Abstract
Objective
To review the data on long-term outcomes in women who underwent prophylactic bilateral oophorectomy, a common surgical procedure that has more than doubled in frequency since the 1960s.
Study design
Literature review of published data on consequences of prophylactic bilateral oophorectomy. Special emphasis was given to the Mayo Clinic Cohort Study of Oophorectomy and Aging.
Main outcome measures
Overall mortality, cardiovascular disease, cognitive impairment and dementia, parkinsonism, osteoporosis, psychological well-being, and sexual function.
Results
There is a growing body of evidence suggesting that the premature loss of ovarian function caused by bilateral oophorectomy performed before natural menopause is associated with several negative outcomes. In particular, studies have revealed an increased risk of premature death, cardiovascular disease, cognitive impairment or dementia, parkinsonism, osteoporosis and bone fractures, decline in psychological well-being, and decline in sexual function. The effects involve different organs (e.g., heart, bone, or brain), and different functions within organs (e.g., cognitive, motor, or emotional brain functions). Estrogen treatment may prevent some of these negative outcomes, but not all.
Conclusion
The potential adverse effects of prophylactic bilateral oophorectomy on heart health, neurologic health, bone health, and quality of life should be carefully weighed against its potential benefits for cancer risk reduction in women at average risk of ovarian cancer.
Keywords: prophylactic bilateral oophorectomy, surgical menopause, estrogen deficiency, aging, mortality, estrogen treatment
Introduction
Approximately 1 in 8 women above age 55 years has undergone bilateral oophorectomy before reaching natural menopause.1,2 Bilateral oophorectomy may be performed for a benign disease or for prophylaxis against ovarian cancer, and is usually performed along with hysterectomy (in nearly 90% of cases).3 Of the more than 600,000 hysterectomies performed annually in the United States, approximately half include bilateral oophorectomy.4 In addition, the practice of prophylactic oophorectomy has increased over time and more than doubled between 1965 and 1990.5 Meanwhile, reports now link premenopausal oophorectomy with serious health consequences including premature death, cardiovascular and neurologic disease, and osteoporosis in addition to menopausal symptoms, psychiatric symptoms, and impaired sexual function. For women undergoing hysterectomy who are not known to be at increased risk for cancer,6,7 these consequences need to be carefully weighed against the potential benefits of preventing ovarian cancer.
Most studies that compared outcomes after surgical menopause with outcomes after natural menopause were observational studies with less than 10-years of follow-up.8 By contrast, the Mayo Clinic Cohort Study of Oophorectomy and Aging involved a follow-up of approximately three decades. That study evaluated a population-based sample of women who underwent unilateral or bilateral oophorectomy between 1950 and 1987 in Olmsted County, Minnesota, U.S.A., and compared them with a cohort of referent women from the same defined population who did not undergo oophorectomy during the 38-year period. These 4,780 women were followed with a combination of active methods (direct or proxy interview and examination) and passive methods (review of medical records in a records-linkage system and review of death certificates). Women were evaluated to assess the incidence of neurologic and psychiatric disease as well as mortality through 2001–2006. Results from these landmark studies,9–13 along with results from other shorter-term studies,8 provide important information to guide decision-making for or against prophylactic bilateral oophorectomy before the onset of natural menopause.
Methods
A literature review of studies related to outcomes following surgical menopause and prophylactic bilateral oophorectomy was completed through 2007. In addition, results from the Mayo Clinic Cohort Study of Oophorectomy and Aging related to bilateral oophorectomy were compared with other studies with regard to overall mortality, cardiovascular, neurologic, and psychiatric outcomes.
Results
Overall mortality
Oophorectomy is associated with increased overall mortality - not limited to cardiovascular disease. In a study using a Markov decision analysis model to assess mortality attributable to oophorectomy at the time of hysterectomy, women undergoing oophorectomy before age 55 years were reported to have an 8.6% excess mortality by age 80 years.14 Even women with oophorectomy up to age 59 years were predicted to have an excess mortality of 3.9%. So, the risk is not confined to premenopausal oophorectomy. Parker and colleagues concluded that women at average risk of ovarian cancer who undergo hysterectomy for a benign disease benefit from ovarian conservation until at least age 65 years.14–16 The Mayo Clinic Cohort Study of Oophorectomy and Aging empirically verified these predictive models by showing that overall mortality was increased in women who underwent prophylactic bilateral oophorectomy before age 45 years compared with referent women (HR 1.67; 95% CI 1.16–2.40).9 The increased mortality in this group of women was mainly observed in those who did not take estrogen up to the age of 45 years (HR 1.93; 95% CI 1.25–2.96). These findings are also consistent with several studies that showed increased overall mortality in women who underwent natural menopause at younger age.17–20
Cardiovascular disease
In 2006, a meta-analysis evaluated 11 studies of postmenopausal status and age at menopause in relation to cardiovascular disease. The study showed that the pooled relative risk of cardiovascular disease in women who underwent bilateral oophorectomy was 2.62 (95% CI 2.05–3.35) compared with women who were premenopausal.21 This compared with a relative risk of 1.14 (95% CI 0.86–1.51) for natural menopause versus premenopausal status. The pooled effect of bilateral oophorectomy before age 50 years was 4.55 (95% CI 2.56–8.01) compared with bilateral oophorectomy after age 50 years. This compared with a relative risk of 1.27 (95% CI 1.14–1.43) for natural menopause before age 50 years versus natural menopause after age 50 years.21
Also in 2006, Lokkegaard and colleagues reported on the Danish Nurse Cohort Study, a prospective cohort study of nearly 20,000 women above 44 years of age who were followed for 5 years.22 In this study, the adjusted risk of ischemic heart disease among women who underwent bilateral oophorectomy before age 40 years was 8.7 (95% CI 2.0–38.1) compared to women with oophorectomy after age 45 years. This compared with a much smaller increased risk for ischemic heart disease among women who experienced natural menopause before age 40 years (HR 2.2; 95% CI 1.0–4.9). Among the women who experienced menopause as a result of bilateral oophorectomy, estrogen therapy was associated with significant protection against ischemic heart disease (HR 5.5 among ever users, versus 16.2 among never users). The benefit from estrogen therapy was most pronounced for women who were current users or who started treatment within 1 year after onset of surgical menopause.
Overall, the preponderance of evidence suggests that bilateral oophorectomy is associated with increased cardiovascular risk and premature death, and that oophorectomy at a young age further increases this risk.23 Estrogen therapy started early after surgical or natural menopause at a young age appears to reduce this risk.22–25
Cognitive impairment or dementia
Current evidence on the association between oophorectomy and cognitive performance comes from observational studies and from small-scale clinical trials. In the Mayo Clinic Cohort Study of Oophorectomy and Aging, women who underwent bilateral oophorectomy before the onset of menopause had an increased risk of cognitive impairment or dementia compared with referent women (HR 1.33; 95% CI 0.98–1.81; P = 0.07). The risk increased with younger age at oophorectomy, and women under age 43 years had the greatest risk (HR 1.74; 95% CI 0.97–3.14; P = 0.06). The trend of increasing risk with younger age at oophorectomy was significant (P=0.01). Interestingly, the risk was restricted to women who underwent oophorectomy before age 49 years and were not treated with estrogen until at least age 50 years (HR 1.89; 95% CI 1.27–2.83; P = 0.002).10
Nappi and colleagues evaluated neuropsychological tasks in 27 surgically menopausal women following hysterectomy with bilateral oophorectomy and 76 naturally menopausal women at a mean age of 52 years. Women who underwent oophorectomy scored significantly worse on recency items from a word-list memory task. Additionally, the recency scores tended to be lower when oophorectomy occurred at younger ages.26 In a longitudinal study from Egypt, 35 premenopausal women at a mean age of 41 years underwent neuropsychological testing before and after oophorectomy with hysterectomy, and results were compared with those of 18 premenopausal women matched for age, education, parity, weight, and height. Surgically menopausal women had significant decreases in global cognitive functioning scores and Wechsler Memory Scale subtests 6 months after oophorectomy, compared with the premenopausal women who experienced no decline. Women with greater declines in estradiol levels had greater declines in performance on one of two verbal memory tasks and two of the four other cognitive tests.27
In the Rancho Bernardo Study, which evaluated older postmenopausal women at a mean age of 74 years in a southern California community, women with prior bilateral oophorectomy with hysterectomy performed significantly poorer on certain memory tests (serial sevens and Trails B), but the difference was reported to be of unlikely clinical significance.28 There were no differences in mean cognitive function scores between women in this cohort who underwent bilateral oophorectomy (n = 190) versus hysterectomy with conservation of one or both ovaries (n = 225), and no differences compared with women who were naturally menopausal (n = 470). Although women who had undergone hysterectomy (with or without oophorectomy) were more likely to be using estrogen at the time of cognitive testing than women who were naturally menopausal, there was no significant difference in cognitive function tests after adjusting for current and past estrogen use. Likewise, in a British cohort of 1,261 women with a mean age of 53 years, there were no significant differences in mean cognitive test scores between women who had undergone hysterectomy, bilateral oophorectomy, or natural menopause.29 However, women who underwent hysterectomy or bilateral oophorectomy and were not taking estrogen therapy had the lowest reading ability scores.
Clinical trials evaluating the effects of oophorectomy and estrogen therapy on cognitive function have reported contrasting results. Sherwin reported a greater decline in cognitive function tests in 40 premenopausal women who underwent hysterectomy with bilateral oophorectomy and were randomized to placebo compared with women randomized to estrogen or testosterone therapy for 3 months following surgery.30 In a subsequent randomized controlled trial evaluating 19 premenopausal women before and after surgical menopause with a larger battery of neuropsychological tests, women were randomized to estradiol versus placebo. The women given estradiol following hysterectomy with oophorectomy performed significantly better on tests of verbal memory than women given placebo.31
Four other randomized controlled trials reported no cognitive benefit of estrogen after surgical menopause, but the trials were not well controlled in that oophorectomy was inferred and not confirmed, estrogen was not given immediately after surgery, and baseline testing before surgical menopause was not performed.32–35
Finally, a series of case-control and cohort studies evaluated the effect of estrogen treatment after menopause, regardless of the cause of menopause. They reported a 20–40% reduction in the risk of Alzheimer’s disease for women starting estrogen therapy early after menopause compared with those not taking estrogen.36–38
Evidence to date suggests that there may be a neuroprotective effect of estrogen on the brain, and that the effect may be age-dependent. For women undergoing surgical menopause as a result of oophorectomy before the age of natural menopause, estrogen therapy may be particularly important for neuroprotection.10,39
Parkinsonism and Parkinson’s disease
In 2001, investigators from the Mayo Clinic reported preliminary associations of Parkinson’s disease with type of menopause, age at menopause, and post-menopause estrogen therapy.40 In that case-control study evaluating 72 patients with Parkinson’s disease and 72 matched controls, women with a history of hysterectomy (but without bilateral oophorectomy) were at significantly increased risk of Parkinson’s disease (OR 3.36; 95% CI 1.05–10.77; P = 0.04) as well as women with menopause before age 47 years (OR 2.18; 95% CI 0.88–5.39; P = 0.09). Finally, there was a trend toward protection against Parkinson’s disease in postmenopausal estrogen therapy users for women who were naturally menopausal as well as surgically menopausal.40 However, other studies did or did not confirm these associations.41,42
In the Mayo Clinic Cohort Study of Oophorectomy and Aging, women who underwent bilateral oophorectomy before the onset of menopause had an increased risk of parkinsonism compared with referent women (HR 1.80; 95% CI 1.00–3.26; P = 0.05), and the risk increased with younger age at oophorectomy (test for linear trend; P = 0.02). The findings were similar regardless of the indication for the oophorectomy (benign condition vs. prophylactic). The findings were also consistent for Parkinson’s disease alone, but did not reach statistical significance.11,12
Psychological well-being and sexual function
While recent data report that hysterectomy performed for a benign disease is generally associated with improved psychological well-being and quality of life,43–49 hysterectomy with bilateral oophorectomy is more commonly associated with worsened psychological well-being. In fact, the link between oophorectomy and depression has been recognized for many years.50–53
However, prospective evaluation of this association has been limited. Nathorst-Boos and colleagues evaluated 101 women following hysterectomy, of whom 35 had their ovaries preserved and were not taking estrogen, 33 underwent bilateral oophorectomy and were not taking estrogen, and 33 underwent oophorectomy and were taking estrogen.54 Women who underwent oophorectomy along with hysterectomy had significantly greater anxiety and depression, and less positive well-being than the women whose ovaries had been preserved. Oophorectomized women taking estrogen reported less anxiety and depression, and their psychological well-being was similar to women whose ovaries had been preserved. Similarly, oophorectomized women reported more impaired sexual function compared to women with intact ovaries; however, sexual symptoms were not ameliorated by taking estrogen.
In contrast, a more recent prospective study evaluating psychological well-being and sexual function in 323 women at baseline and one year after simple hysterectomy versus hysterectomy with oophorectomy found no difference in psychological well-being changes and no overall difference in reported sexual function changes from baseline between the two groups.55 Of note, 98% of women who underwent hysterectomy with bilateral oophorectomy were taking estrogen at the time of testing while only 26% of women in the simple hysterectomy group were taking estrogen, and estradiol levels in the simple hysterectomy group declined significantly following surgery suggesting a decline in ovarian function in these women.
The Mayo Clinic Cohort Study of Oophorectomy and Aging followed 666 women with bilateral oophorectomy and 673 referent women using structured questionnaires and telephone interviews to assess depressive and anxiety symptoms. Women who underwent premenopausal bilateral oophorectomy were found to have an increased risk of developing de novo depressive symptoms (HR 1.54; 95% CI 1.04–2.26) and de novo anxiety symptoms (HR 2.29; 95% CI 1.33–3.95) compared with referent women.13 This increase in depressive and anxiety symptoms occurred in women who did not suffer from depression or anxiety before the surgery, and persisted even many years after surgery.
Among women at increased risk for ovarian cancer, such as a woman with a family history of ovarian cancer or with a BRCA mutation, several studies have reported on the negative psychosocial and sexual consequences of prophylactic oophorectomy. Madalinska and colleagues compared quality of life after prophylactic oophorectomy versus surveillance in their survey of 846 women, and found that the most common adverse effects of surgery were an increase in hot flashes, dyspareunia, and a decrease in sexual satisfaction.56 In other smaller cohort studies, prophylactic oophorectomy has also been associated with a decrease in sexual satisfaction and dyspareunia.57–62
In 2006, Dennerstein and colleagues reported that in their survey of 1,345 European women aged 20–70 years, women who underwent bilateral oophorectomy were twice as likely to meet diagnostic criteria for hypoactive sexual desire disorder compared with women who were premenopausal or experienced natural menopause.63
Osteoporosis
Numerous studies through the years have shown that bone loss accelerates following menopause. The earlier in life that menopause occurs, the lower the bone density will be later in life.64 Oophorectomy before age 45 is a well-established risk-factor for osteoporosis.65 In addition, even in women who undergo bilateral oophorectomy after natural menopause, the risk of osteoporotic fracture may be increased compared with women with intact ovaries.66 Estrogen therapy reduces this risk, and there are now also numerous non-estrogen treatment options for postmenopausal osteoporosis.67 The frequency of use of these non-estrogen treatments in women discontinuing estrogen therapy following surgical menopause is unknown.
Discussion
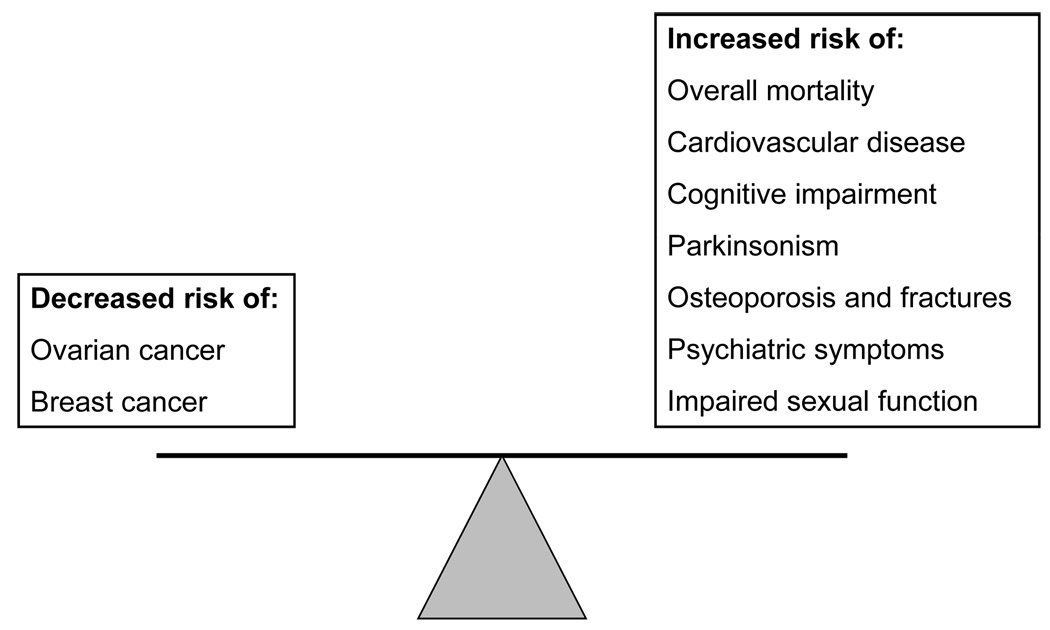
Women facing the decision to undergo prophylactic bilateral oophorectomy at the time of hysterectomy are confronted with a difficult dilemma. Guidance regarding the decision generally focuses on a reduction in risk for ovarian cancer. But, evidence shows that a woman’s lifetime risk for premature death, cardiovascular disease, cognitive impairment or dementia, symptoms of parkinsonism, and osteoporosis may be adversely impacted by premature loss of ovarian function. Additionally, the potential adverse effects on quality of life, sexual function, and mental health are rarely discussed with women considering this surgical procedure. The risk-benefit balance for prophylactic bilateral oophorectomy in younger women is outlined schematically in Figure 1.
Figure 1.
The risk-benefit balance for prophylactic bilateral oophorectomy in younger women.
Premature loss of ovarian function by elective oophorectomy before natural menopause is associated with an increased risk for premature death, cardiovascular disease, cognitive impairment or dementia, parkinsonism, a decline in psychological well-being and, in some studies, a decline in sexual function. Whether these consequences are due to the abrupt drop in estrogen, testosterone, or progesterone levels, or whether the changes might be mediated through effects on the hypothalamic-pituitary axis via an increase in gonadatropins remains unknown. Estrogen levels are higher in women with ovaries intact than in women after bilateral oophorectomy, even in older women.68,69 Now that women are discouraged from initiating estrogen therapy, those who undergo oophorectomy at a young age and do not initiate or continue estrogen therapy until at least the age of natural menopause are at significantly increased risk for several chronic diseases of aging.
In the specialty of Obstetrics and Gynecology, the predominant teaching in recent years has been that prophylactic bilateral oophorectomy should be avoided in women under the age of 40 years, should routinely be performed above age 55 years, and should be considered and discussed with the patient in the interval between.70 More research is needed to appropriately inform women between the ages of 40 and 55 years about the risks and benefits of prophylactic oophorectomy. The findings summarized in this review argue for thoughtful scrutiny of the practice of prophylactic oophorectomy at all ages, for women who are not known to be at increased risk for ovarian cancer. Additionally, more research is needed to determine the relative benefits and risks of hormone therapy for this group of women. The studies summarized in this review article argue for caution against thoughtlessly applying the results of the Women’s Health Initiative clinical trials to hormone therapy decisions for women with prophylactic bilateral oophorectomy.71
Finally, the primary consideration favoring prophylactic bilateral oophorectomy is the lack of effective screening tools for ovarian cancer. When diagnosed early, cure rates for ovarian cancer are as high as 90% to 95%. However, this disease has appropriately earned a dreaded reputation because vague and non-specific symptoms lead to late stage diagnosis in 75% of patients.72 Unfortunately, advanced ovarian cancer has a low cure rate in spite of aggressive medical and surgical therapy. An effective and affordable screening test that allows early diagnosis, when ovarian cancer is most treatable, will eliminate the justification for prophylactic bilateral oophorectomy. Fortunately, considerable research is being directed toward that goal.73
In the meantime, elective oophorectomy in women not known to be at increased risk for ovarian or breast cancer needs to be carefully considered in the context of each woman’s health risks, goals, and personal preferences.
Acknowledgements
The authors thank Ms. Barbara J. Balgaard for her secretarial assistance.
Sources of Funding: The Mayo Clinic Cohort Study of Oophorectomy and Aging was funded by NIH grant R01 NS33978 from the National Institute of Neurological Disorders and Stroke and was made possible by the NIH grant R01 AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Competing interests: None
References
- 1.Howe HL. Age-specific hysterectomy and oophorectomy prevalence rates and the risks for cancer of the reproductive system. Am J Public Health. 1984;74:560–563. doi: 10.2105/ajph.74.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Bergstralh EJ, Malkasian GD, O'Fallon WM. Bilateral oophorectomy trends in Olmsted County, Minnesota, 1950–1987. Epidemiology. 1991;2:149–152. [PubMed] [Google Scholar]
- 4.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198(34):e1–e7. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance--United States, 1994–1999. MMWR Surveill Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 6.Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 7.Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7:223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 8.Wild RA. Introduction to special issue on surgical menopause. Menopause. 2007;14:556–561. [Google Scholar]
- 9.Rocca WA, Grossardt BR, de Andrade M, et al. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 10.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 12.Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis. 2008;5:257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms following early bilateral oophorectomy. Menopause. 2008 doi: 10.1097/gme.0b013e318174f155. in press. [DOI] [PubMed] [Google Scholar]
- 14.Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219–226. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 15.Shoupe D, Parker WH, Broder MS, et al. Elective oophorectomy for benign gynecological disorders. Menopause. 2007;14:580–585. doi: 10.1097/gme.0b013e31803c56a4. [DOI] [PubMed] [Google Scholar]
- 16.Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Clin Obstet Gynecol. 2007;50:354–361. doi: 10.1097/GRF.0b013e31804a838d. [DOI] [PubMed] [Google Scholar]
- 17.Snowdon DA, Kane RL, Beeson WL, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79:709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157:923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 19.Ossewaarde ME, Bots ML, Verbeek AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 20.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–1097. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 21.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 22.Lokkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226–233. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Lobo RA. Surgical menopause and cardiovascular risks. Menopause. 2007;14:562–566. doi: 10.1097/gme.0b013e318038d333. [DOI] [PubMed] [Google Scholar]
- 24.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 25.Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Nappi RE, Sinforiani E, Mauri M, et al. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- 27.Farrag AK, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13:193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- 28.Kritz-Silverstein D, Barrett-Connor E. Hysterectomy, oophorectomy, and cognitive function in older women. J Am Geriatr Soc. 2002;50:55–61. doi: 10.1046/j.1532-5415.2002.50008.x. [DOI] [PubMed] [Google Scholar]
- 29.Kok HS, Kuh D, Cooper R, et al. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- 30.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 31.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 32.Polo-Kantola P, Portin R, Polo O, et al. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, cross-over trial in postmenopausal women. Obstet Gynecol. 1998;91:459–466. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- 33.Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. A randomised placebo controlled pilot cross-over study. Psychoneuroendocrinology. 2005;30:309–315. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Almeida OP, Lautenschlager NT, Vasikaran S, et al. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 37.LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 38.Hogervorst E, Williams J, Budge M, et al. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 39.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- 40.Benedetti MD, Maraganore DM, Bower JH, et al. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord. 2001;16:830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 41.Ragonese P, D'Amelio M, Salemi G, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology. 2004;62:2010–2014. doi: 10.1212/wnl.62.11.2010. [DOI] [PubMed] [Google Scholar]
- 42.Popat RA, Van Den Eeden SK, Tanner CM, et al. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology. 2005;65:383–390. doi: 10.1212/01.wnl.0000171344.87802.94. [DOI] [PubMed] [Google Scholar]
- 43.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women's Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83:556–565. doi: 10.1097/00006250-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women's Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566–572. doi: 10.1097/00006250-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915–921. doi: 10.1016/s0029-7844(98)00541-9. [DOI] [PubMed] [Google Scholar]
- 46.Ryan MM, Dennerstein L, Pepperell R. Psychological aspects of hysterectomy. A prospective study. Br J Psychiatry. 1989;154:516–522. doi: 10.1192/bjp.154.4.516. [DOI] [PubMed] [Google Scholar]
- 47.Lambden MP, Bellamy G, Ogburn-Russell L, et al. Women's sense of well-being before and after hysterectomy. J Obstet Gynecol Neonatal Nurs. 1997;26:540–548. doi: 10.1111/j.1552-6909.1997.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 48.Kjerulff KH, Langenberg PW, Rhodes JC, et al. Effectiveness of hysterectomy. Obstet Gynecol. 2000;95:319–326. doi: 10.1016/s0029-7844(99)00544-x. [DOI] [PubMed] [Google Scholar]
- 49.Kjerulff KH, Rhodes JC, Langenberg PW, Harvey LA. Patient satisfaction with results of hysterectomy. Am J Obstet Gynecol. 2000;183:1440–1447. doi: 10.1067/mob.2000.107731. [DOI] [PubMed] [Google Scholar]
- 50.McKinlay JB, McKinlay SM, Brambilla D. The relative contributions of endocrine changes and social circumstances to depression in mid-aged women. J Health Soc Behav. 1987;28:345–363. [PubMed] [Google Scholar]
- 51.McKinlay JB, McKinlay SM, Brambilla DJ. Health status and utilization behavior associated with menopause. Am J Epidemiol. 1987;125:110–121. doi: 10.1093/oxfordjournals.aje.a114492. [DOI] [PubMed] [Google Scholar]
- 52.Shoupe D, Lobo RA. Prolactin response after gonadotropin-releasing hormone in the polycystic ovary syndrome. Fertil Steril. 1985;43:549–553. [PubMed] [Google Scholar]
- 53.Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. J Clin Endocrinol Metab. 1985;60:178–183. doi: 10.1210/jcem-60-1-178. [DOI] [PubMed] [Google Scholar]
- 54.Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy--effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283–293. doi: 10.3109/01674829309084451. [DOI] [PubMed] [Google Scholar]
- 55.Aziz A, Brannstrom M, Bergquist C, Silfverstolpe G. Perimenopausal androgen decline after oophorectomy does not influence sexuality or psychological well-being. Fertil Steril. 2005;83:1021–1028. doi: 10.1016/j.fertnstert.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Madalinska JB, van Beurden M, Bleiker EM, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol. 2006;24:3576–3582. doi: 10.1200/JCO.2005.05.1896. [DOI] [PubMed] [Google Scholar]
- 57.Elit L. Familial ovarian cancer. Can Fam Physician. 2001;47:778–784. [PMC free article] [PubMed] [Google Scholar]
- 58.Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149–156. doi: 10.1023/a:1021119405814. [DOI] [PubMed] [Google Scholar]
- 59.Elit L, Jack E, Kwan E, et al. A unique BRCA1 mutation identified in Mongolia. Int J Gynecol Cancer. 2001;11:241–243. doi: 10.1046/j.1525-1438.2001.01020.x. [DOI] [PubMed] [Google Scholar]
- 60.Elit L, Lukka H, Friedman E. Cutaneous metastasis of papillary serous uterine cancer. Gynecol Oncol. 2001;82:208–211. doi: 10.1006/gyno.2001.6224. [DOI] [PubMed] [Google Scholar]
- 61.Elit L, Rosen B, Goel V, et al. Prophylactic oophorectomy in Ontario. Fam Cancer. 2001;1:143–148. doi: 10.1023/a:1021174604905. [DOI] [PubMed] [Google Scholar]
- 62.Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281–287. doi: 10.1016/s0090-8258(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 63.Dennerstein L, Koochaki P, Barton I, Graziottin A. Hypoactive sexual desire disorder in menopausal women: a survey of Western European women. J Sex Med. 2006;3:212–222. doi: 10.1111/j.1743-6109.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 64.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–571. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 65.National Osteoporosis Foundation. Physician's guide to prevention and treatment of osteoporosis. Washington, D.C.: National Osteoporosis Foundation; 1998. American Academy of Orthopaedic Surgeons. [Google Scholar]
- 66.Melton LJ, 3rd, Khosla S, Malkasian GD, et al. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900–905. doi: 10.1359/jbmr.2003.18.5.900. [DOI] [PubMed] [Google Scholar]
- 67.MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 68.Sluijmer AV, Heineman MJ, De Jong FH, Evers JL. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab. 1995;80:2163–2167. doi: 10.1210/jcem.80.7.7608272. [DOI] [PubMed] [Google Scholar]
- 69.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 70.Olive DL. Dogma, skepsis, and the analytic method: the role of prophylactic oophorectomy at the time of hysterectomy. Obstet Gynecol. 2005;106:214–215. doi: 10.1097/01.AOG.0000173317.49461.52. [DOI] [PubMed] [Google Scholar]
- 71.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 72.ACOG Committee Opinion: number 280, December 2002. The role of the generalist obstetrician-gynecologist in the early detection of ovarian cancer. Obstet Gynecol. 2002;100:1413–1416. doi: 10.1016/s0029-7844(02)02630-3. [DOI] [PubMed] [Google Scholar]
- 73.Aletti GD, Gallenberg MM, Cliby WA, et al. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–770. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]