Abstract
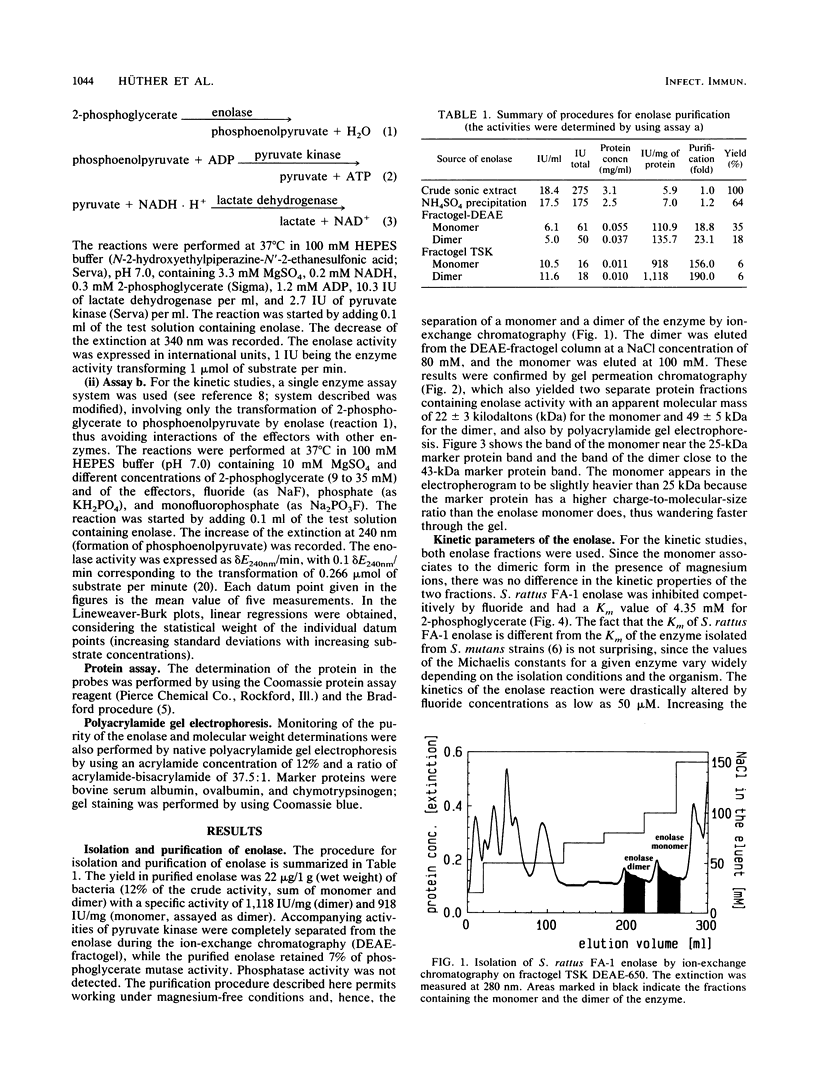
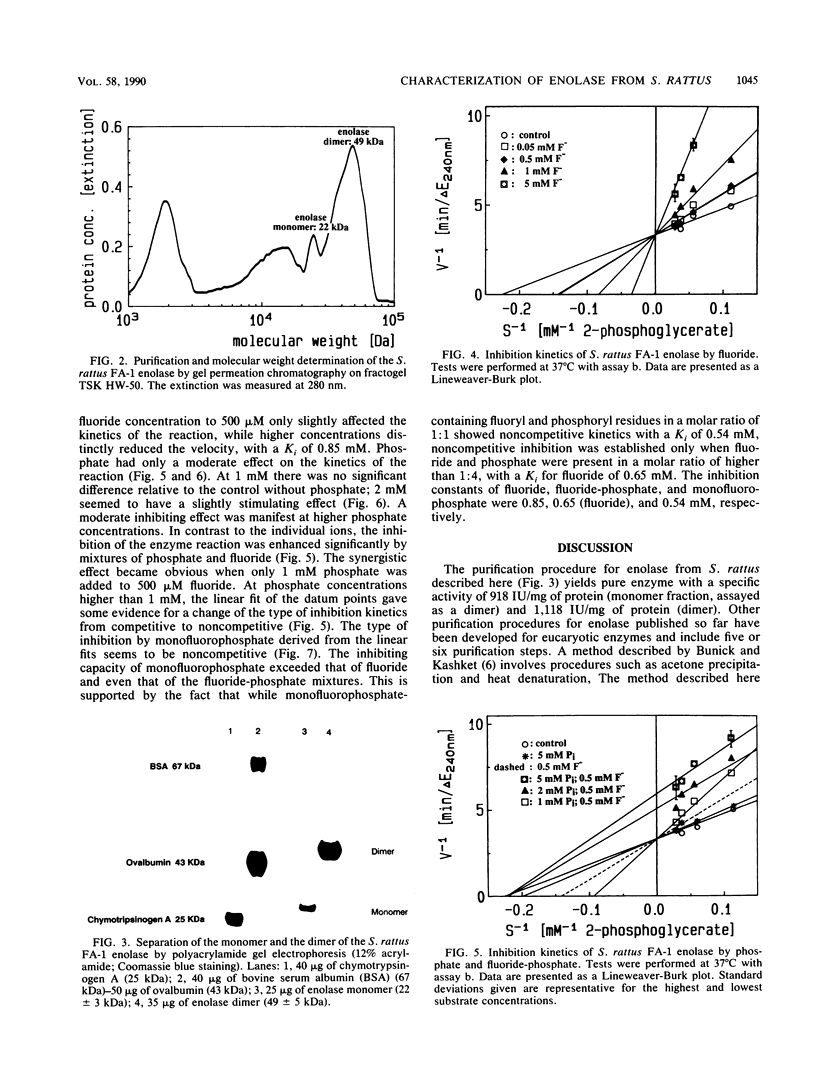
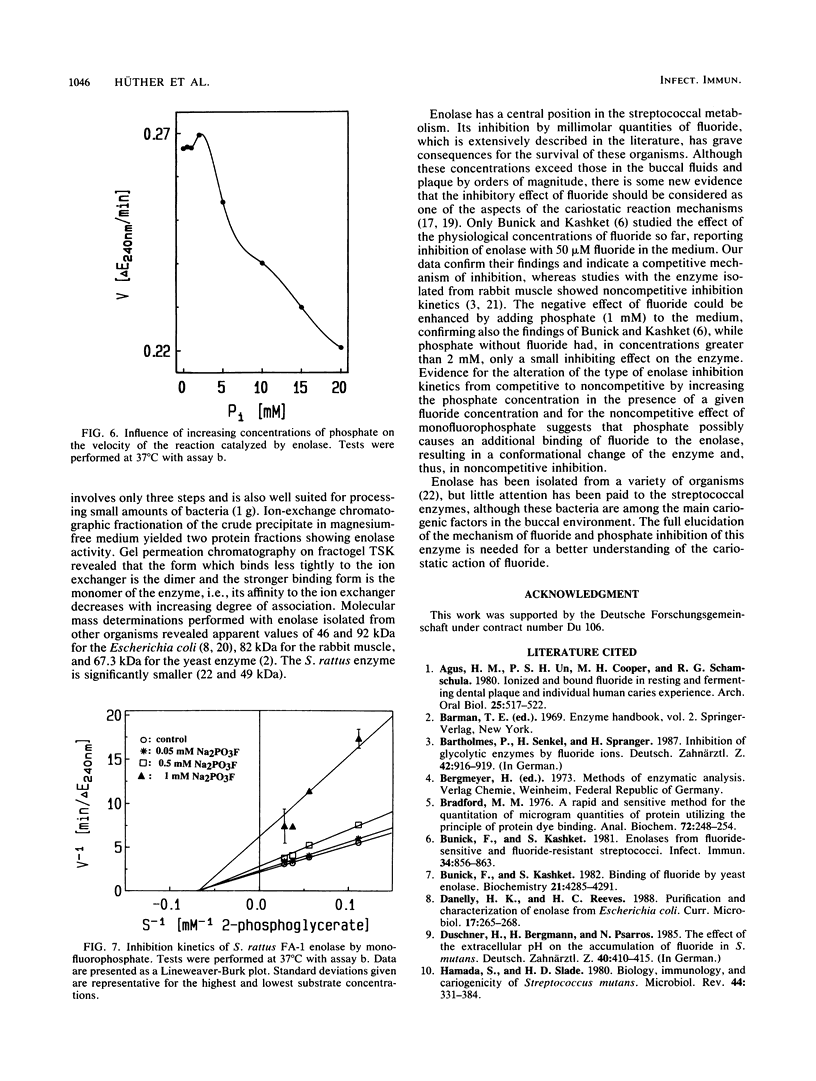
One aspect in a broad spectrum of possible mechanisms of cariostatic reactions of fluoride is its interaction with the metabolism of oral bacteria. Information on the mechanisms and kinetics of fluoride inhibition of essential enzymes of the glycolytic pathway of the relevant bacteria is lacking. In this work, the isolation and purification of enolase from Streptococcus rattus and its characterization are described. The enzyme has been isolated in a monomeric (22 kilodaltons) and dimeric (49 kilodaltons) form. The Km for 2-phosphoglycerate is 4.35 mM. Fluoride inhibition kinetics have competitive character, while phosphate in concentrations above 2 mM and in the presence of 0.5 mM fluoride alters the inhibition kinetics from competitive to noncompetitive. Without fluoride, 2 mM phosphate has a slight stimulatory effect on the enzyme. Monofluorophosphate has a noncompetitive inhibiting effect on the enzyme. This finding suggests that the effect of phosphate may be due to an additional binding of fluoride to the enolase, resulting in a conformational change of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus H. M., Un P. S., Cooper M. H., Schamschula R. G. Ionized and bound fluoride in resting and fermenting dental plaque and individual human caries experience. Arch Oral Biol. 1980;25(8-9):517–522. doi: 10.1016/0003-9969(80)90063-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bunick F. J., Kashket S. Binding of fluoride by yeast enolase. Biochemistry. 1982 Aug 31;21(18):4285–4290. doi: 10.1021/bi00261a017. [DOI] [PubMed] [Google Scholar]
- Bunick F. J., Kashket S. Enolases from fluoride-sensitive and fluoride-resistant streptococci. Infect Immun. 1981 Dec;34(3):856–863. doi: 10.1128/iai.34.3.856-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Ochiai K., Shiota T. Taxonomy of the oral Streptococcus mutans based on colonial characteristics and serological, biochemical and genetic features. Arch Oral Biol. 1979;24(10-11):863–867. doi: 10.1016/0003-9969(79)90052-9. [DOI] [PubMed] [Google Scholar]
- Jenkins G. N., Edgar W. M. Distribution and forms of F in saliva and plaque. Caries Res. 1977;11 (Suppl 1):226–242. doi: 10.1159/000260302. [DOI] [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Kashket S., Bunick F. J. Binding of fluoride in oral streptococci. Arch Oral Biol. 1978;23(11):993–996. doi: 10.1016/0003-9969(78)90255-8. [DOI] [PubMed] [Google Scholar]
- Nowak T., Maurer P. J. Fluoride inhibition of yeast enolase. 2. Structural and kinetic properties of the ligand complexes determined by nuclear relaxation rate studies. Biochemistry. 1981 Nov 24;20(24):6901–6911. doi: 10.1021/bi00527a025. [DOI] [PubMed] [Google Scholar]
- Spring T. G., Wold F. The purification and characterization of Escherichia coli enolase. J Biol Chem. 1971 Nov 25;246(22):6797–6802. [PubMed] [Google Scholar]
- Wang T., Himoe A. Kinetics of the rabbit muscle enolase-catalyzed dehydration of 2-phosphoglycerate. Fluoride and phosphate inhibition. J Biol Chem. 1974 Jun 25;249(12):3895–3902. [PubMed] [Google Scholar]



