Abstract
Hypothalamic–pituitary–adrenal axis dysregulation after stress was found to be associated with borderline personality disorder (BPD). Nine female BPD young adults and 12 control subjects were investigated for stress reactivity and recovery after an interpersonal conflict discussion with their mothers. BPD subjects showed a delayed cortisol response after psychosocial stress.
Keywords: Borderline personality disorder, Psychosocial stress, Cortisol response
1. Introduction
The prevalence of borderline personality disorder (BPD) is estimated to be 1–2.5% in the general population and 10–50% in psychiatric outpatient and inpatient settings [25]. Subjects with BPD suffer from three sectors of psychopathology: affective instability, interpersonal instability, and impulsivity [8].
Patients with BPD report more stressful interpersonal situations than do individuals suffering from other types of personality disorders [18]. It has also been clinically described that stressful interpersonal situations are associated with higher affective instability among individuals with BPD [17].
BPD subjects showed cortisol hypersuppression after Dexamethasone Suppression Test (DST) [21]. In response to DST, cortisol hypersuppression has been found to be associated with sustained childhood abuse [21] and comorbid PTSD [9,15] rather than with the BPD diagnosis. Nevertheless, it has been argued that the DST is not a useful test for stress response in BPD subjects [14]. Using the Trier Social Stress Test, BPD subjects with higher dissociation scores showed significantly higher stress responses [22], which suggested that a higher severity of BPD psychopathology might be associated with heightened vulnerability to stress.
No published study has examined yet the association between BPD psychopathology and stress response after a naturalistic interpersonal stress situation. The aim of this pilot study was to investigate the relation of BPD diagnosis to the cortisol response in young adulthood, when BPD onset usually occurs [7]. We hypothesized that BPD diagnosis would be associated with greater cortisol reactivity after interpersonal stress, and with delayed recovery from the stressor. Hypotheses for the study were derived from a previous investigation of 104 low-income non-patient young adults, among whom borderline personality traits were related to disorganized attachment patterns in interaction with the parent in the same interpersonal stress situation (Lyons-Ruth et al., in preparation). Disorganized attachment patterns earlier in development have been related to elevated cortisol responses 30 min after the offset of the stressful situation [10,23].
2. Subjects and methods
2.1. Participants
Nine young adults were recruited from the Two Brattle Center, a private mental health clinic in Cambridge, MA, specializing in the treatment of young adults with self-destructive behavior and borderline personality disorder. Subjects eligible for participation were all outpatients referred for borderline personality disorder features or self-destructive behaviors. Twelve control subjects recruited via advertisements were also included in the study. Consistent with the gender distribution among BPD subjects [6], 16 (76%) of the total sample were female, and 5 (24%) male. The mean age was 18.7 years (SD = 2.1).
2.2. Study procedures
Upon arriving at the laboratory, subjects and their mothers were separated and asked to fill out a series of self-report forms. While separated, both mothers and young adults were then asked to identify areas of disagreement in their relationship. Following procedures described by Kobak et al. [13] and Klimes-Dougan et al. [12], the young adult taped a brief statement of her position regarding the area of conflict. Next, the subjects and their mothers came together to be videotaped in two brief interactions: a 5-min unstructured reunion followed by a 10-min discussion of the conflict topic. These psychological stressors are capable to activate the HPA axis [2].
2.3. Interviews and psychological measurements
The Structured Clinical Interview for Axis I (SCID-I) [4] and II Disorders (SCID-II) [5] was used to assess psychiatric disorders and BPD diagnosis according the DSM-IV criteria.
All subjects were also administered the Dissociative Experiences Scale (DES) [1]. To assess physical and emotional abuse, the Conflict Tactics Scales (CTS) [24] was used. Depressive symptoms were assessed with the Center for Epidemiological Studies Depression Scale (CES-D) [20].
2.4. Biochemical measurements
Three saliva samples were collected from each subject: a baseline sample collected 40 min after arriving in the lab, between 2 pm and 4 pm, a reactivity sample collected 20 min after the start of the conflict discussion, and a recovery sample collected 40 min after the start of the conflict discussion. The saliva samples were thawed and spun at 3000 rpm for 5 min to obtain samples with low viscosity. Clear saliva (100 μl) was removed for duplicate analysis of cortisol levels using a time resolved fluorescence immunoassay (DELFIA) that has been previously described in detail [3], The lower limit of sensitivity was <0.003 μg/dl, and the inter- and intra-assay coefficients of variance of less than 10%.
2.5. Statistical analyses
All statistical analyses were performed with SPSS/15.0 for Windows. Analysis of variance was performed with BPD versus healthy subjects as the main factor, dissociation score, depression score and childhood abuse history as covariates. Post hoc t-tests were performed with no Bonferroni corrected p-values.
3. Results
BPD subjects had significantly higher dissociation (t = −2.38, p = 0.037) and depression scores (t = −2.99, p = 0.010) than the controls, whereas their reported abuse histories on the CTS showed no difference (t = −1.24, ns). 11 subjects (52%) – all nine outpatients and two control subjects – fulfilled the criterion “self-injurious behavior and suicidality” which was the most common BPD criterion in the study sample.
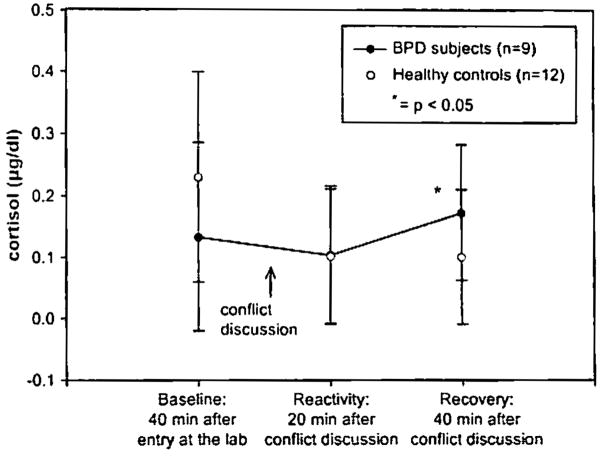
The variance analysis revealed significant group effect (F=5.99, df= 1, p = 0.016). There was no significant difference between BPD and healthy subjects on baseline cortisol level, on reactivity cortisol level, but there was a significant difference on recovery cortisol level; BPD subjects showed elevated cortisol levels 40 min after stress onset (t=−2.29, p = 0.037) (Fig. 1). The recovery sample was taken 30 min after the conflict discussion had ended (40 min after its start). Depression level, dissociation score, and reported abuse history had no significant influence on either cortisol reactivity or recovery.
Fig. I.
Salivary cortisol levels of BPD subjects and healthy subjects before and after conflict discussion wilh their mothers.
4. Discussion
In BPD, higher baseline cortisol levels have been identified as compared to healthy control subjects [16,27]. In other psychiatric disorders, an association between higher cortisol levels and negative stress-coping styles has been described [26]. This pilot study investigated the associations between BPD diagnosis and cortisol course after a stressful interpersonal interaction between mother and young adult offspring.
The young adults meeting criteria for BPD displayed a delayed stress response to the conflict discussion, with elevated cortisol seen during the recovery period. The conflict discussion appears to be a specific stressor for BPD subjects, because the healthy subjects showed a decrease of cortisol level during the study period with an initial higher cortisol concentration. These findings indicate that there may be a relationship between BPD diagnosis and delayed recovery from interpersonal stressors in BPD. Powers and McArdle [19] reported a relation between self-injurious behavior, stress-coping strategies, and elevated cortisol reactivity among a non-clinical sample of college students after conflict with a romantic partner. Among BPD subjects, conflict in intimate relationships produced delayed cortisol secretion with onset after the stressful situation.
Processes that delay the stress response among BPD subjects may account for some of the discrepancies in the literature related to cortisol response in BPD subjects. These results may indicate a complex process of stress responding among BPD subjects in which initial response is inhibited while dealing with a significant social stressor and only become manifest after relief from the interpersonal situation. We have hypothesized that the delayed cortisol response may be a form of blunted stress response that is related to social hypervigilance during the discussion.
In contrast to results from other studies, we did not find an association between depression symptom score or dissociation scores and cortisol responses [22], or between childhood abuse and cortisol level [11]. While we measured childhood physical and emotional abuse by mother, we did not look at sexual abuse history – a factor that seems to play an important role in BPD psychopathology [28]. However, the findings are not consistently; other results have indicated that childhood abuse is not directly related to neurobiological abnormalities in BPD [28]. Moreover, Rinne et al. [21] found an association between sustained childhood abuse and enhanced cortisol response after DST, which was independent from BPD psychopathology indicating that childhood abuse but not BPD psychopathology can be related to higher cortisol response. Until now, it remains unclear whether sustained childhood abuse, the BPD diagnosis, or specific BPD psychopathology (e.g. depression, impulsivity) influences the cortisol response. Regarding the different BPD psychopathology, it should be considered that we included BPD outpatients without co-occurring psychiatric disorders in the study.
The strength of the study was the investigation of a natural psychosocial stressor in subjects with BPD. Study subjects were exposed to a specific interpersonal stress situation in which young adults and their mothers decided on a specific conflict area before their discussion. Nevertheless the findings of the present study are limited by the small sample size, the missing values for subjective stress measurements, and the missing values for morning cortisol samples, ACTH, CRH, and DHEA, which would allow a more precise examination of the stress perception and the HPA axis activity. We did not control for the menstrual cycle or the intake of oral contraceptives in female BPD subjects which might influence the cortisol release. However, these findings indicated for the first time a delayed cortisol response to interpersonal stress in subjects with BPD. Controlled studies conducted with larger study samples are needed to enhance our understanding of the link between BPD psychopathology and the course of the stress response over time to more naturalistic interpersonal stress situations.
Acknowledgments
This study was supported by a pilot project grant from the Borderline Foundation and by NIMH grant #062030 to K. Lyons-Ruth.
References
- 1.Carlson EB, Putnam FW, Ross CA, Torem M, Coons P, Dill DL, et al. Validity of the Dissociative Experience Scale in screening for multiple personality disorder: a multicenter study. Am J Psychiatry. 1993;150:1030–6. doi: 10.1176/ajp.150.7.1030. [DOI] [PubMed] [Google Scholar]
- 2.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 3.Dressendoerfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as tracer in an imunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1990;43:683–92. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 4.First MB, Spitzer RL, Gibbon M, William JBW. Structured clinical interview for DSM-IV Axis I disorders/patient edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 5.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured clinical interview for DSM-IV personality disorders (SCID-II): interview and questionnaire. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 6.Gunderson JG, Shea MT, Skodol AE, McGlashan TH, Morey LC, Stout B, et al. The Collaborative Longitudinal Personality Disorders Study: development, aims, design, and sample characteristics. J Personal Disord. 2000;14:300–15. doi: 10.1521/pedi.2000.14.4.300. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson JG. Borderline personality disorder: a clinical guide. Washington, DC: American Psychiatric Press; 2001. [Google Scholar]
- 8.Gunderson JG, Lyons-Ruth K. BPD’s interpersonal hypersensitive phenotype: a gene-environment transactional model. J Personal Disord. doi: 10.1521/pedi.2008.22.1.22. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman R, Yehuda R, New A, Schmeidler J, Silverman J, Mitropoulou V, et al. Dexamethasone suppression test findings in subjects with personality disorders: associations with posttraumatic stress disorder and depression. Am J Psychiatry. 2003;160:1291–7. doi: 10.1176/appi.ajp.160.7.1291. [DOI] [PubMed] [Google Scholar]
- 10.Hertsgaard L, Gunnar M, Erickson MF, Nachmius M. Adrenocortical response to the strange situation in infants with disorganized/disoriented attachment relationships. Child Dev. 1995;66:1100–6. [PubMed] [Google Scholar]
- 11.Jogems-Kosterman BJ, de Knijff DW, Kusters R, van Hoof JJ. Basal cortisol and DHEA levels in women with borderline personality disorder. J Psychiatr Res. 2006;41:1019–26. doi: 10.1016/j.jpsychires.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- 13.Kobak RR, Cole HE, Ferenz-Gillies R, Fleming WS, Gamble W. Attachment and emotion regulation during mother-teen problem solving: a control theory analysis. Child Dev. 1993;64:231–45. [PubMed] [Google Scholar]
- 14.Korzekwa M, Steiner M, Links P, Eppel A. The dexamethasone suppression test in borderlines: is it useful? Can J Psychiatry. 1991;36:26–8. doi: 10.1177/070674379103600106. [DOI] [PubMed] [Google Scholar]
- 15.Langc W, Wulff H, Berea C, Beblo T, Saavedra AS, Mensebach C, et al. Dexamethasone suppression test in borderline personality disorder – effects of posttraumatic stress disorder. Psychoneuroendocrinology. 2005;30:919–23. doi: 10.1016/j.psyneuen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Lieb K, Rexhausen JE, Kahl KG, Schweiger U, Philipsen A, Hellhammer DH, et al. Increased diurnal salivary cortisol in women with borderline personality disorder. J Psychiatry Res. 2004;38:559–65. doi: 10.1016/j.jpsychires.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Linehan MM. Cognitive-behavioral treatment in borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- 18.Pagano ME, Skodol AE, Stout RL, Shea MT, Yen S, Grilo CM, et al. Stressful life events as predictors of functioning: findings from the Collaborative Longitudinal Personality Disorders Study. Acta Psychiatr Scand. 2004;110:421–9. doi: 10.1111/j.1600-0447.2004.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers SI, McArdle ET. Coping strategies moderate the relation of the hypothalamic–pituitary–adrenal axis reactivity to self-injurious behavior. Ann N Y Acad Sci. 2003;1008:285–8. doi: 10.1196/annals.1301.033. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 21.Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of hypothalamic–pituitary–adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52:1102–12. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- 22.Simeon D, Knutelska M, Smith L, Baker BB, Hollander E. A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Res. 2007;149:177–84. doi: 10.1016/j.psychres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Dev. 1993;64:1439–50. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 24.Straus MA. Measuring intrafamily conflict and violence: the conflict tactics scales. J Marriage Fam. 1979;41:75–88. [Google Scholar]
- 25.Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58:590–6. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- 26.Walter M, Gerhard U, Gerlach M, Weijers H-G, Boening J, Wiesbeck GA. Cortisol concentrations, stress-coping styles after withdrawal, and long-term abstinence in alcohol dependence. Addict Biol. 2006;54:100–6. doi: 10.1111/j.1369-1600.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 27.Wingenfeld K, Driessen M, Adam B, Hill A. Overnight urinary cortisol release in women with borderline personality disorder depends on comorbid PTSD and depressive psychopathology. Eur Psychiatry. 2007;22:309–12. doi: 10.1016/j.eurpsy.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Zweig-Frank H, Paris J, Ng Ying Kin NMK, Schwartz G, Steiger H, Nair NPV. Childhood sexual abuse in relation to neurobiological challenge tests in patients with borderline personality disorder and normal controls. Psychiatry Res. 2006;141:337–41. doi: 10.1016/j.psychres.2005.02.009. [DOI] [PubMed] [Google Scholar]