Impact of tissue lipid partitioning on insulin sensitivity in the obese child
Although obesity is the most common cause of insulin resistance in children and adolescents, some obese youth may be relatively insulin sensitive and thus be at reduced risk for the development of the adverse cardiovascular and metabolic outcomes driven by insulin resistance. Studies from our group demonstrated that obese youth with impaired glucose tolerance (IGT) are significantly more insulin resistant than those with normal glucose tolerance (NGT), despite having an overall equal degree of adiposity (25). The difference in insulin sensitivity was attributed to different patterns of lipid partitioning in which those with severe insulin resistance were characterized by increased deposition of lipid in the visceral and intramyocellular (IMCL) compartments. The question that arises is: what determines the accumulation of IMCL in the obese child?
Recent studies indicate that increased IMCL deposition occurs early in childhood obesity and is directly associated with peripheral insulin sensitivity (24,25). Importantly, not all obese children have increased IMCL levels, and those who do not are much more insulin sensitive (26). Why some individuals who are seemingly equally obese and share common lifestyle and dietary habits tend to accumulate more IMCL lipid than others is unclear. The effects of IMCL accumulation on insulin sensitivity are not caused by the stored triglyceride per se but rather by fatty acid derivates such as diacylglycerol or ceramide, which have been found to alter the insulin signal transduction pathway, eventually leading to reduced glucose uptake and glycogen synthesis (27,28).
A putative explanation for the tendency to accumulate lipids in skeletal muscle may be due to differences in the number and function of the mitochondria within the myocyte (29). Simoneau et al. (30) were the first to report that impaired mitochondrial function might contribute to the reduction in the activity of the oxidative pathway. More recent studies reported that mitochondrial metabolism in muscle and adipocytes is disturbed in adult patients with insulin resistance and type 2 diabetes mellitus (T2DM) (31,32). This includes a reduction in the expression of peroxisome proliferator-activated receptor coactivator-1α (31,32). Additionally, lower expression of peroxisome proliferator-activated receptor coactivator-1α-responsive genes involved in oxidative phosphorylation was noted in morbid obesity and the prediabetes state in skeletal muscle (31,32). Petersen et al. (33) found that when offspring of diabetics were compared with age- and activity-matched insulin sensitive controls, they had an approximately 30% reduction rate of ATP production in mitochondria of skeletal muscle and a reduced ratio of inorganic phosphate to phosphocreatine ratio, which may reflect a lower ratio of type I fibers (mostly oxidative) to type II fibers (mostly glycolytic) in the insulin-resistant subjects.
A second factor leading to IMCL accumulation may be related to the fat constituents of the diet. High-fat diets of varying durations have been shown to increase IMCL content by 36–90%, depending on their duration and baseline IMCL level (34,35). In physically inactive obese individuals, a continuous increased supply of fatty acids by way of excess energy intake, alongside a reduced capacity to oxidize fat may lead to overall fat storage, specifically in skeletal muscle. An obvious third source of increased IMCL is an increase in circulating free fatty acid concentration, characteristic of obese insulin-resistant individuals. These observations indicate that the tendency to accumulate IMCL may be genetically determined as well as be influenced by diet and activity and may be associated with a reduced quantity and altered function of myocellular mitochondria. A tendency for increased IMCL deposition, which is partially genetically determined, predisposes individuals to greater insulin resistance, whereas obesity with low IMCL deposition seems to be more metabolically benign.
Abdominal fat patterning: a determinant of an adverse metabolic phenotype in obese adolescents
Previous studies from our group (25,26) suggest that, in obese youth, insulin resistance may be a result of the imbalance of fat distribution in both the abdominal fat depot and skeletal muscle tissues. Moreover, recently we found that the ability of peripheral sc fat tissue to vary its storage capacity may be critical for modulating insulin sensitivity (36). It is noteworthy that the two abdominal fat depots are distinct not only metabolically but also in their role in releasing key hormones and cytokines, including adiponectin, leptin and IL-6 that regulate insulin sensitivity. Thus, if the proportion of abdominal visceral fat were to be altered in favor of an increased amount of visceral, one can envision a scenario whereby low leptin and adiponectin levels would emerge, which together might negatively influence insulin sensitivity, given their known positive effects on stimulating glucose and lipid metabolism. Indeed, our recent study further expanded these earlier findings in a much larger group of subjects (36).
In this study we found that a high proportion of visceral fat relative to sc fat in the abdominal region was associated with hepatic steatosis and marked insulin resistance in obese adolescents. Concurrent with the altered partitioning of fat in the abdominal cavity, muscle, and liver, there was a worsening of the metabolic phenotype characterized by deterioration in insulin sensitivity, increased triglycerides, and decreased HDL-cholesterol, leptin, and adiponectin levels. Notably, the risk for the metabolic syndrome was 5 times higher in the adolescents with this peculiar fat deposition compared with those with a low proportion of visceral to sc fat. Our studies support the hypothesis that the ability to retain fat in the sc depot seems to be beneficial in obese adolescents because it is associated with decreased visceral fat and reduced ectopic fat deposition and, more importantly, a more favorable metabolic profile.
β-Cell function in the obese child with NGT
By convention, NGT is defined as a plasma glucose level less than 140 mg/dl after a standard oral glucose load. In our multiethnic population of obese youth with NGT, 2-h glucose levels ranged between 60 and 139 mg/dl. Insulin sensitivity and early insulin responses to the glucose load also varied over a large range in these subjects (37). These observations led us to question whether increases in 2-h plasma glucose levels, even in the normal range are primarily related to β-cell dysfunction or insulin resistance. To examine this question, we first divided our large cohort of obese youth with NGT into three strata based on 2-h plasma glucose concentrations. This stratification allowed us to examine the impact of altered insulin responsiveness on the 2-h glucose level in obese children and adolescents in general. Within each stratum, we subdivided the subjects into moderate, low, and very low insulin sensitivity groups to control for the large magnitude changes in overall insulin response with increasing insulin resistance. Importantly, we examined our large cohort of obese youth to see whether even discrete changes in 2-h glucose within the normal range could be detected with the insulin feedback model of glucose tolerance.
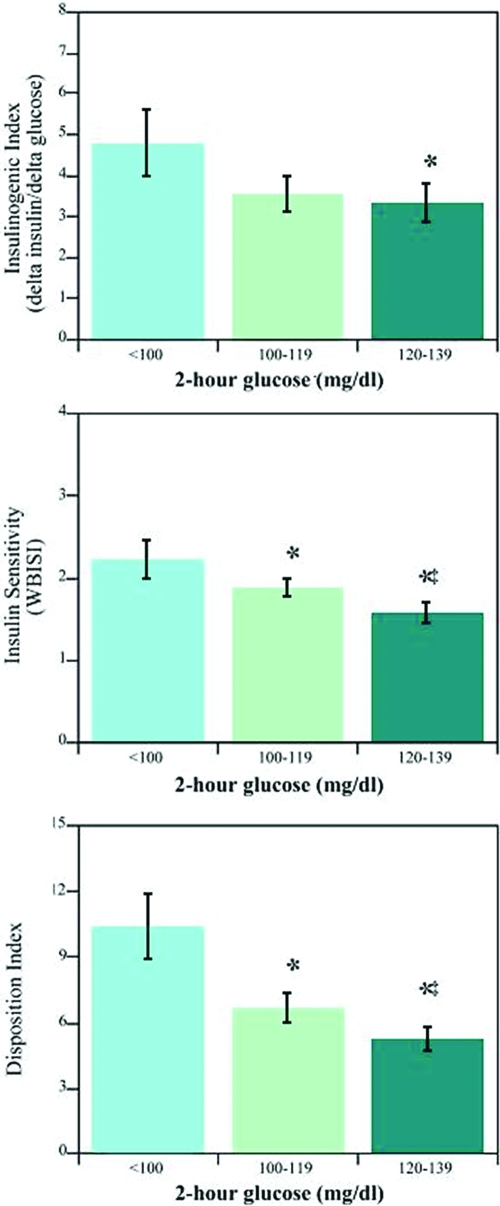
At first glance, the 2-h glucose category data suggest that worsening insulin resistance may contribute to early deterioration in oral glucose tolerance. On further analysis, however, insulin secretion, as reflected by the insulinogenic index, appears to have a strong impact on glucose tolerance (albeit still normal), irrespective of insulin sensitivity. As illustrated in Fig. 2, at any level of insulin sensitivity (WBISI), the IGI was lowest in the group with the highest 2-h glucose level. The same result is obtained if C-peptide replaces insulin in the equation. These data provide support for the notion that even obese youth with NGT who are more insulin sensitive can have a perturbed β-cell response to a normal physiological stimulus (glucose ingestion). Interestingly, this pattern of β-cell dysfunction could be replicated if fasting insulin tertiles were used in place of the WBISI strata (highest insulin tertile corresponding to lowest WBISI tertile).
Figure 2.
Relationship between β-cell function (insulinogenic index; top panel) and insulin sensitivity (WBISI; middle panel) as a function of 2-h glucose category. Data are expressed as least-squares means and 95% confidence intervals (adjusted for age, gender, race/ethnicity, and BMI). For comparisons made between the lowest and more elevated 2-h glucose levels (P < 0.02, P < 0.05 for moderate to high glucose levels). The net response on the insulin feedback system was a decrease in disposition index (bottom panel) at each level of increasing hour glucose category (<0.01 for all comparisons). Modified from Yeckel CW et al. (2005, J Clin Endocrinol Metab 747–754).
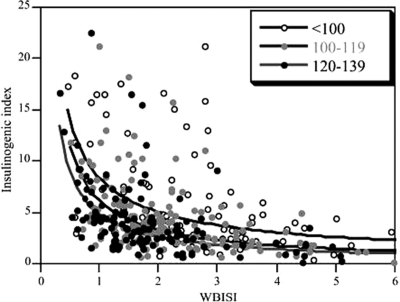
The net result from our data would indicate that insulin resistance is likely a necessary component for large-magnitude changes in insulin secretory response to a glucose load, whatever the mechanism for adaptive plasticity within the β-cells to increase insulin secretion. This concept fits within the general framework of the hyperbolic feedback curves (38,39,40). We identified a systematic leftward shift in each hyperbolic curve (Fig. 3) as glucose increased across 2-h glucose categories toward IGT. This demonstrates that the hyperbolic model for glucose tolerance can distinguish even finer levels of perturbation in glucose homeostasis besides the traditional broad categories of NGT, IGT, and T2DM.
Figure 3.
Summary hyperbolic feedback curves constructed from all participants grouped by 2-h glucose category. Feedback curves were compared using liner regression in which the logarithm of the IGI was modeled as a function of the logarithm of the WBISI. There was a significant leftward shift (toward IGT) in the insulin feedback curve for increasing 2-h glucose category. This leftward shift remained significant after adjustment for age, gender, race/ethnicity, and BMI. Group comparisons were: less than 100 mg/dl to 100–119 mg/dl (P < 0.001); less than 100 mg/dl to 120–139 mg/dl (P < 0.001); and 100–119 mg/dl to 120–139 mg/dl (P = 0.03). Modified from Yeckel CW et al. (2005, J Clin Endocrinol Metab 747–754).
In summary, increased 2-h glucose concentrations during an oral glucose tolerance test in NGT obese youth reflect the apparent inability of the pancreatic β-cells to fully compensate for early increases in glucose. We observed the same pattern of dysfunction at every level of insulin sensitivity. Furthermore, we determined that the insulin feedback curves were sensitive enough to identify differences in 2-h glucose level even in the NGT range. Consequently, in obese children and adolescents, the transition from NGT to IGT and ultimately T2DM more likely represents a gradual deterioration in glucose-stimulated insulin response rather than a threshold effect or an all-or-none phenomenon.
Implications
The growing number of obese children and adolescents worldwide is of great concern. Many obese children and adolescents already manifest some metabolic complications such as impaired glucose regulation, hypertension, dyslipidemia, fatty liver disease, and systemic low-grade inflammation. Many of these complications are silent and often go undiagnosed. However, these children are at high risk for the development of early morbidity. Recent studies suggest that there is a particular obese phenotype that is linked to alterations in insulin sensitivity and cardiometabolic complications. This obese phenotype is characterized by a high proportion of visceral fat and relatively low abdominal sc fat, increased intrahepatic fat, and intramyocellular fat. These adolescents are not necessarily the most severely obese, yet they suffer from severe metabolic complications and are at a high risk of developing diabetes and its associated cardiometabolic complications. Understanding the underlying pathogenesis of this peculiar phenotype is of critical importance.
Acknowledgments
We are grateful to all adolescents who participated in the study; the research nurses for the excellent care given to our subjects; and Dr. Ram Weiss, Sara Taksali, and Cathrine Yeckel for conducting these studies.
Footnotes
This work was supported by Grants R01-HD40787, R01-HD28016, and K24-HD01464 from the National Institutes of Health (NIH) (to S.C.), Grant M01-RR00125 to the Yale Clinical Research Center, and Grant R01-EB006494 (Bioimage Suite). This publication (or project described) was supported by Clinical and Translational Science Awards (CTSA) Grant UL1 RR0249139 from the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
A.M.G.C. and S.C. have nothing to declare.
Abbreviations: BMI, Body mass index; HDL, high-density lipoprotein; IGT, impaired glucose tolerance; IMCL, intramyocellular; NGT, normal glucose tolerance; NHANES, National Health and Nutrition Examination Survey; T2DM, type 2 diabetes mellitus; WBISI, whole-body insulin sensitivity index.
References
- Ogden CL, Flegal KM, Carroll MD, Johnson CL 2002 Prevalence and trends in overweight among U.S. children and adolescents, 1999–2000. JAMA 288:1728–1732 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM 2008 High body mass index for age among U.S. children and adolescents, 2003–2006. JAMA 299:2401–2405 [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WPT 2005 Obesity. Lancet 366:1197–1209 [DOI] [PubMed] [Google Scholar]
- Uauy R, Albala C, Kain J 2001 Obesity trends in Latin America: transiting from under- to overweight. J Nutr 131:893S–899S [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobstein T 2006 Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 1:11–25 [DOI] [PubMed] [Google Scholar]
- Sturm R 2003 Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med 163:2146–2148 [DOI] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S 2002 Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 346:802–810 [DOI] [PubMed] [Google Scholar]
- Daniels S 2006 The consequences of childhood overweight and obesity. Future Child 16:47–67 [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S 2004 Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS 2005 Racial differences in the tracking of childhood BMI to adulthood. Obes Res 13:928–935 [DOI] [PubMed] [Google Scholar]
- Harris KM, Gordon-Larsen P, Chantala K, Udry R 2006 Longitudinal trends in race/ethnic disparities in leading health indicators from adolescence to young adulthood. Arch Pediatr Adolesc Med 160:74–81 [DOI] [PubMed] [Google Scholar]
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T 1993 Do obese children become obese adults? A review of the literature. Prev Med 22:167–177 [DOI] [PubMed] [Google Scholar]
- M Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG 2006 Prevalence and determinants of insulin resistance among U.S. adolescents. A population-based study. Diabetes Care 29:2427–2432 [DOI] [PubMed] [Google Scholar]
- Bell LM, Byrne S, Thompson A, Ratnam N, Blair E, Bulsara M, Jones TW, Davis EA 2007 Increasing body mass index z-score is continuously associated with complications of overweight in children, even in the healthy range. J Clin Endocrinol Metab 92:517–522 [DOI] [PubMed] [Google Scholar]
- Reaven GM 1988 Banting lecture. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L 2001 Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24:683–689 [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman III WP, Tracy RE, Wattigney WA 1998 Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med 338:1650–1656 [DOI] [PubMed] [Google Scholar]
- Li S, Chen W, Srinivasan SR, Bond Gene M, Tang R, Urbina EM, Berenson GS 2003 Childhood cardiovascular risk factors and carotid vascular changes in adulthood. JAMA 290:2271–2276 [DOI] [PubMed] [Google Scholar]
- Raitakari TO, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS 2003 Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood. JAMA 290:2277–2283 [DOI] [PubMed] [Google Scholar]
- Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM 2001 Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine study. J Am Coll Cardiol 27:277–284 [DOI] [PubMed] [Google Scholar]
- Davis PH, Dawson JD, Riley WA, Lauer RM 2001 Carotid intimal media thikness is related to cardiovascular risk factors measured from childhood through middle age. The Muscatine Study. Circulation 104:2815–2819 [DOI] [PubMed] [Google Scholar]
- 1990 Relationships in young men to serum lipoprotein cholesterol concentrations and smoking. Pathobiological Determinants of Arthrosclerosis in Youth (PDAY) Research Group. JAMA 264:3018–3024 [DOI] [PubMed] [Google Scholar]
- Grundy SM 2002 Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation 105:2696–2698 [DOI] [PubMed] [Google Scholar]
- Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S 2003 Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, Tamborlane WV, Dziura J, Shulman GI, Caprio S 2005 The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 90:3731–3737 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster BH, Storlien L 2002 Muscle triglyceride and insulin resistance. Annu Rev Nutr 22:325–346 [DOI] [PubMed] [Google Scholar]
- Shulman GI 2000 Cellular mechanism of insulin resistance. J Clin Invest 106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB 2002 Dysfunction in mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Colberg SR, Thaete FL, Kelley D 1995 Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB 9:273–278 [PubMed] [Google Scholar]
- Mootha VK, Lingren CM, Eriksson KF, Subramania A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstadele M, Laurila E, Houstis N, Daly MK, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α responsive genes involved in oxidative phosphorylation are coordinately down-regulated in human diabetes. Nat Genet 34:267–273 [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zderic TW, Davidson CJ, Schenk S, Byerley LO, Coyle EF 2003 High-fat diet elevates resting intramuscular triglyceride concentration and whole-body lipolysis during exercise. Am J Physiol Endocrinol Metab 286:E217–E225 [DOI] [PubMed] [Google Scholar]
- Helge JW, Watt PW, Richter EA, Rennie MJ, Kiens B 2001 Fat utilization during exercise: adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J Physiol 537:1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taksali S, Caprio S, Dziura J, Dufour S, Cali A, Goodman R, Papademetris X, Burgert T, Pierpont B, Savoye M, Shaw M, Seyal A, Weiss R 2008 High visceral and low abdominal subcutaneous fat stores in the obese adolescent. A determinant of an adverse metabolic phenotype. Diabetes 57:367–371 [DOI] [PubMed] [Google Scholar]
- Yeckel CW, Taksali SE, Dziura J, Weiss R, Burgert TS, Sherwin RS, Tamborlane WV, Caprio S 2005 The normal glucose tolerance continuum in obese youth: evidence for impairment in β-cell function independent of insulin resistance. J Clin Endocrinol Metab 89:1096–1101 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte Jr D 1993 Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes 42:1663–1672 [DOI] [PubMed] [Google Scholar]
- Bergman RN 1989 Lilly lecture. Toward physiological understanding of glucose tolerance: minimal-model approach. Diabetes 38:1512–1527 [DOI] [PubMed] [Google Scholar]
- Kahn SE 2003 The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19 [DOI] [PubMed] [Google Scholar]